Abstract
In contrast to their polymyxin-susceptible parent strains, polymyxin-resistant Salmonella typhimurium mutants (pmrA strains) did not lose their outer membrane permeability barrier to macromolecules such as lysozyme and periplasmic proteins upon polymyxin treatment. The sensitization of pmrA strains to deoxycholate-induced lysis required 10-times-higher polymyxin concentrations than did the sensitization of the parent strains. These findings indicate that the pmrA mutation affects the outer membrane and decreases its susceptibility to polymyxin. By contrast, the pmrA mutants did not differ from their parents in the uptake of gentian violet after treatment with polymyxin, suggesting a degree of specificity in the pmrA effect in the outer membrane.
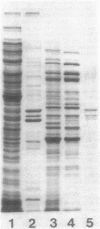
Full text
PDF
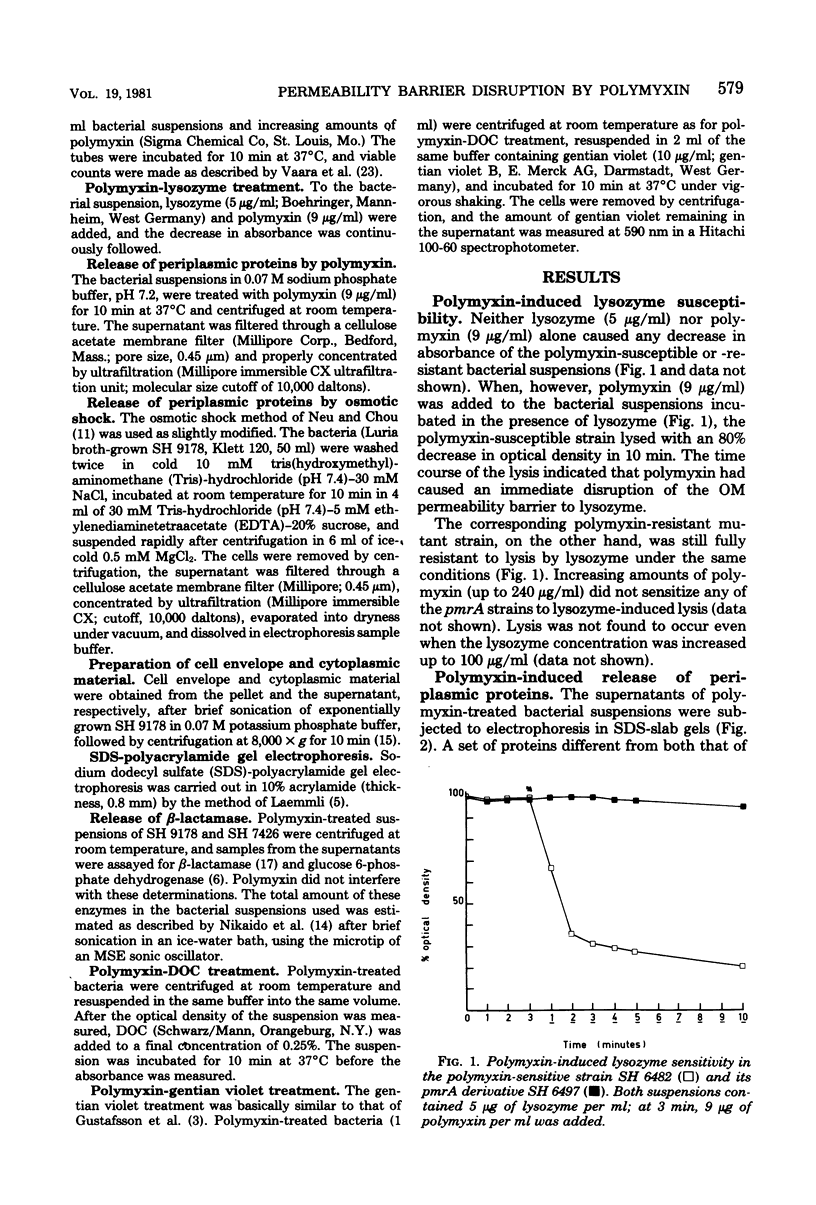
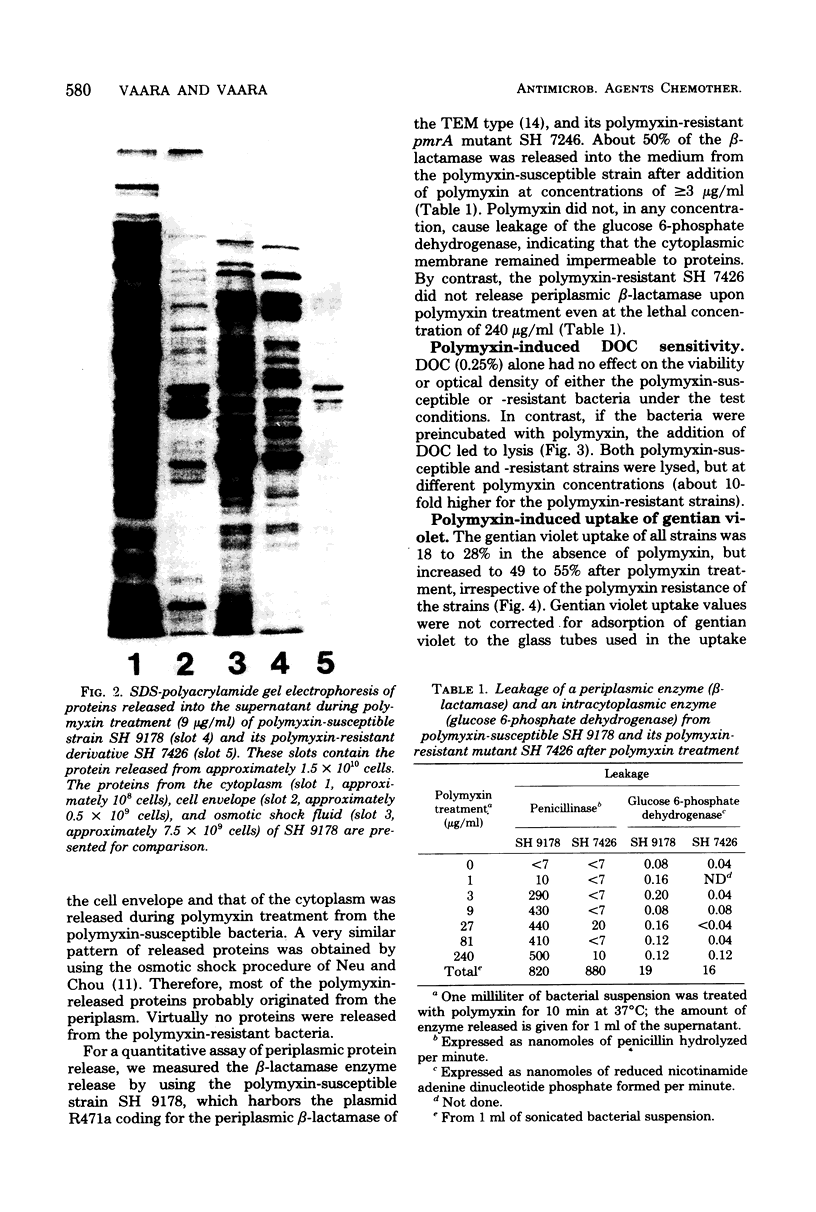
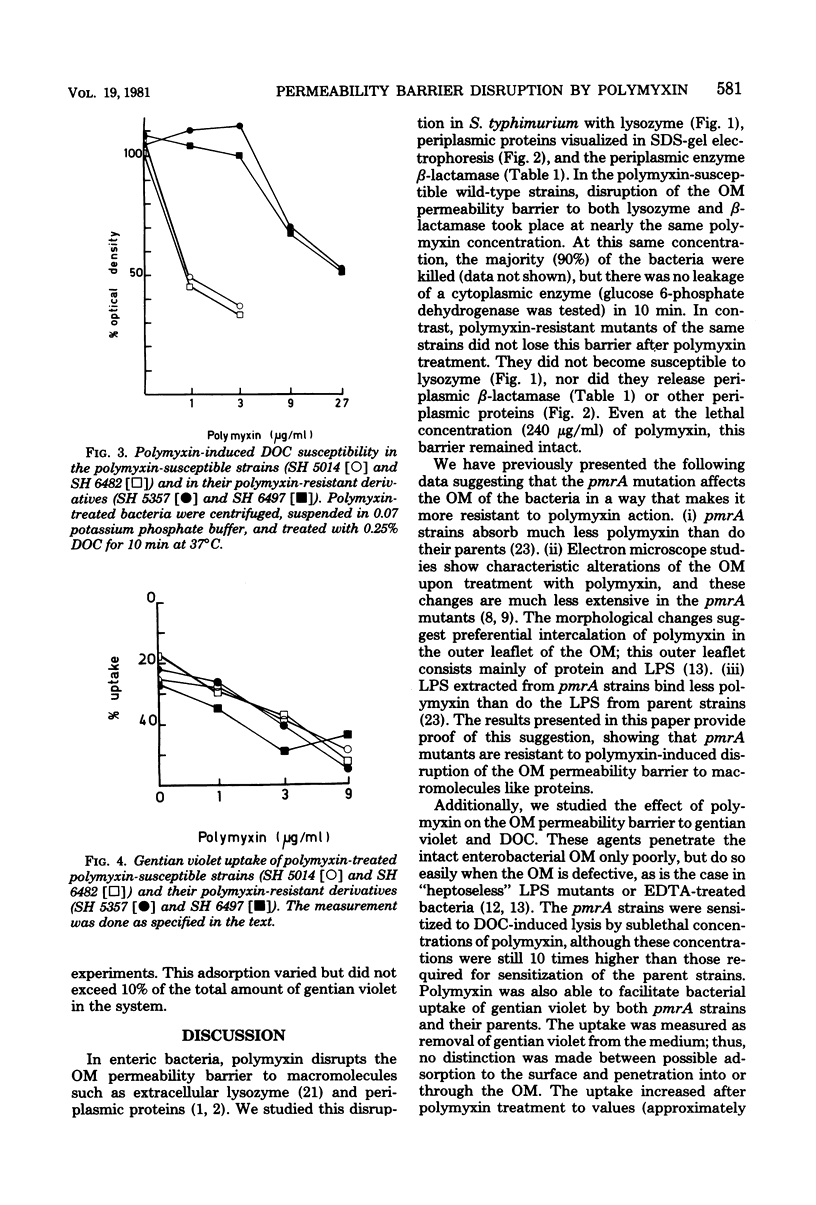


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerny G., Teuber M. Comparative polyacrylamide electrophoresis of periplasmic proteins released from gram-negative bacteria by polymyxin B. Arch Mikrobiol. 