Abstract
Recent studies have suggested that formation of Golgi membrane tubules involves the generation of membrane-associated lysophospholipids by a cytoplasmic Ca2+-independent phospholipase A2 (PLA2). Herein, we provide additional support for this idea by showing that inhibition of lysophospholipid reacylation by a novel Golgi-associated lysophosphatidylcholine acyltransferase (LPAT) induces the rapid tubulation of Golgi membranes, leading in their retrograde movement to the endoplasmic reticulum. Inhibition of the Golgi LPAT was achieved by 2,2-dimethyl-N-(2,4,6-trimethoxyphenyl)dodecanamide (CI-976), a previously characterized antagonist of acyl-CoA cholesterol acyltransferase. The effect of CI-976 was similar to that of brefeldin A, except that the coatomer subunit β-COP remained on Golgi-derived membrane tubules. CI-976 also enhanced the cytosol-dependent formation of tubules from Golgi complexes in vitro and increased the levels of lysophosphatidylcholine in Golgi membranes. Moreover, preincubation of cells with PLA2 antagonists inhibited the ability of CI-976 to induce tubules. These results suggest that Golgi membrane tubule formation can result from increasing the content of lysophospholipids in membranes, either by stimulation of a PLA2 or by inhibition of an LPAT. These two opposing enzyme activities may help to coordinately regulate Golgi membrane shape and tubule formation.
INTRODUCTION
Recent studies have shown that modification of the phospholipid content on one side of a membrane bilayer can have biologically relevant consequences on both membrane structure and function. For example, inhibition of a cytoplasmic Ca2+-independent phospholipase A2 (PLA2) activity has been shown to prevent the formation of Golgi membrane tubules that form both constitutively and in response to brefeldin A (BFA) treatment (de Figueiredo et al., 1998, 1999, 2000). These PLA2 antagonists also inhibited retrograde trafficking from the Golgi to the endoplasmic reticulum (ER), and a late step leading to the reassembly of an intact Golgi complex (Drecktrah and Brown, 1999; de Figueiredo et al., 2000). Moreover, stimulation of a cytoplasmic PLA2 activity had the opposite effect, that of inducing Golgi membrane tubules (Polizotto et al., 1999). Other recent studies have shown that endosome tubule formation and endocytic recycling are also inhibited by PLA2 antagonists (de Figueiredo et al., 2001). These results suggest a direct biological role for the phospholipid (PL) products of PLA2 hydrolysis, lysophospholipids (LPLs), and/or fatty acids, in mediating the curvature of membranes. Specifically, increasing the ratio of LPL/PL in the outer leaflet of a membrane produces an outward curvature that at its most extreme leads to tubule formation (Fujii and Tamura, 1979; Christiansson et al., 1985; Mui et al., 1995). This curvature may result because LPLs have a more inverted cone shape, compared with cylindrical or cone-shaped PLs (for review, see Scales and Scheller, 1999).
Other studies have recently demonstrated that LPL acyltransferases (LPATs), which reacylate LPLs back to PLs, have the opposite effect of PLA2. That is, conversion of LPLs back to PLs apparently causes inward curvature of biological membranes, resulting in important physiological consequences. For example, the cytosolic lysophosphatidic acid (LPA)-specific LPAT CtBP/BARS was shown to induce fission and vesicle formation from Golgi membrane tubules (Weigert et al., 1999). Likewise, inhibition of the intrinsic LPA-specific LPAT activity of endophilin was shown to reduce its ability to induce endocytic vesicle formation (Schmidt et al., 1999), although subsequent studies question whether endophilin's LPAT activity is required for vesiculation (Farsad et al., 2001). For both proteins, it has been proposed that conversion of inverted cone-shaped LPAs to cone-shaped phosphatidic acid by LPA-specific LPAT activity may contribute to the inward curvature of a membrane at the neck of a budding vesicle, thus aiding in its fission (Scales and Scheller, 1999). Together, these studies strongly suggest that cytosolic LPATs and PLA2 seem to play an important role in modulating membrane lipid composition and structure, with resultant consequences for intracellular trafficking.
