Abstract
Prions are self-perpetuating and, in most cases, aggregation-prone protein isoforms that transmit neurodegenerative diseases in mammals and control heritable traits in yeast. Prion conversion requires a very high level of identity of the interacting protein sequences. Decreased transmission of the prion state between divergent proteins is termed “species barrier” and was thought to occur because of the inability of divergent prion proteins to coaggregate. Species barrier can be overcome in cross-species infections, e.g., from “mad cows” to humans. We studied the counterparts of yeast prion protein Sup35, originated from three different species of the Saccharomyces sensu stricto group and exhibiting the range of prion domain divergence that overlaps with the range of divergence observed among distant mammalian species. All three proteins were capable of forming a prion in Saccharomyces cerevisiae, although prions formed by heterologous proteins were usually less stable than the endogenous S. cerevisiae prion. Heterologous Sup35 proteins coaggregated in the S. cerevisiae cells. However, in vivo cross-species prion conversion was decreased and in vitro polymerization was cross-inhibited in at least some heterologous combinations, thus demonstrating the existence of prion species barrier. Moreover, the barrier between the S. cerevisiae protein and its Saccharomyces paradoxus and Saccharomyces bayanus counterparts was asymmetric both in vivo and in vitro. Our data show that a decreased cross-species prion transmission does not necessarily correlate with a lack of cross-species coaggregation, suggesting that species-specificity of prion transmission is controlled at the level of conformational transition rather than coaggregation.
Keywords: amyloid, Saccharomyces bayanus, Saccharomyces cerevisiae, Saccharomyces paradoxus, Sup35
Amyloids are highly ordered self-seeded fibrous β-rich protein aggregates associated with some diseases in mammals and humans. Amyloid-based prions (infectious proteins) are thought to propagate via nucleated polymerization so that aggregated prion “seeds” immobilize a monomeric protein of the same amino acid sequence and convert it into a prion (1). Mammalian prion proteins cause fatal transmissible encephalopathies such as bovine spongiform encephalopathy (“mad cow disease”) and Creutzfeld–Jacob disease (for review, see refs. 2–4), while yeast prion proteins Sup35, Ure2, and Rnq1 control inheritance of non-Mendelian traits transmitted via cytoplasmic infection (for review, see refs. 5 and 6).
Yeast prion proteins contain prion domains (PrDs) that are terminally located, dispensable for the normal cellular function of a respective protein, and responsible for prion properties (5). PrDs are more variable in evolution compared with the functional domains of the same proteins (7). Divergent yeast PrDs (8–12) and even artificially generated PrDs with the randomly reshuffled amino acid sequences (13, 14) retain the ability to generate and maintain a prion state in Saccharomyces cerevisiae. However, conversion of a prion state from a given preexisting prion to the newly mobilized protein molecules is a strictly sequence-specific process in each case. Cross-species prion transmission in mammals is reduced because of so-called “species barrier,” which is overcome in certain cases, e.g., between mad cows and humans (3). Despite its obvious importance for prediction of the potential cross-species infectivity of heterologous prions, the mechanism of the sequence-specificity in prion conversion is poorly understood. While in vitro and in situ assays for cross-species “seeding” of the mammalian prion protein aggregation were developed (15–18), some features observed in vitro disagreed with the in vivo data (for comments, see ref. 19).
In yeast, prion species barrier between highly divergent proteins from distantly related yeast genera Saccharomyces and Pichia or Candida coincided with the inability of heterologous PrDs to coaggregate in vivo and in vitro (9, 10, 20, 21). Homologs of prion protein Ure2 from the closely related Saccharomyces species exhibited no species barrier, although researchers disagreed on whether Saccharomyces paradoxus Ure2 is capable of forming a prion at all (22–24). Therefore, it remained unclear whether prion species barrier exists in yeast at the levels of sequence divergence that are comparable to those observed in mammals.
We have developed a new experimental system for studying prion species barrier that employs the closely related Sup35 proteins from the Saccharomyces sensu stricto group (25). Levels of sequence divergence among these proteins overlap the range of divergence detected among the prion proteins of the distantly related mammalian species. By using this system we have demonstrated that closely related yeast proteins capable of coaggregation still exhibit a species barrier.
Results and Discussion
Conservation and Divergence of the S. sensu stricto Sup35 Proteins.
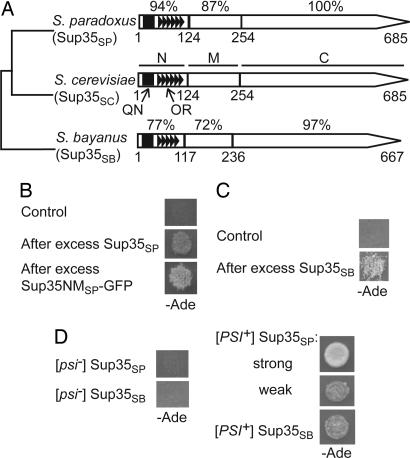
Sup35 is a translation termination factor composed of the following regions: (i) N-proximal PrD (Sup35N), required for [PSI+], the prion form of Sup35, and containing the N-terminal QN-rich stretch (QN) and the region of oligopeptide repeats (ORs) (5); (ii) middle region (Sup35M) of unknown function; and (iii) C-proximal region (Sup35C), required and sufficient for termination of translation (26, 27). In agreement with previous reports demonstrating the higher rate of Sup35NM evolution in comparison to Sup35C (7), the amino acid sequences of the N, M, and C regions [Fig. 1A and supporting information (SI) Fig. 5] show, respectively, 94%, 87%, and 100% of identity between S. cerevisiae and S. paradoxus (28) and, respectively, 77%, 72%, and 97% of identity between S. cerevisiae and Saccharomyces bayanus (29, 30). S. bayanus OR region is shortened by one repeat.
