Abstract
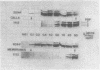
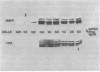
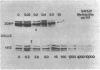
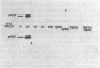
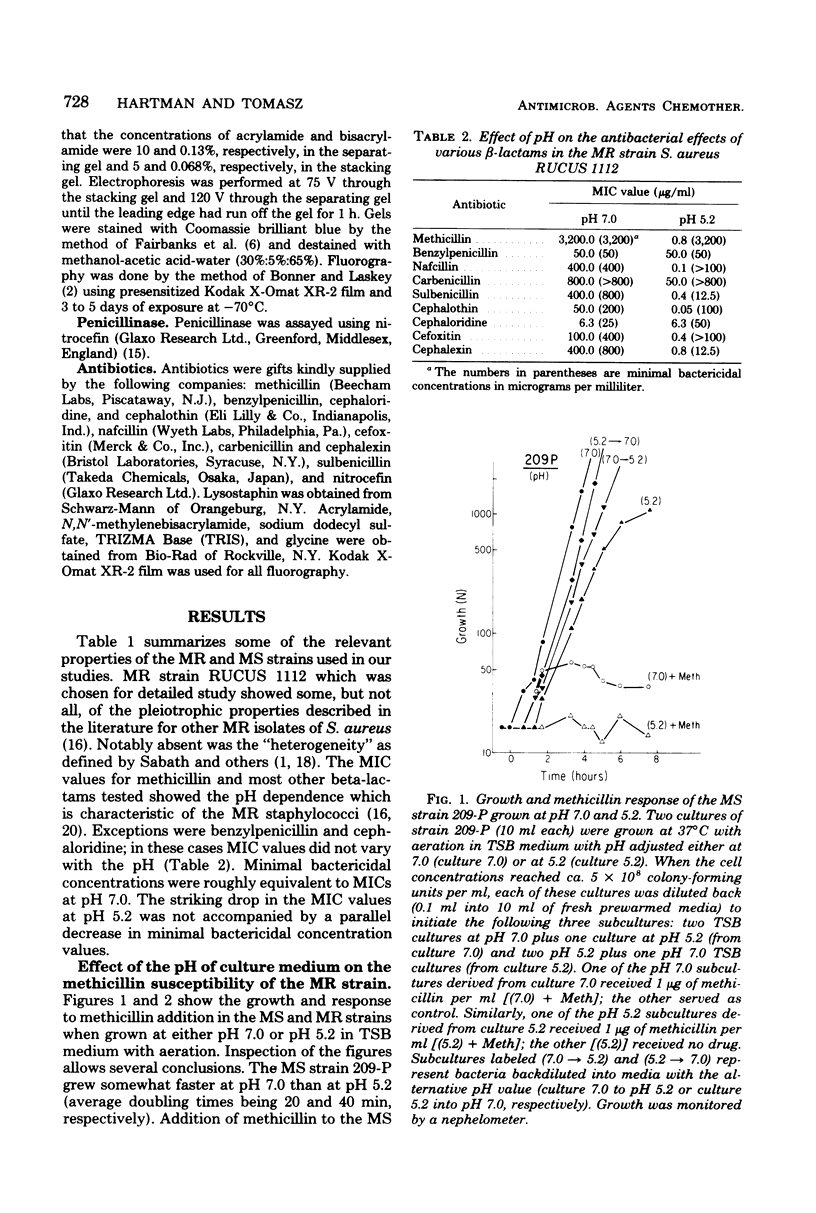
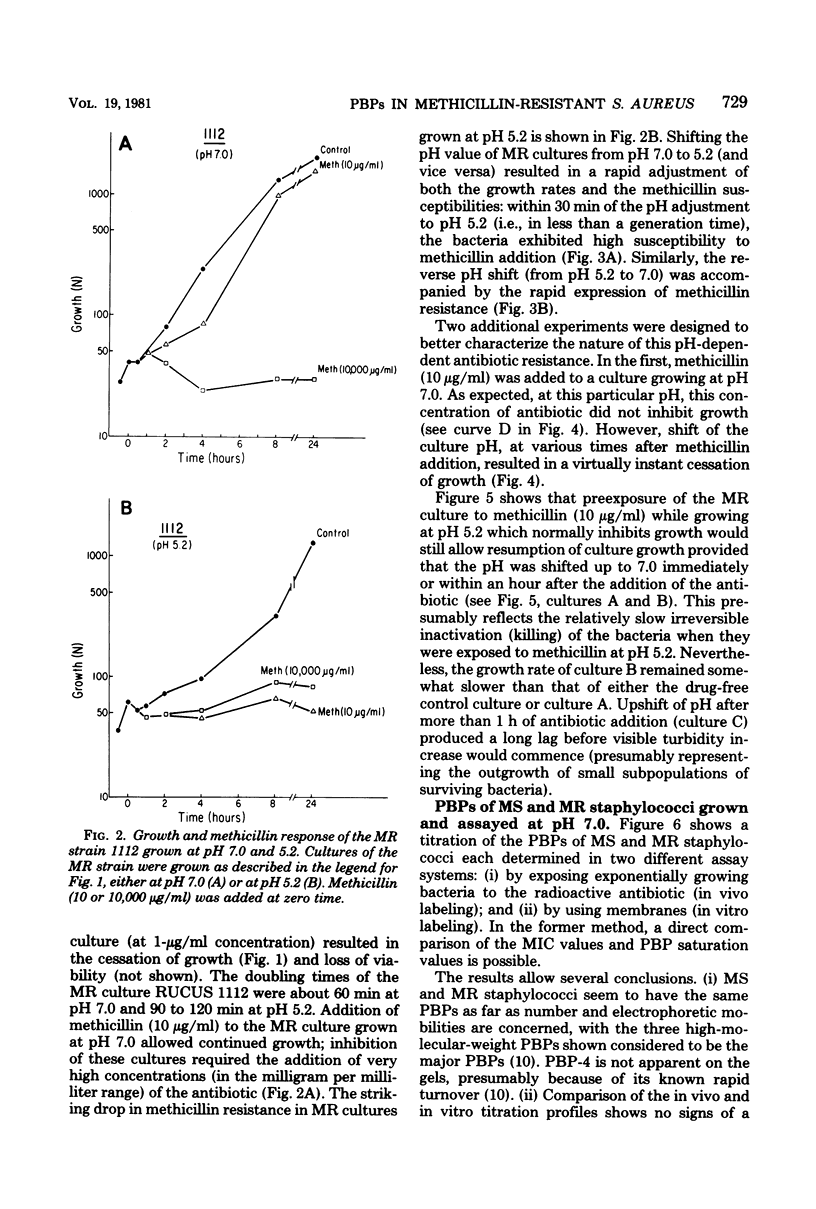
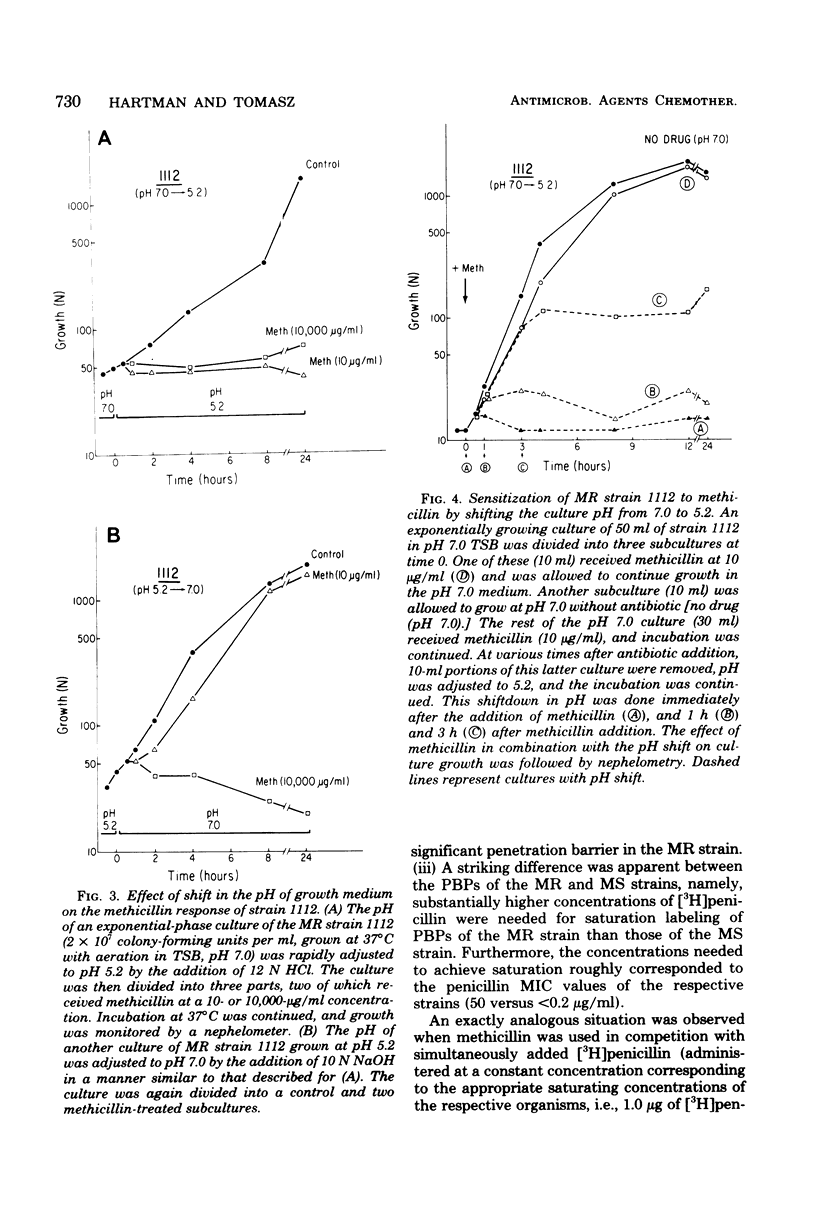
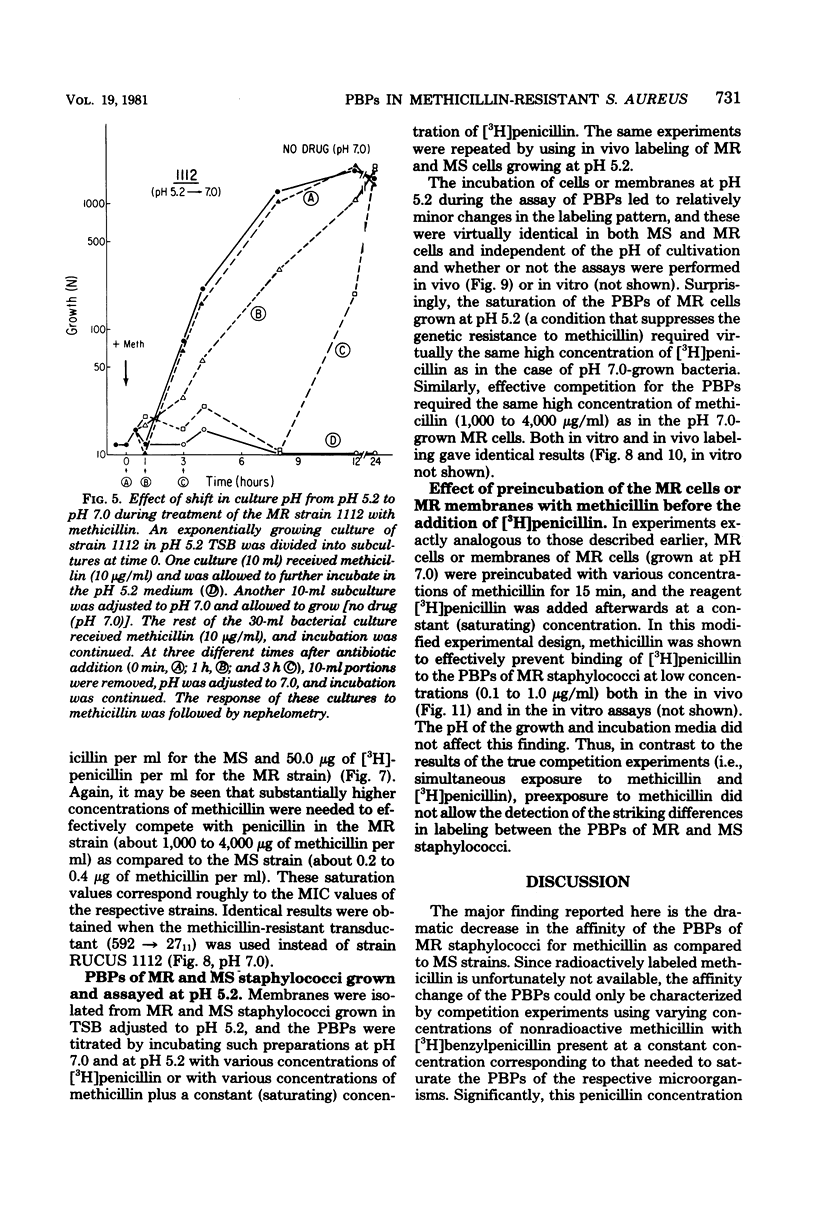
The penicillin-binding proteins (PBPs) of a methicillin-resistant (MR) and a methicillin-susceptible (MS) Staphylococcus aureus were compared by various approaches involving the use of high-specific-activity [3H]penicillin as a reagent. The MR and MS strains were found to contain PBPs of the same number and electrophoretic mobilities. However, saturation of PBPs 1, 2, and 3 by methicillin in the MR strain required the use of several thousands of micrograms of antibiotic per milliliter, whereas 0.2 to 0.4 micrograms of methicillin per ml was sufficient to effectively compete with [3H]penicillin for the PBPs for the MS