To better understand the role that phospholipid metabolism plays in the formation of membrane tubules from the Golgi complex and to explore the functional role of tubules in membrane-trafficking events, we screened for inhibitors of LPAT activity that also influenced membrane trafficking from the Golgi complex. We found that 2,2-dimethyl-N-(2,4,6-trimethoxyphenyl)dodecanamide (CI-976), a previously characterized inhibitor of acyl-CoA cholesterol acyltransferase (ACAT) (Harte et al., 1995), was also a potent antagonist of a Golgi-associated LPAT activity. Remarkably, CI-976 also stimulated the rapid tubulation of Golgi membranes and their redistribution to the ER. These results are consistent with the idea that Golgi membrane tubules form, at least in part, by regulating the ratio of LPL/PL.
MATERIALS AND METHODS
Reagents and Antibodies
The PLA2 antagonists ONO-RS-082 and HELSS (also known as BEL) were purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). ONO-RS-082 and BEL were freshly prepared as 5 mM stock solutions in 100% ethanol or 100% dimethyl sulfoxide (DMSO), respectively, and then diluted into media or buffer as described below. l-α-Lysophosphatidylcholine solutions at 1 mg ml–1 in chloroform/methanol (1:1) and in 100% chloroform were prepared as required. Lysopalmitoyl phosphatidylcholine-l-1-[palmitoyl-1-14C] and palmitoyl CoA [palmitoyl-1-14C] were purchased from PerkinElmer Life Sciences (Boston, MA) and stored at –80°C. Lysophosphatidic acid (16:0 and 18:1) was purchased from Avanti Polar Lipids (Alabaster, AL). Flexible thin layer chromatography (TLC) silica gel plates were purchased from Whatman (Clifton, NJ). Solvents for extraction of Golgi membranes and high-performance TLC were purchased from Burdick and Jackson (Muskegon, MI). High-performance thin layer chromatography (HPTLC) plates (silica gel 60, 10 × 10 cm, without fluorescent indicator) were from Alltech Associates (Deerfield, IL). ACAT inhibitors CI-976 and N′-(2,4-difluorophenyl)-N-[5-(4,5-diphenyl-1H-imidazol-2-ylthio)pentyl]-N-hepthylurea (DuP-128) were provided by GlaxoSmithKline Pharmaceuticals (Essex, United Kingdom), and 3-(decyldimethylsilyl)-N-[2-(4-methylphenyl)-1-phenylethyl]propanamide was provided by Novartis Pharmaceuticals (Summit, NJ). All were stored as 5 mM stock solutions in DMSO at 4°C. Analytical grade ethanol and chloroform were obtained from Mallinckrodt (Phillipsburg, NJ) All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
The following antibodies were generously supplied to us: rabbit polyclonal anti-α-mannosidase II (ManII) (Dr. Kelley Moremen, University of Georgia, Athens, GA); rabbit polyclonal anti-p58 (ER-Golgi-intermediate compartment-53) (Dr. Jaakko Saraste, University of Bergen, Bergen, Norway); and mouse monoclonal M3A5 anti-β-COP (Dr. William Balch, Scripps Research Institute, La Jolla, CA). Polyclonal anti-TGN38 antibody and monoclonal anti-protein disulfide isomerase (PDI) were purchased from Affinity Bioreagents (Golden, CO). All secondary fluorescent antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG and sheep anti-mouse IgG were obtained from Amersham Biosciences (Piscataway, NJ). The expression vector that encodes a galactosyltransferase-green fluorescent protein chimera (GalT-GFP) was kindly provided by Dr. Jennifer Lippincott-Schwartz (National Institute of Child Health and Human Development, Bethesda, MD).