Fig. 1.
S. paradoxus and S. bayanus Sup35 proteins retain prion-forming abilities despite divergence of PrD sequences. (A) Structural and functional organization of the S. sensu stricto Sup35 proteins. N, M, C, QN, and OR refer to the Sup35N, Sup35M, Sup35C, QN-rich stretch, and ORs, respectively. Numbers correspond to amino acid positions; the percentage of amino acid identity to S. cerevisiae is shown for each region of S. paradoxus and S. bayanus proteins individually. For sequence alignment of the Sup35N regions, see SI Fig. 5. Data are from refs. 28–30 and the Yeast Genome Database (www.yeastgenome.org), confirmed by sequencing of our PCR-amplified clones. (B and C) Transient overproduction of S. paradoxus Sup35 protein (Sup35SP) or the Sup35NMSP-GFP fusion protein (B), or transient overproduction of S. bayanus Sup35 protein (Sup35SB) (C) induces prion formation in the [psi− PIN+] S. cerevisiae strains bearing the SUP35SP (B) or SUP35SB (C) gene instead of SUP35SC. Empty plasmids pmCUP-GFP (B) or pRS316GAL (C) were used as controls. Prion formation was detected by growth on −Ade medium after induction on PCUP1-SUP35SP or PCUP1-SUP35NMSP-GFP constructs on the medium with 100 μM CuSO4 (B) or PGAL-SUP35SB construct on Gal medium (C). (D) Sup35SP generates both strong and weak prion variants, whereas Sup35SB generates only weak prion variants in S. cerevisiae, as judged from the efficiency of ade1–14 suppression reflected by growth on −Ade. Note that both strong and weak variants of the Sup35SP prion show low mitotic stability (see SI Table 2). Plates were photographed after 5 (B), 8 (C), and 7 (D) days of incubation.
Heterologous S. sensu stricto Sup35 Proteins Are Capable of Forming a Prion in S. cerevisiae.
The SUP35 ORFs of S. paradoxus (SUP35SP) and S. bayanus (SUP35SB), placed onto a low-copy (centromeric, or CEN) shuttle plasmid under the endogenous S. cerevisiae SUP35 promoter (PSUP35), produced the respective Sup35 proteins at the same level as did the analogous S. cerevisiae SUP35 (SUP35SC) construct (data not shown) and conferred viability to the S. cerevisiae strain lacking the endogenous chromosomal SUP35SC gene.
To check whether S. sensu stricto Sup35 proteins can be turned into a prion state in the S. cerevisiae cell environment, we used the UGA reporter allele ade1–14 (31). The [psi−] ade1–14 cells do not grow on −Ade medium and accumulate a red pigment on the complete medium [yeast extract/peptone/dextrose (YPD)]. In the [PSI+] cells, sequestration of the Sup35 termination factor into prion aggregates causes read-through (suppression) of UGA, resulting in growth on −Ade and a white color on YPD. Transient overproduction of endogenous Sup35- or Sup35N-containing fragments induces de novo formation of [PSI+] in the S. cerevisiae [psi−] cells bearing another QN-rich prion, [PIN+] (31–34). Likewise, we have shown that transient overproduction of Sup35SP or its NM-containing derivatives (Fig. 1B), or transient overproduction of Sup35SB (Fig. 1C), induced generation of the Ade+ cells in the S. cerevisiae strain, lacking endogenous Sup35 and maintained alive by CEN plasmids bearing, respectively, SUP35SP or SUP35SB. The resulting Ade+ phenotype was curable by guanidine hydrochloride (data not shown) as typical of the [PSI+] prion (see ref. 31). Prion induction by overproduction was also detected in all possible heterologous combinations of the “inducer” and “inducee” S. sensu stricto Sup35 proteins (data not shown). This observation is not surprising because even the highly divergent Sup35NM region of Pichia methanolica is capable of inducing Sup35SC into a prion when overproduced (8, 9).
Suppression Efficiency and Mitotic Stability of Heterologous Prion Isolates in S. cerevisiae.
In S. cerevisiae, multiple variants or “strains” of the endogenous [PSI+] prion have been found (32). “Strong” [PSI+] variants are white on YPD medium and grow fast (in 2–3 days) on −Ade, whereas “weak” variants are medium pink on YPD and grow slowly (in 4–7 days) on −Ade. Strong variants are usually 100% stable in mitotic divisions, whereas weak variants produce some [psi−] colonies in nonselective conditions. Like Sup35SC, Sup35SP generated both strong and weak prion variants in S. cerevisiae as judged by growth (Fig. 1D) and color, but weak isolates were more abundant, and both strong and weak prion variants of Sup35SP accumulated 10–25% of [psi−] colonies after 24–25 cell divisions in nonselective conditions (SI Table 2). Prion variants of Sup35SB were always characterized by weak suppression (Fig. 1D) and low stability (SI Table 2).