strain. Additional experiments indicate that these differences most likely reflect a greatly decreased affinity of the PBPs of the MR strain as compared to those of the MS strain. Shift of the pH of the culture medium of the MR strain from pH 7.0 to 5.2 resulted in an immediate drop in phenotypic resistance to methicillin (from a minimal inhibitory concentration value of 3,200 micrograms/ml at pH 7.0 to 0.8 microgram/ml at pH 5.2). Examination of the methicillin affinities of PBPs in MR bacteria grown at pH 5.2 showed the presence of the same low-affinity PBPs as in bacteria grown at pH 7.0. Thus, the pH-dependent resensitization to methicillin cannot be explained by a parallel increase in the antibiotic affinities of the PBPs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annear D. I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibioics. Med J Aust. 1968 Mar 16;1(11):444–446. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Bruns W., Keppeler H. Mechanism of intrinsic penicillin-resistance in Staphylococcus aureus. Binding of penicillin G to the cytoplasmic membrane of resistant staphylococci. Arzneimittelforschung. 1980;30(9):1469–1475. [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. W., James A. M. Effect of growth temperature on the surface properties of cells of Staphylococcus aureus with particular reference to methicillin-resistance. Microbios. 1972 Sep-Oct;6(22):169–178. [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Leaf C. D., Gerstein D. A., Finland M. Altered cell walls of Staphylococcus aureus resistant to methicillin. Nature. 1970 Mar 14;225(5237):1074–1074. doi: 10.1038/2251074a0. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Byers K., Toftegaard I. Resistance of Staphylococcus aureus to penicillins and cephalosporins: reversal of intrinsic resistance with some chelating agents. Ann N Y Acad Sci. 1974 Jul 31;236(0):435–443. doi: 10.1111/j.1749-6632.1974.tb41508.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Gerstein D. A. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Nov;2(5):350–355. doi: 10.1128/aac.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J. The problems of drug-resistant pathogenic bacteria. Factors influencing methicillin resistance in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:258–266. doi: 10.1111/j.1749-6632.1971.tb30662.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Vernon G. N., Russell A. D. Surface properties of cells of some methicillin-resistant strains of Staphylococcus aureus. J Antibiot (Tokyo) 1977 Nov;30(11):974–979. doi: 10.7164/antibiotics.30.974. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Dorian K. J., Sabath L. D. Cell wall composition and associated properties of methicillin-resistant Staphylococcus aureus strains. J Bacteriol. 1978 Dec;136(3):976–982. doi: 10.1128/jb.136.3.976-982.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]