LPAT Assays
The LPAT activity present in isolated Golgi complexes prepared from rat liver (Cluett and Brown, 1992) was measured as described previously (Kerkhoff et al., 1996). Golgi membranes (3–10 μg) were incubated with fatty acyl-CoA and LPL in a final volume of 200 μl of LPAT buffer (150 mM NaCl, 1 mM EDTA, 10 mM Tris, pH 7.4). The LPL was added as liposomes by drying the LPL under argon, resuspending in LPAT buffer, and sonicating before adding to the reactions. The reaction mixtures were incubated with ACAT inhibitors or solvent controls at 37°C for 5 min before the addition of the 14C-labeled substrate. The reaction was incubated at 37°C for the indicated amount of time and then stopped by adding 1.0 ml of chloroform/methanol/water mixture, to a final ratio of 1:2:0.8. The lipids were extracted according to the method of Bligh and Dyer, 1959 by the addition of 300 μl of chloroform and 300 μl of water. Nonlabeled phosphatidylcholine (PC) (10 μg) and lysophosphatidylcholine (LPC) (10 μg) were added as standards. The extracted lipids were dried under nitrogen or argon. The samples were resuspended in 1:1 chloroform/methanol and separated by TLC on silica gel plates by developing in chloroform/methanol/water (65:25:4). The radioactivity of individual spots containing LPL or PL was measured by phosphorimaging (PhosphorImager and ImageQuant; Amersham Biosciences) or by scintillation counting. For determination by scintillation, lipids were visualized by staining with Zinzade's reagent. Spots containing PC and LPC were cut out of the TLC plates, allowed to fade, and dissolved in EcoLume scintillation fluid (ICN Pharmaceuticals, Costa Mesa, CA). The radioactivity was measured in a Beckman Coulter LS-230 scintillation counter. Values represent the average of two experiments performed in duplicate. When testing LPC as a substrate, 6.25 nmol of arachidonyl-CoA and 50 nCi of [14C]LPC were used per reaction and the reactions were incubated at 37°C for 1 h after addition of the 14C-labeled substrate. In experiments testing various LPL substrates, 6.0 nmol of LPL and 100 nCi of [14C]palmitoyl-CoA were used per reaction, and the reactions were incubated at 37°C for 5 min after addition of the 14C-labeled substrate.
Immunofluorescence Microscopy
Rat Clone 9 hepatocytes were grown on glass coverslips for 2 d before the experiments were performed. Cells were washed twice in minimal essential medium (MEM) without serum and incubated in MEM without serum containing inhibitors at the concentrations and for the times indicated under RESULTS. Immunofluorescence microscopy was performed as described previously (Wood et al., 1991). To ensure that the change in distribution of membrane markers, e.g., ManII, was not due to new protein synthesis, all trafficking experiments were done in the presence of cycloheximide (2 μg ml–1) to inhibit protein synthesis. We note that CI-976 was not active in media containing serum.
Transfection and Live Cell Confocal Microscopy
HeLa cells were grown on glass coverslips and transfected with the plasmid expressing GalT-GFP described above by the Ca2+ phosphate precipitation method. Coverslips were inverted on a slide with a CoverWell silicon gasket (Molecular Probes, Eugene, OR) to form a chamber filled with media containing CI-976 or control solvent. Cells were kept at 37°C on a heated stage, and GalT-GFP was visualized by laser scanning confocal microscopy (MRC-600; Bio-Rad, Hercules, CA). Image collection was controlled using COMOS software (Bio-Rad).
Subcellular Fractionation
Clone 9 cells were grown to near confluence in 210-mm2 dishes. Cells were rinsed three times with MEM, and half the dishes were treated with 20 μM CI-976 (in MEM) for 1 h at 37°C and the other half with a control solvent. Cells were harvested with a rubber policeman, homogenized, and organelles were separated by differential centrifugation followed by continuous sucrose density centrifugation as described previously (Brown and Farquhar, 1987). Fractions were collected and analyzed by Western blotting by using antibodies against the resident ER marker PDI and the Golgi marker ManII. Protein transfers were made using a semidry blotting apparatus onto polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat dry milk (NFDM) in Tris-buffered saline (TBS), pH 7.4, for 2 h at room temperature (RT), rinsed once with 2.5% NFDM in TBS (NFDM/TBS), and incubated in rabbit anti-ManII diluted 1:1000 in NFDM/TBS for 1 h at RT and then overnight at 4°C. Membranes were washed three times for 10 min each in the following solutions: NFDM/TBS, TBS containing 1% Tween 20, and TBS containing 0.5 M NaCl. After a brief rinse in TBS, membranes were incubated in sheep anti-rabbit-HRP diluted 1:1000 in NFDM/TBS for 3 h at RT. Membranes were then washed as described above after the first antibody. Bands were visualized using enhanced chemiluminescence development (Amersham Biosciences) according to the manufacturer's directions.
To ensure that a direct comparison could be made in the distribution of ManII and PDI on the same fractions, the membranes described above were stripped and reprobed with antibodies against PDI. After enhanced chemiluminescence development and film exposure, membranes were rinsed in TBS, incubated in stripping buffer (62.5 mM Tris, pH 6.8, 2% SDS, 0.7% β-mercaptoethanol) for 30 min at 50°C, and then washed two times with TBS containing 1% Tween 20. Membranes were then blocked and processed as described above except that the first antibody was now mouse anti-PDI (1:1000 dilution) and the second antibody was sheep anti-mouse-HRP (1:1000 dilution).