To identify the region of Sup35 responsible for low prion stability, we constructed chimeric genes in which the SUP35N region of SUP35SC was substituted with either SUP35NSP or SUP35NSB. Chimeric protein with Sup35NSB continued to produce mitotically unstable prions, whereas chimeric protein with Sup35NSP produced prions of various mitotic stabilities, and some of these prion isolates were stable (SI Table 3). Therefore, decreased mitotic stability of heterologous prions is primarily determined by the differences in Sup35N region for Sup35SB but not for Sup35SP.
Divergent Sup35 Proteins Coaggregate in S. cerevisiae.
Sup35SC protein is precipitated at high speed from the extracts of the prion-containing ([PSI+]) strain, whereas the nonprion ([psi−]) strain retains a significant fraction of Sup35SC in the soluble phase (31). Centrifugation analysis confirmed that the proportion of the Sup35SP or Sup35SB protein in the aggregated state was increased in a culture containing respective protein in the prion form, compared with the isogenic nonprion culture (data not shown).
In extracts of the S. cerevisiae [PSI+] strain, bearing both endogenous SUP35SC and newly introduced SUP35SB or SUP35SP, the Sup35-reacting material was shifted to the pellet, in contrast to the isogenic [psi−] strain (Fig. 2A). This indicates that heterologous protein is precipitated together with the endogenous Sup35SC prion aggregates. Shift of Sup35SB could be visualized directly because this protein is distinguishable by size from Sup35SC. To visualize the Sup35SP-based construct, we used a chimeric protein composed of the N and M regions of Sup35SP fused to the green fluorescent protein (GFP). This protein, distinguishable by size from intact Sup35SC, was also shifted to the insoluble phase together with Sup35SC in the [PSI+] compared with the [psi−] extracts (Fig. 2B).
Fig. 2.
Coaggregation of heterologous Sup35 proteins in S. cerevisiae. (A) The S. cerevisiae [PSI+] strain simultaneously expressing both endogenous (Sup35SC) and heterologous (Sup35SP or Sup35SB) proteins shows all of the Sup35-reacting material in the pellet (P) after centrifugation at 39,000 × g, whereas the isogenic [psi−] strain retains a fraction of Sup35 in the supernatant (S). Shift of Sup35SB to pellet can be monitored directly because of its lower molecular weight, compared with Sup35SC. T, total lysate. (B) The chimeric Sup35NMSP-GFP protein, expressed from the PCUP1 promoter in the presence of background levels (2 μM) of CuSO4, shifts to pellet together with the endogenous Sup35SC protein in the [PSI+] extract, in contrast to the [psi−] extract. Designations are as in A. (C–F) The GFP-tagged NM fragments of Sup35SC (C), Sup35SP (D), and Sup35SB (E), but not the GFP-tagged Sup35NM fragment of P. methanolica (Sup35NMPM-GFP, F), colocalize with the aggregated clumps of RFP-tagged Sup35SC in the S. cerevisiae [PSI+] cells. GFP- and RFP-tagged constructs were expressed from the PCUP1 and PGAL promoters, respectively, in the Gal+Raf medium supplemented with 150 μM CuSO4. In each case, >100 cells containing both GFP and RFP aggregates were scored, and the percentage of cells with colocalization is shown. Scale bars are indicated.
Coaggregation of the heterologous Sup35 proteins was also confirmed by fluorescence microscopy. We used the Sup35NM fragments tagged with GFP or red fluorescent protein (RFP). These tagged proteins usually show diffuse fluorescence in the nonprion cells (as confirmed by us for the constructs based on Sup35NMSP or Sup35NMSB) but generate cytologically detectable clumps in yeast cells bearing Sup35 in a prion state (for review, see ref. 31). The constructs producing GFP-tagged Sup35NMSC, Sup35NMSP, or Sup35NMSB were introduced into the strain containing endogenous Sup35SC in a prion form and bearing the plasmid that produces Sup35SC-RFP. Green and red clumps colocalized with each other in all or most cells where both types of clumps were detected (Fig. 2 C–E). In contrast, highly divergent Sup35NM of P. methanolica, tagged with GFP, never colocalized with Sup35SC-RFP (Fig. 2F).
Species Barrier in Prion Transmission Between the Divergent Sup35 Proteins Is Detected Despite Coaggregation.
When an extra copy of the homologous SUP35 gene was introduced into the strain containing the respective protein in a prion form, it did not affect suppression of ade1–14. In contrast, introduction of a plasmid with heterologous SUP35 gene usually decreased or eliminated suppression, as detected by inhibition of growth on −Ade medium in the presence of such a plasmid (Fig. 3A). Suppression was restored on the medium not selective for the heterologous plasmid (data not shown), indicating that endogenous Sup35SC prion was not lost. Thus, heterologous Sup35 protein remained functional despite its aggregation (see above and Fig. 2).
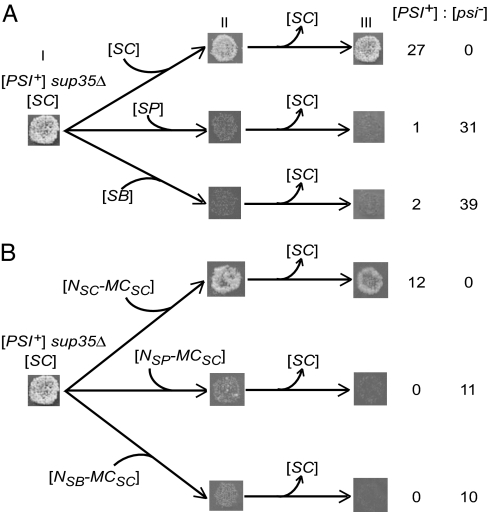
Fig. 3.
