Abstract
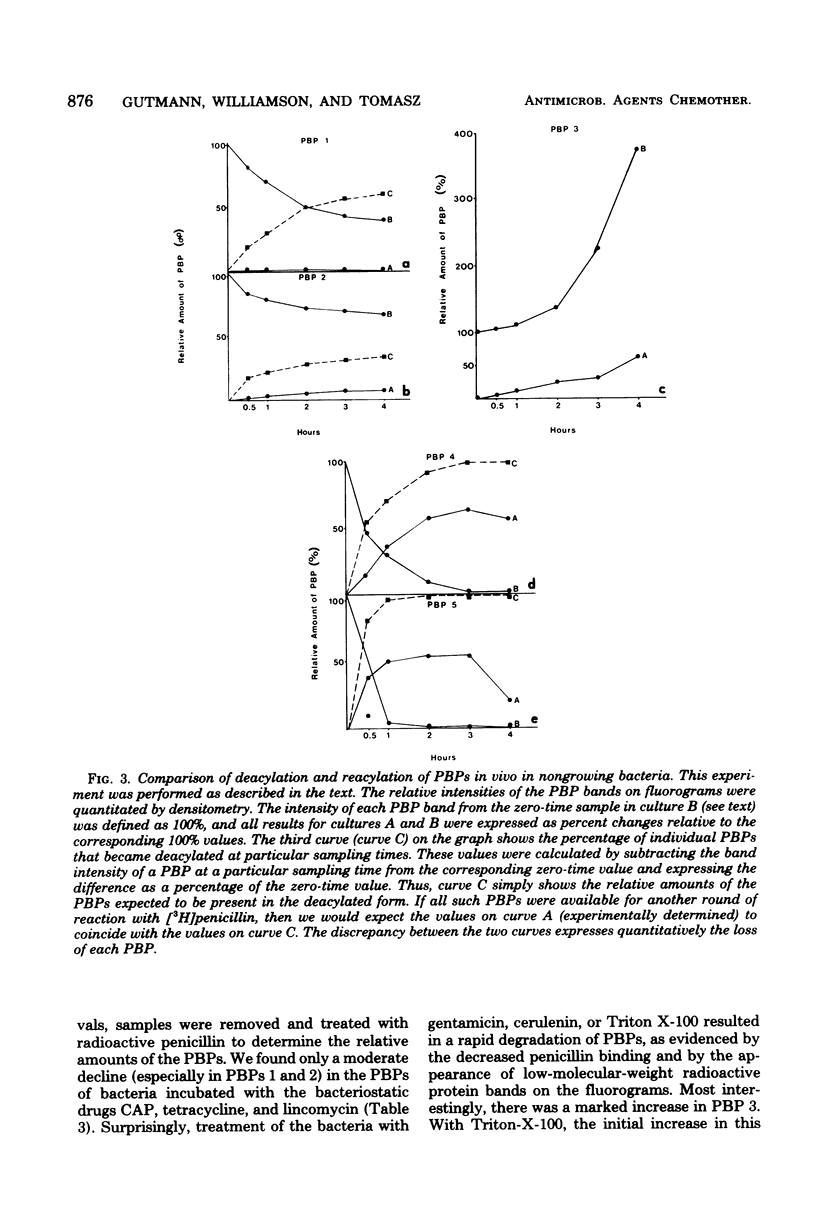
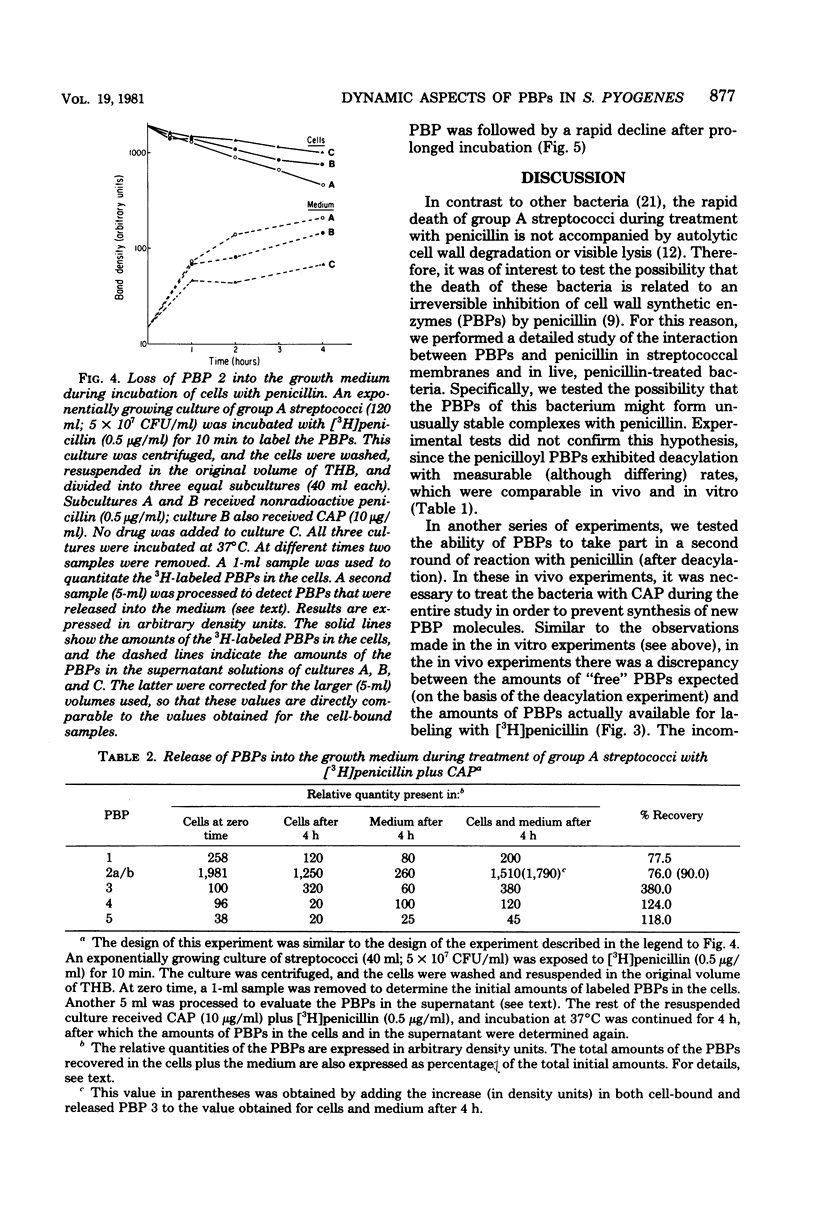
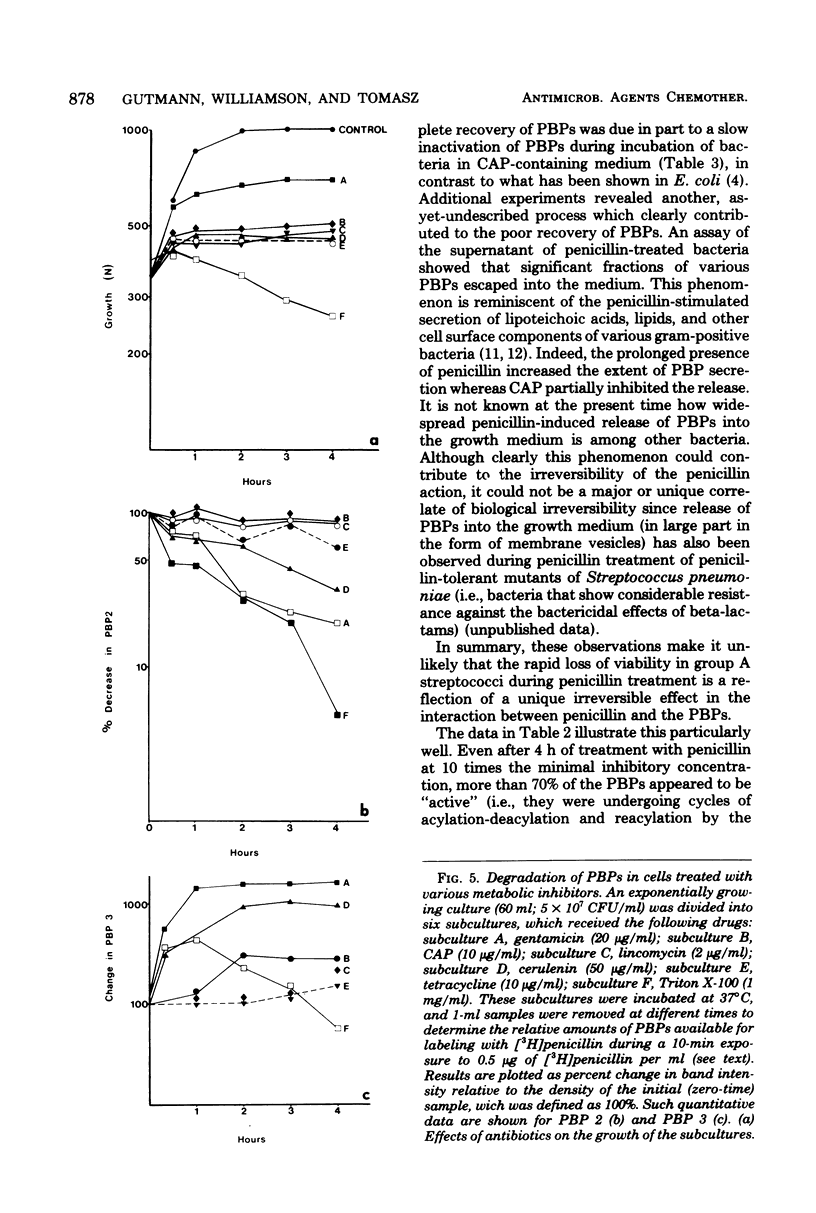
We detected five major penicillin-binding proteins (PBPs) in group A streptococci by labeling either cell membrane preparations or live bacteria with tritiated penicillin. All PBPs appeared to be equally accessible to penicillin in vitro and in vivo. Individual PBPs differed in their rates of deacylation, and four of the five PBPs underwent rapid inactivation both in vivo and in vitro. At least two processes seemed to contribute to in vivo inactivation; these were (i) a penicillin-induced release of all five PBPs into the growth medium and (ii) degradation, as evidenced by the appearance of penicillin-labeled protein band of lower molecular weight and also by a gradual increase in material migrating with the same electrophoretic mobility