1972;82(4):361–370. doi: 10.1007/BF00424939. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lounatmaa K., Mäkelä P. H., Sarvas M. Effect of polymyxin on the ultrastructure of the outer membrane of wild-type and polymyxin-resistant strain of Salmonella. J Bacteriol. 1976 Sep;127(3):1400–1407. doi: 10.1128/jb.127.3.1400-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounatmaa K., Nanninga N. Effect of polymyxin on the outer membrane of Salmonella typhimurium: freeze-fracture studies. J Bacteriol. 1976 Nov;128(2):665–667. doi: 10.1128/jb.128.2.665-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Major outer membrane protein in Salmonella typhimurium induced by maltose. J Bacteriol. 1978 Oct;136(1):286–294. doi: 10.1128/jb.136.1.286-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. S., Storm D. R. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J Antibiot (Tokyo) 1977 Dec;30(12):1087–1092. doi: 10.7164/antibiotics.30.1087. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan-Lotter H., Gupta M., Sanderson K. E. The influence of cations on the permeability of the outer membrane of Salmonella typhimurium and other gram-negative bacteria. Can J Microbiol. 1979 Apr;25(4):475–485. doi: 10.1139/m79-070. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Effect of polymyxin B on antibiotic-resistant Proteus mirabilis. Antimicrob Agents Chemother. 1972 May;1(5):417–421. doi: 10.1128/aac.1.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Teuber M. Lysozyme-dependent production of spheroplast-like bodies from polymyxin B-treated Salmonella typhimurium. Arch Mikrobiol. 1970;70(2):139–146. doi: 10.1007/BF00412204. [DOI] [PubMed] [Google Scholar]
- Teuber M. Susceptibility to polymyxin B in penicillin G-induced Proteus mirabilis L forms and spheroplasts. J Bacteriol. 1969 May;98(2):347–350. doi: 10.1128/jb.98.2.347-350.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Sarvas M. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):664–667. doi: 10.1128/jb.139.2.664-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN G. H., GRAY J., YURCHENCO J. A. Effect of polymyxin on the lysis of Neisseria catarrhalis by lysozyme. J Bacteriol. 1957 Dec;74(6):788–793. doi: 10.1128/jb.74.6.788-793.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]