To quantify the band intensities, films were scanned at 300 dpi by using a flatbed scanner and saved as TIFF files and then band intensities were analyzed using NIH Image Gel Electrophoresis software. The amount of each protein in a lane was expressed as the percentage of the total mean pixel intensities.
Effect of CI-976 on In Vitro Golgi Tubulation and LPC Levels
Bovine brain cytosol (BBC) was prepared as described previously (Banta et al., 1995). A frozen aliquot of BBC was thawed on ice, centrifuged at 70,000 rpm for 20 min in a TLA100.3 rotor in a Beckman Coulter tabletop ultracentrifuge, and the supernatant was used for experiments. Increasing concentrations of BBC, or 2.5 mg ml–1 bovine serum albumin, were prepared in tubulation buffer (25 mM Tris-HCl, 50 mM KCl, 10 mM HEPES, 50 μM ATP, and 1 mM MgCl2, pH 7.4), containing either 50 μM CI-976, or a 1:100 dilution of DMSO, as the solvent control. Mixtures were prepared in a final volume of 100 μl and placed on ice for 30 min. Next, 20 μl of these mixtures was mixed with 20 μl of isolated, intact Golgi complexes (Cluett and Brown, 1992) and incubated for 15 min at 37°C. A 10-μl aliquot of each mixture was prepared for negative staining, and tubulation of Golgi stacks was quantified as described previously (Banta et al., 1995). A Golgi stack was considered tubulated if it possessed at least one membrane tubule that was 50–70 nm in diameter and at least 3 times as long as its diameter.
To determine whether CI-976 caused an increase in the levels of LPLs in Golgi membranes, normal tubulation reactions were scaled up to obtain sufficient material for analysis by HPTLC. Aliquots of BBC and Golgi membranes (prepared as described above) were pretreated with varying concentrations of CI-976 for 15 min at 4°C. For these experiments, CI-976 was dissolved in ethanol to avoid interference from DMSO during TLC. Before the start of the reaction, Mg2+-ATP was added to all aliquots of BBC to ensure a final concentration of 50 μM in the reaction mix. Pretreated BBC (400 μl) was added to an equal volume of pretreated Golgi membranes, resulting in a final concentration of 1.5 mg/ml cytosol. Samples were incubated for 15 min at 37°C, transferred to glass test tubes, and the lipids were extracted as described previously (Bligh and Dyer, 1959). Chloroform/methanol [3 ml, 1:2 (vol/vol)] were added to 0.8 ml of reaction mix, samples were incubated on ice for 20 min, and 1 ml each of chloroform and water were added to induce phase separation. The lipid-enriched chloroform layer was saved, and the aqueous phase was reextracted with 1 ml of chloroform. The chloroform aliquots were pooled and concentrated by drying under nitrogen for separation by HPTLC as described previously (Macala et al., 1983). Lipids were dissolved in cholorform:methanol [19:1 (vol/vol)], 5-μl samples were spotted onto a washed 10 × 10-cm HPTLC plate, and run in chloroform/methanol/acetic acid/formic acid/water [35:15:6:2:1 (vol/vol)] to 7 cm. After drying, samples were run in the same direction in hexane/diethyl ether/acetic acid [80:20:1 (vol/vol)] to the top of the plate. The plate was dipped in 3% CuSo4/8% H3PO4 and charred to visualize bands. Lipids were identified by running standards in adjacent lanes. A digitized image of the plate was created and bands were quantified using NIH Image.
RESULTS
The ACAT inhibitor, CI-976, Is Also an LPAT Inhibitor
From previous studies, we hypothesized that Golgi tubulation results from increased membrane curvature by PLA2-catalyzed conversion of PLs to LPLs (de Figueiredo et al., 1998, 1999, 2000). A prediction of this hypothesis is that inhibition of LPL acylation should result in a state that favors tubule formation. Because we could find no specific LPAT inhibitors, we screened various antagonists of ACATs, which perform a similar enzymatic reaction, but with a different substrate, to see whether any also inhibited LPAT activity.