Prion species barrier between the closely related Sup35 proteins in the plasmid shuffle assay. Designations SC, SP, and SB refer to the SUP35SC, SUP35SP, and SUP35SB genes, respectively. Donor [PSI+] sup35Δ strain with SC gene on the CEN plasmid, which grew on −Ade before the experiment (stage I), was transformed individually with CEN plasmids either bearing the intact SC, SP, or SB genes (A) or containing the chimeric constructs composed of the SUP35MC region of S. cerevisiae (MCSC) and SUP35N regions (N) of various origins (B). In B, reconstructed SUP35 gene with SUP35NSC origin was used as a control. In contrast to intact or reconstructed SUP35SC, heterologous genes (A) or chimeric genes with the heterologous N regions (B) inhibited suppression of ade1–14 by [PSI+], as judged by decreased growth on −Ade medium selective for both plasmids (stage II). After elimination of the original SC plasmid (stage III), all colonies with the new SC plasmid or reconstructed NSC-MCSC plasmid retained [PSI+], whereas most or all colonies with SP, SB, or chimeric plasmids containing NSP or NSB lost [PSI+], as seen by growth/no growth on −Ade medium, respectively. Numbers of [PSI+] and [psi−] colonies obtained are given in each case.
To check whether the prion state can be transmitted between the heterologous Sup35 proteins, plasmid shuffle was performed. For this purpose, the [PSI+] sup35Δ strain bearing SUP35SC on a CEN plasmid has been individually transformed with CEN plasmids bearing SUP35SC, SUP35SP, or SUP35SB with a different marker. Transformation was followed by the loss of the original SUP35SC plasmid. Whereas shuffle for SUP35SC to SUP35SC exclusively produced [PSI+] progeny, shuffle from SUP35SC to SUP35SP or SUP35SB almost exclusively produced the [psi−] progeny (Fig. 3A). As confirmed by centrifugation analysis, these [psi−] colonies contained the Sup35SP or Sup35SB protein in the soluble phase and did not restore the [PSI+] state after reintroduction of the SUP35SC plasmid, followed by the loss of SUP35SP or SUP35SB plasmid (data not shown). In the rare exceptional cases when cross-species conversion from Sup35SC to Sup35SP or Sup35SB occurred, it generated weak [PSI+] strains with low mitotic stability (data not shown) similar to the majority class prions generated by these proteins as a result of self-induction (see above), even if the strong Sup35SC prion variant was used as the initial prion source.
Moreover, the [PSI+] state was not transmitted by shuffle from SUP35SC to the chimeric constructs containing the SUP35N regions of S. paradoxus or S. bayanus and the SUP35MC region of S. cerevisiae (Fig. 3B). This shows that the Sup35N region is responsible for the species barrier.
Species barrier was also confirmed when diploid [PSI+] strain homozygous by ade1–14 and heterozygous by sup35Δ::HIS3 has been transformed with a CEN plasmid containing SUP35SC, SUP35SP, or SUP35SB, sporulated, and dissected. All sup35Δ [SUP35SC] spore clones remained [PSI+], whereas all sup35Δ spore clones with the SUP35SP or SUP35SB plasmid became [psi−] (SI Table 4).
Prions generated by Sup35SP and Sup35SB were frequently lost during transformation that complicated use of the above mentioned techniques for these strains. To check whether prion state is transmitted from Sup35SP or Sup35SB to Sup35SC, we mated the [PSI+] sup35Δ strains bearing a SUP35SP or SUP35SB plasmid to the isogenic [psi−] sup35Δ strains bearing either homologous or heterologous (SUP35SC) gene on a plasmid with a different marker. Resulting diploids were cured of the original plasmid and checked for the presence of [PSI+]. [PSI+] cells were obtained in heterologous combinations, although with at least several-fold lower frequency than in homologous combinations (data not shown). Remarkably, while Sup35SB prions produced a variety of Sup35SC prion isolates of different stringencies, the Sup35SP prion usually produced strong and stable Sup35SC prion isolates. This phenomenon formally resembles so-called “adaptation” of the heterologous prion strains after conversion of prion state to the host protein in the mammalian systems (3).
Finally, we compared efficiencies of prion transmission between all combinations of the S. sensu stricto Sup35 proteins “used in this work” in one and the same type of assay by employing cytoplasm transfer, or cytoduction (35). Cytoplasm was transferred from each of the [PSI+] sup35Δ donor strains with different SUP35 genes to the set of recipient karyogamy-deficient [psi−] sup35Δ strains with different SUP35 genes. The [PSI+] transmission was highly efficient in each homologous combination but very inefficient in most heterologous combinations, specifically from Sup35SC to Sup35SP or Sup35SB, from Sup35SP to Sup35SB, and from Sup35SB to Sup35SP (Table 1). Interestingly, the prion state was transferred with high frequency from Sup35SP to Sup35SC, and with only moderately decreased frequency from Sup35SB to Sup35SC, indicating that prion species barrier is not completely symmetric. Cross-species prion conversion detected in these combinations by cytoduction was certainly higher than one detected by mating and shuffle assay (see above). This could be due to different genotype of the cytoduction recipient strains or, more likely, different experimental design. In cytoduction assay, the number of cell divisions in nonselective conditions both before and after cytoplasm transfer was minimized, thus effectively eliminating the possibility of the spontaneous loss of prion state. Therefore, we believe that cytoduction provides a more accurate assessment of the efficiency of cross-species conversion. Observed asymmetry of prion species barrier in yeast resembles the situation described in some mammalian systems (15).