as PBP 3. Inactivation of the PBPs was stimulated greatly by pretreatment of bacteria with gentamicin, cerulenin, or Triton X-100, whereas chloramphenicol, tetracycline, and lincomycin treatments had no such effect.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Yocum R. R., Willoughby E., Strominger J. L. Binding of (14C)penicillin G to the membrane-bound and the purified D-alanine carboxypeptidases from Bacillus stearothermophilus and Bacillus subtilis and its release. J Biol Chem. 1974 Nov 10;249(21):6828–6835. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. The penicillin-binding proteins in Streptococcus faecalis ATCC 9790. Eur J Biochem. 1980 Sep;110(2):445–456. doi: 10.1111/j.1432-1033.1980.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Perkins H. R., Nieto M. The active centres in penicillin-sensitive enzymes. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):285–301. doi: 10.1098/rstb.1980.0046. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. The concept of the penicillin target from 1965 until today. The thirteenth marjory stephenson memorial lecture. J Gen Microbiol. 1977 Jul;101(1):13–33. doi: 10.1099/00221287-101-1-13. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarich J. W., Nishino T., Willoughby E., Strominger J. L. Hydroxylaminolysis of penicillin binding componenets is enzymatically catalyzed. J Biol Chem. 1977 Nov 10;252(21):7525–7529. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Weinberg A. N. Studies on antibiotic syngerism against enterococci. II. Effect of various antibiotics on the uptake of 14 C-labeled streptomycin by enterococci. J Clin Invest. 1971 Dec;50(12):2580–2584. doi: 10.1172/JCI106758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]