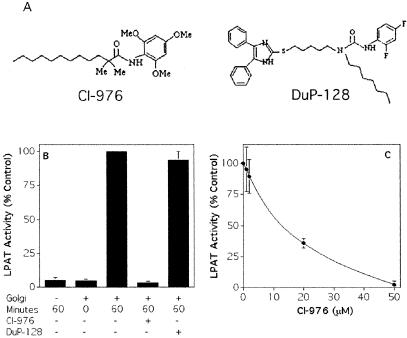
Golgi membranes have been reported to contain LPAT activity (Lawrence et al., 1994), and we used these membranes to screen ACAT inhibitors such as CI-976 and DuP-128 (Harte et al., 1995) (Figure 1A). Isolated rat liver Golgi complexes were incubated with arachidonyl-CoA as an acyl chain donor, and [14C]LPC as an acceptor in the presence or absence of inhibitors. In the absence of Golgi membranes, no LPAT activity was detected (Figure 1B). Under linear conditions of the assay, Golgi membranes converted up to ∼15% of the LPC to PC, corresponding to 0.4 nmol of PC/min/mg membrane protein (Figure 1B). Interestingly, this Golgi LPAT activity was completely inhibited by 50 μM CI-976, which exhibited an IC50 value of ∼15 μM (Figure 1C), a concentration range that similarly inhibits ACAT activity (Harte et al., 1995). Importantly, the more specific and potent ACAT inhibitor DuP-128 had no effect on Golgi LPAT activity (Figure 1B), even at concentrations (5 μM) that were 100-1000 times higher than the IC50 value for ACAT inhibition (Harte et al., 1995). Another ACAT inhibitor, PKF058035 (Patankar and Jurs, 2000), also had no effect on the Golgi LPAT activity (our unpublished data). Therefore, CI-976 and DuP-128 can be used to distinguish between LPAT- and ACAT-dependent cellular processes.
Figure 1.
CI-976, but not DuP-128, inhibits Golgi-associated LPAT activity in vitro. (A) Chemical structures of CI-976 and DuP-128. (B) LPAT activity in the absence and presence of Golgi membranes, and the effect of CI-976 (50 μM) and DuP-128 (5 μM). LPAT activity is represented as the percentage of the control (Golgi membranes alone for 1 h at 37°C). (C) Dose response of CI-976 inhibition of LPAT activity. The conversion of LPC to PC was monitored by incubating isolated rat liver Golgi membranes, arachidonyl CoA, inhibitors, and [14C]LPC for 1 h at 37°C. Each data point represents the mean plus 1 SD of quadruplicate samples.
CI-976 Induces Tubulation of Golgi Membranes and Retrograde Transport to the ER
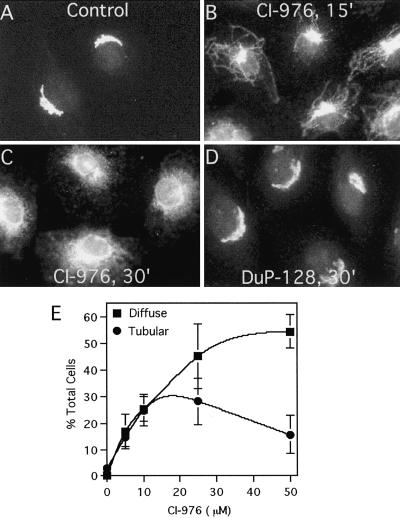
Given that CI-976 inhibits a Golgi-associated LPAT in vitro, we asked whether CI-976 had any effect on the morphology of the Golgi complex. At a concentration (25 μM) that almost completely inhibits LPAT activity in vitro, CI-976 dramatically altered Golgi morphology as seen by immunofluorescence of the resident Golgi enzyme ManII. After 15 min in CI-976, numerous thin membrane tubules emanated from the Golgi complex (Figure 2B). By 30 min, in many cells ManII was diffuse throughout the cytoplasm and in the nuclear envelope, consistent with its movement to the ER (Figure 2C). Conversely, DuP-128 (Figure 2D) and PKF058035 (our unpublished data) had no such effect on ManII localization.
Figure 2.
CI-976 stimulates the tubulation of Golgi membranes as seen by ManII immunofluorescence. Rat clone 9 hepatocytes were treated with solvent control (A), CI-976 (25 μM) for 15 min (B) or 30 min (C), or DuP-128 (5 μM) (D) for 30 min. Cells were then fixed and stained for immunofluorescence localization of the Golgi enzyme ManII. (E) Dose response to CI-976 of changes in Golgi morphology. Cells were treated with various concentrations of CI-976 for 30 min at 37°C, fixed, and processed for immunofluorescence of ManII, and scored as either containing ManII in obvious Golgi tubules (as in B) or as diffuse throughout the cytoplasm (as in C). Each data point is the mean plus 1 SD from three independent experiments.