Table 1.
Transmission of prion state by cytoduction
| [PSI+] donor | [psi−] recipient | Cytoductants |
||
|---|---|---|---|---|
| [PSI+] (%) | [psi−] | Total | ||
| SUP35SC | SUP35SC | 64 (100) | 0 | 64 |
| SUP35SP | 7 (13.0) | 47 | 54 | |
| SUP35SB | 1 (2.1) | 46 | 47 | |
| SUP35SP | SUP35SC | 43 (95.6) | 2 | 45 |
| SUP35SP | 39 (97.5) | 1 | 40 | |
| SUP35SB | 1 (3.8) | 25 | 26 | |
| SUP35SB | SUP35SC | 33 (76.7) | 10 | 43 |
| SUP35SP | 2 (6.9) | 27 | 29 | |
| SUP35SB | 39 (97.5) | 1 | 40 | |
The donor [PSI+] sup35Δ strains with a SUP35 gene of S. cerevisiae, S. paradoxus, or S. bayanus were grown on −Ade medium to minimize the spontaneous loss of [PSI+] and mated on YPD medium individually to each representative of the set of cytoduction recipient [psi−] sup35Δ strains bearing a SUP35 plasmid with a different marker. After mating, cells were plated onto the medium with ethanol, glycerol, and cycloheximide, selective for cytoductants. Resulting colonies were analyzed for the presence of [PSI+] by growth on −Ade medium. Numbers of [PSI+] and [psi−] cytoductants are given. Exceptional Ura+cytoductants in which the SUP35 plasmid was transferred from the donor were excluded from analysis. For the [PSI+] SUP35SC donor, the same results were obtained with the other (weak) prion variant, as well as in the version of the experiment where the donor strain was grown under nonselective conditions (data not shown). The control [psi−] donor strains did not produce Ade+ cytoductants (data not shown).
Cross-Seeding and Cross-Inhibition of Each Other's Polymerization by the Divergent Sup35 Proteins in Vitro.
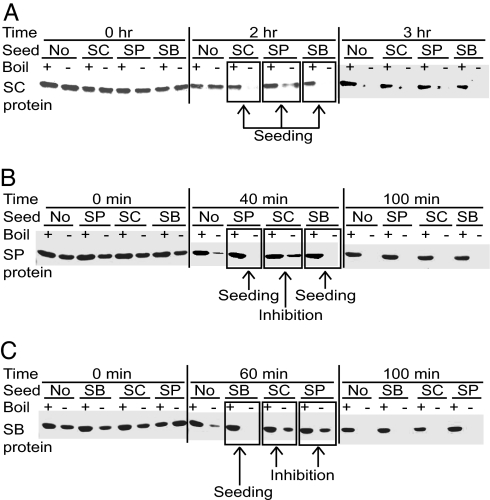
In vitro polymerization of a purified Sup35NM fragment has been routinely used to study prion properties of Sup35 (36–38). Our data (Fig. 4) show that Sup35NMSP and Sup35NMSB are spontaneously polymerized in vitro in the nondenaturing conditions even more rapidly than Sup35NMSC. For each of these three proteins, lag period of polymerization reaction was decreased by addition of the preformed homologous Sup35NM polymers (seeds) at a 1:20 ratio. Likewise, polymerization of Sup35NMSC was accelerated by the heterologous preformed polymers of Sup35NMSP or Sup35NMSB (Fig. 4A), confirming that divergent Sup35NM domains of S. sensu stricto interact with each other in vitro as well as in vivo. Polymerization of Sup35NMSP was accelerated by the preformed Sup35NMSB seed but delayed by the preformed Sup35NMSC seed (Fig. 4B), whereas polymerization of Sup35NMSB was delayed by the preformed heterologous polymers of Sup35NMSC or Sup35NMSP (Fig. 4C). Thus, species barrier between the S. sensu stricto proteins is detected in vitro, although not in all reciprocal combinations. Moreover, in vitro assay confirms the asymmetry of the species barrier between Sup35SC and other S. sensu stricto Sup35 protein, detected by cytoduction.
Fig. 4.
In vitro polymerization of the S. sensu stricto Sup35NM protein fragments. The purified (His)6-tagged Sup3NM regions of S. cerevisiae (Sup35NMSC or SC, A), S. paradoxus (Sup35NMSP or SP, B), and S. bayanus (Sup35NMSB or SB, C) spontaneously polymerize in the nondenaturing conditions after a certain lag period, as detected by a decrease of the monomeric fraction remaining soluble in SDS and capable of entering the SDS/PAGE gel without boiling. Boiled samples where all protein enters the gel are shown in each case as controls. Addition of preformed polymers at the ratio of 1:20 leads to the following results: Sup35NMSC promotes polymerization of Sup35NMSC (A) and Sup35NMSP (B) but delays polymerization of Sup35NMSB (C); Sup35NMSP promotes polymerization of Sup35NMSP (B) but delays polymerization of both Sup35NMSC (A) and Sup35NMSB (C); Sup35NMSB promotes polymerization of both Sup35NMSC (A) and Sup35NMSB (C) but delays polymerization of Sup35NMSP (B).