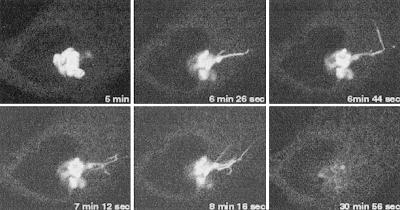
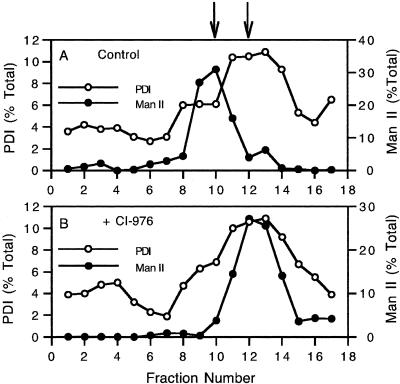
Quantitation of dose-response, immunofluorescence experiments of cells treated for a fixed period of time showed that as the concentration of CI-976 increased, the percentage of cells with Golgi tubules increased until a plateau was reached (Figure 2E). At higher concentrations, Golgi staining began to decrease as reflected in an increase in diffuse/nuclear envelope stained cells. These results are consistent with a quantitative analysis of time-course studies that found that the percentage of cells with Golgi tubules increases until ∼15–20 min. The percentage of cells with Golgi tubules then decreases as tubules fuse with the ER, ultimately resulting in >90% of the cells having diffuse/ER type staining after ∼1 h (our unpublished data). To extend these results, time-lapse confocal microscopy of the transiently expressed trans-Golgi marker GalT-GFP in living HeLa cells showed that GalT-GFP entered tubules within ∼6 min after addition of CI-976. The GalT-GFP tubules then quickly disappeared, as did the entire Golgi complex, and was replaced by a diffuse, ER-like pattern throughout the cytoplasm by 15–30 min (Figure 3). To confirm that CI-976 stimulated the tubule-mediated retrograde trafficking of Golgi membranes to the ER, control and CI-976–treated cells were fractionated to separate Golgi and ER membranes by sucrose density gradient centrifugation. In fractions from control cells, Golgi ManII and ER PDI were separated into discrete peaks; however, in CI-976–treated cells, the ManII distribution was shifted and coincided with PDI in denser fractions (Figure 4). These results show that Golgi markers redistribute to the ER in the presence of CI-976.
Figure 3.
Time-lapse microscopy of CI-976–stimulated cells shows Golgi tubulation and redistribution to a diffuse, ER-like pattern. HeLa cells were transiently transfected to express GalTase-GFP and visualized by confocal microscopy. The cell shown herein was treated with 20 μM CI-976 at 37°C and images were collected at the times indicated in each panel.
Figure 4.
Redistribution of ManII to the ER in CI-976–treated cells can be seen by cell fractionation. Golgi (ManII) and ER (PDI) markers in sucrose gradient fractions from control (A) and CI-976–treated cells (B) are shown. Fraction 1 represents the top of each gradient. Arrows indicate the position of Golgi (ManII; closed circles) and ER (PDI; open circles) peak fractions in control cells.