Potential Mechanism of Prion Species Barrier at Low Levels of Sequence Divergence.
Although mammalian prion proteins with 90% or higher amino acid identity exhibit species barrier (3), previous reports of yeast prion species barrier dealt with highly divergent PrDs retaining only 30–40% of identity (7). By detecting prion species barrier in yeast at the levels of identity up to 94%, we demonstrate that yeast prion conversion also requires nearly a precise correspondence of amino acid sequences.
Although inefficient cross-species conversion between proteins with highly divergent PrDs was apparently due to their inability to coaggregate both in vivo and in vitro (9, 10, 20, 21), yeast proteins with closely related PrDs exhibit species barrier (Fig. 3) even in combinations for which efficient in vivo coaggregation was observed (Fig. 2). In vitro, purified S. sensu stricto PrD-containing fragments either cross-seed or inhibit each other's polymerization, depending on the combination (Fig. 4). Either effect is hard to explain by a mechanism that would not involve direct interactions between heterologous proteins. Taken together, our data clearly demonstrate that mechanical association of the heterologous proteins is not sufficient for efficient transmission of a prion state. This also agrees with the previous observation that heterologous mammalian prion proteins can bind each other without efficient conversion to the proteinase-resistant state and that heterologous binding may inhibit homologous conversion (16). Therefore, in both yeast and mammalian systems, prion species barrier between closely related proteins appears to be controlled at levels other than simple coaggregation.
Because heterologous coaggregation fails to efficiently produce a heritable prion state, it appears that sequence divergence impairs conformational transition. Difference by only a few (Sup35NMSC and Sup35NMSP PrDs) or even by one (some mammalian prion proteins, e.g., ref. 18) amino acid residue is sufficient for prion species barrier in some assays, suggesting that interactions between certain specific amino acid residues play a crucial role in achieving the maximal efficiency of the conformational transition. Further investigation of the prion species barrier between the closely related PrDs may help to identify these positions.
Most heterologous prions generated by the S. paradoxus and S. bayanus proteins in the S. cerevisiae cell environment were weak and exhibited a high frequency of mitotic loss (Fig. 1D and SI Table 2). Propagation of yeast prions is thought to occur via generation of new polymerization seeds in result of chaperone-mediated fragmentation of amyloid polymers (5), whereas mitotically unstable prions apparently are defective in their ability to be fragmented by the chaperones (39–41). It is an intriguing possibility that polymers generated by heterologous PrDs are not capable of efficient fragmentation in the S. cerevisiae cell environment because they are not adjusted to the levels or activities of the S. cerevisiae chaperones. Indeed, Sup35SB PrD has a shortened OR region (Fig. 1A and SI Fig. 5), previously implicated in control of prion fragmentation and propagation (42). Moreover, combination of S. paradoxus Sup35N region with S. cerevisiae Sup35MC increases prion mitotic stability (SI Table 3), in agreement with the observation that Sup35M influences interactions with the disaggregating chaperone Hsp104 (43). Interestingly, prions generated by a chimeric Sup35 protein with Pichia PrD also exhibited low mitotic stability (8, 9) and decreased sensitivity to Hsp104 (9). Defective prion propagation in a heterologous cell environment may represent an additional mechanism contributing to prion species barrier, but cannot explain it completely because restoration of prion mitotic stability in chimeric S. paradoxus–S. cerevisiae constructs did not eliminate the barrier (Fig. 3B).
Cross-inhibition of the in vitro Sup35 polymerization in some combinations by a small proportion (≈5%) of the preformed heterologous seed suggests that the spontaneously arisen fraction of polymerization-proficient Sup35NM is initially very small. Remarkably, asymmetric patterns of in vitro cross-inhibition generally resemble asymmetry of the species barrier observed in the cytoduction assay in vivo (Table 1) because Sup35NMSC inhibits polymerization of Sup35NMSP or Sup35NMSB, but not vice versa (Fig. 4). The only exception was the S. paradoxus–S. bayanus combination exhibiting a strong species barrier in both directions in vivo (Table 1) but only in one direction in vitro (Fig. 4). This may reflect the difference in protein ratios between the in vivo and in vitro systems and/or involvement of cell components other than Sup35 in the in vivo species barrier.
Overall, our data establish a yeast model for studying the mechanism of prion species barrier at low levels of sequence divergence and pave the way for understanding the molecular processes responsible for this phenomenon.
Materials and Methods
Yeast Strains.
With the exception of cytoduction recipients, all S. cerevisiae strains were isogenic [PSI+] and [psi−] derivatives of GT81 (8) of the following genotype: MATa (or MATα) ade1–14 his3 leu2 lys2 trp1 ura3. Strains bearing the sup35Δ::HIS3 transplacement on the chromosome and maintained alive by the SUP35-containing plasmids were constructed as described previously (8, 39). The recipient strains for cytoduction were derivatives of 1B-D910 (MATa ade1–14 his3 leu2 trp1 ura3 cyhR kar1–1 [rho− psi− pin−]), kindly provided by A. Galkin (St. Petersburg University, Russia) and containing the sup35Δ::HIS3 deletion on the chromosome with various SUP35-containing plasmids introduced by plasmid shuffle. The S. paradoxus strain SP7-1D, kindly provided by G. Naumov (State Institute for Genetics and Selection of Industrial Microorganisms, Moscow, Russia), and S. bayanus strain FM361, kindly provided by M. Johnston (Washington University, St. Louis, MO), were the source of the SUP35SP and SUP35SB genes, respectively.
Plasmids.
Plasmids used in this work are described in SI Table 5. All PCR-generated fragments were verified by sequencing. Sequences of primers used for PCR are provided in SI Table 6.
Genetic and Microbiological Techniques.
Standard media, cultivation conditions, and yeast genetic techniques were used (44). Gal and Gal+Raf media contained, respectively, 2% galactose or 2% galactose and 2% raffinose instead of glucose. Assays for [PSI+] were described previously (31). Plasmid shuffle and cytoduction were performed as described previously (39, 40), with modifications specified in the Fig. 3 and Table 1 legends. DNA sequencing was performed at the Nevada Genomics Center and Georgia Institute of Technology School of Biology Genomics Facility. Fluorescence microscopy was performed and images of the live yeast cells analyzed by using the LSM510 laser confocal microscope (Carl Zeiss, Inc., Jena, Germany) as described previously (45).
Protein Analysis.
The Sup35NM antibodies were described previously (46). The Sup35C antibodies were a gift of D. Bedwell (University of Alabama at Birmingham, Birmingham, AL). Protein isolation from yeast and centrifugation analysis were in accordance with the previously published protocol (31) using the centrifuge TL-100 (Beckman Counter, Fullerton, CA).
In Vitro Protein Polymerization and Cross-Seeding.
Sup35NM fragments with His tag at the C terminus were expressed in Escherichia coli, isolated as described previously (47), with the only difference that Ni-NTA resin (Novagen, La Jolla, CA) was used, concentrated to 500 μM by the microcon filter devices (Millipore, Billerica, MA), and stored in 20 mM Tris·HCl (pH 8.0) with 6 M guanidine hydrochloride and 300 mM NaCl. For polymerization experiments (31), protein extracts were diluted 100-fold to 5 μM in 1 ml of 150 mM NaCl with 5 mM potassium phosphate (pH 7.4) and one tablet per 20 ml of Roche (Mannheim, Germany). Complete (proteinase inhibitor mixture) and incubated at room temperature with shaking at 12 rpm on Fisher Scientific (Pittsburgh, PA) chemistry mixer, model 346. Aliquots were taken after a specified period and mixed with SDS to the final concentration of 2%. Half of each aliquot was boiled for 10 min to disaggregate polymers. Both boiled and nonboiled samples were run on SDS/PAGE and stained with Coomassie brilliant blue R-250. Polymerization was detected by a decrease in the proportion of protein entering the gel in the nonboiled sample versus the boiled sample. Polymers not capable of entering the gel without boiling were usually seen at the start of the gel in the nonboiled samples (data not shown).
Supplementary Material
Acknowledgments
We thank D. Bedwell, A. Galkin, S. Inge-Vechtomov (St. Petersburg University, Russia), M. Johnston, S. Lindquist (Whitehead Institute, Cambridge, MA), G. Naumov, and J. Weissman (University of California, San Francisco, CA), for strains and antibodies; K. Gokhale for help with plasmid constructions; and K. Lobachev and M. Tanaka for useful suggestions on the experimental protocols. This work was supported by National Science Foundation Grant MCB-0614772 (to Y.O.C.). B.C. was a recipient of the Suddath Award from the Institute for Bioengineering and Bioscience (Georgia Institute of Technology).
Abbreviations
- PrD
prion domain
- RFP
red fluorescent protein
- OR
oligopeptide repeat.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611158104/DC1.
References
- 1.Lansbury PT, Jr, Caughey B. Chem Biol. 1995;2:1–5. doi: 10.1016/1074-5521(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 2.Weissmann C. Nat Rev Microbiol. 2004;2:861–871. doi: 10.1038/nrmicro1025. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris DA, True HL. Neuron. 2006;50:353–357. doi: 10.1016/j.neuron.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Chernoff YO. Curr Opin Chem Biol. 2004;8:665–671. doi: 10.1016/j.cbpa.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Wickner RB, Edskes HK, Ross ED, Pierce MM, Shewmaker F, Baxa U, Brachmann A. Cold Spring Harbor Symp Quant Biol. 2004;69:489–496. doi: 10.1101/sqb.2004.69.489. [DOI] [PubMed] [Google Scholar]
- 7.Zhouravleva GA, Alenin VV, Inge-Vechtomov SG, Chernoff YO. In: Recent Research Developments in Molecular and Cellular Biology. Pandalai SG, editor. Vol 3. Trivandrum, India: Research Signpost; 2002. pp. 185–218. [Google Scholar]
- 8.Chernoff YO, Galkin AP, Lewitin E, Chernova TA, Newnam GP, Belenkiy SM. Mol Microbiol. 2000;35:865–876. doi: 10.1046/j.1365-2958.2000.01761.x. [DOI] [PubMed] [Google Scholar]
- 9.Kushnirov VV, Kochneva-Pervukhova NV, Chechenova MB, Frolova NS, Ter-Avanesyan MD. EMBO J. 2000;19:324–331. doi: 10.1093/emboj/19.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoso A, Chien P, Osherovich LZ, Weissman JS. Cell. 2000;100:277–288. doi: 10.1016/s0092-8674(00)81565-2. [DOI] [PubMed] [Google Scholar]
- 11.Nakayashiki T, Ebihara K, Bannai H, Nakamura Y. Mol Cell. 2001;7:1121–1130. doi: 10.1016/s1097-2765(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 12.Zadorskii SP, Sopova Iu, V, Inge-Vechtomov SG. Genetika. 2000;36:1322–1329. [PubMed] [Google Scholar]
- 13.Ross ED, Baxa U, Wickner RB. Mol Cell Biol. 2004;24:7206–7213. doi: 10.1128/MCB.24.16.7206-7213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross ED, Edskes HK, Terry MJ, Wickner RB. Proc Natl Acad Sci USA. 2005;102:12825–12830. doi: 10.1073/pnas.0506136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocisko DA, Priola SA, Raymond GJ, Chesebro B, Lansbury PT, Jr, Caughey B. Proc Natl Acad Sci USA. 1995;92:3923–3927. doi: 10.1073/pnas.92.9.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horiuchi M, Priola SA, Chabry J, Caughey B. Proc Natl Acad Sci USA. 2000;97:5836–5841. doi: 10.1073/pnas.110523897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskakov IV. J Biol Chem. 2004;279:7671–7677. doi: 10.1074/jbc.M310594200. [DOI] [PubMed] [Google Scholar]
- 18.Vanik DL, Surewicz KA, Surewicz WK. Mol Cell. 2004;14:139–145. doi: 10.1016/s1097-2765(04)00155-8. [DOI] [PubMed] [Google Scholar]
- 19.Chernoff YO. Mol Cell. 2004;14:147–148. doi: 10.1016/s1097-2765(04)00208-4. [DOI] [PubMed] [Google Scholar]
- 20.Chien P, Weissman JS. Nature. 2001;410:223–227. doi: 10.1038/35065632. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Chien P, Yonekura K, Weissman JS. Cell. 2005;121:49–62. doi: 10.1016/j.cell.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Edskes HK, Wickner RB. Proc Natl Acad Sci USA. 2002;99:16384–16391. doi: 10.1073/pnas.162349599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudin-Baillieu A, Fernandez-Bellot E, Reine F, Coissac E, Cullin C. Mol Biol Cell. 2003;14:3449–3458. doi: 10.1091/mbc.E03-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talarek N, Maillet L, Cullin C, Aigle M. Genetics. 2005;171:23–34. doi: 10.1534/genetics.105.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandula J, Vojtkova-Lepsikova A. Folia Microbiol. 1974;19:94–101. doi: 10.1007/BF02872841. [DOI] [PubMed] [Google Scholar]
- 26.Ter-Avanesyan MD, Kushnirov VV, Dagkesamanskaya AR, Didichenko SA, Chernoff YO, Inge-Vechtomov SG, Smirnov VN. Mol Microbiol. 1993;7:683–692. doi: 10.1111/j.1365-2958.1993.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 27.Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. Genetics. 1994;137:671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen MA, True HL, Chernoff YO, Lindquist S. Genetics. 2001;159:527–535. doi: 10.1093/genetics/159.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cliften P, Sudarsanam P, Desikan A, Fulton L, Fulton B, Majors J, Waterston R, Cohen BA, Johnston M. Science. 2003;301:71–76. doi: 10.1126/science.1084337. [DOI] [PubMed] [Google Scholar]
- 30.Kellis M, Patterson N, Endrizzi M, Birren B, Lander ES. Nature. 2003;423:241–254. doi: 10.1038/nature01644. [DOI] [PubMed] [Google Scholar]
- 31.Chernoff YO, Uptain SM, Lindquist SL. Methods Enzymol. 2002;351:499–538. doi: 10.1016/s0076-6879(02)51867-x. [DOI] [PubMed] [Google Scholar]
- 32.Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chernoff YO, Derkach IL, Inge-Vechtomov SG. Curr Genet. 1993;24:268–270. doi: 10.1007/BF00351802. [DOI] [PubMed] [Google Scholar]
- 34.Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetics. 1997;147:507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conde J, Fink GR. Proc Natl Acad Sci USA. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S. Cell. 1997;89:811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 37.King CY, Diaz-Avalos R. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 39.Borchsenius AS, Wegrzyn RD, Newnam GP, Inge-Vechtomov SG, Chernoff YO. EMBO J. 2001;20:6683–6691. doi: 10.1093/emboj/20.23.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchsenius AS, Mèuller S, Newnam GP, Inge-Vechtomov SG, Chernoff YO. Curr Genet. 2006;49:21–29. doi: 10.1007/s00294-005-0035-0. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Collins SR, Toyama BH, Weissman JS. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 42.Osherovich LZ, Cox BS, Tuite MF, Weissman JS. PLoS Biol. 2004;2:E86. doi: 10.1371/journal.pbio.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JJ, Sondheimer N, Lindquist SL. Proc Natl Acad Sci USA. 2002;99:16446–16453. doi: 10.1073/pnas.252652099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherman F. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- 45.Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. Mol Cell Biol. 2006;26:617–629. doi: 10.1128/MCB.26.2.617-629.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO. Mol Cell Biol. 2001;21:4656–4669. doi: 10.1128/MCB.21.14.4656-4669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen KD, Wegrzyn RD, Chernova TA, Müller S, Newnam GP, Winslett PA, Wittich KB, Wilkinson KD, Chernoff YO. Genetics. 2005;169:1227–1242. doi: 10.1534/genetics.104.037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.