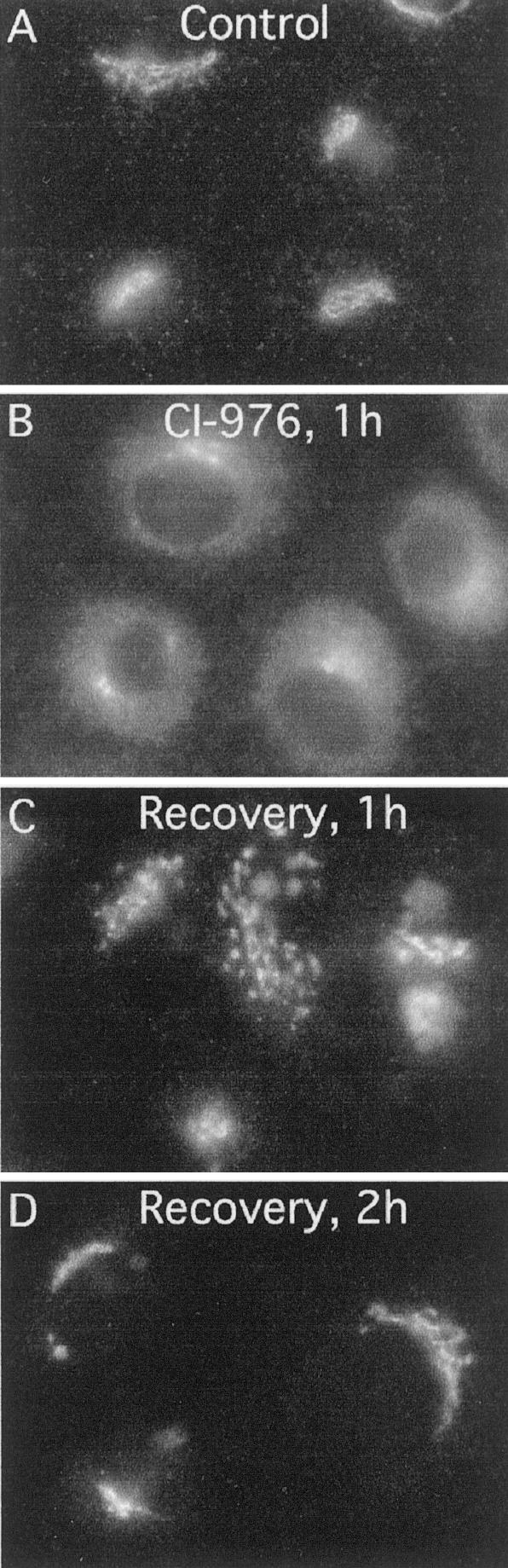
To determine whether the effects of CI-976 on the Golgi complex are reversible, cells were treated with 20 μM CI-976 for 1 h, extensively washed, and then incubated for various periods of time in media containing cycloheximide. As described above, treatment with CI-976 resulted in ManII redistribution to a diffuse, ER localization (Figure 5B). After ∼30 min of recovery, ManII was starting to be located in discrete but faint puncta located throughout the cytoplasm (our unpublished data). By 1 h of recovery, some cells had brightly stained puncta located in the juxtanuclear region and other cells that had nearly intact Golgi complexes (Figure 5C). By 2 h of recovery, the majority of cells had reassembled Golgi ribbons (Figure 5D). We note that recovery occurred much more slowly in serum-free media. These results show that the Golgi complex can fully recover from the effects of CI-976. Also, because recovery occurred in the presence of cycloheximide, CI-976 must be reversibly inhibiting its enzymatic target.
Figure 5.
The Golgi complex reassembles after removal of CI-976 as revealed by ManII immunofluorescence. (A) Cells treated with DMSO for 1 h as a solvent control have normal juxtanuclear Golgi staining patterns. (B) Cells treated with 20 μM CI-976 for 1 h. (C and D) Cells were treated with 20 μM CI-976 for 1 h, quickly washed in MEM containing 10% Nu-Serum, and subsequently incubated in MEM (with 10% Nu-Serum and 50 μg/ml cycloheximide) for1h(C) or 2 h (D). All treatments were done at 37°C. n, nucleus.
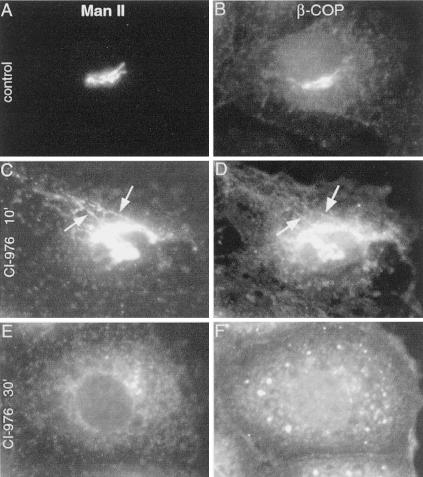
Previous studies have suggested that the BFA-induced loss of coatomer proteins, e.g., β-COP (Donaldson et al., 1990), is a prerequisite for tubule-mediated redistribution of Golgi markers to the ER (Scheel et al., 1997). However, in cells treated with CI-976 for 10 min, β-COP remained Golgi associated, and colocalized with ManII-positive tubules (Figure 6, C and D). In cells exposed to CI-976 for 30 min, ManII was found in a diffuse ER-like pattern, whereas β-COP was localized to both the cytoplasm and to specific punctate structures throughout the cytoplasm (Figure 6, E and F). Thus, in CI-976–treated cells, dissociation of β-COP from Golgi membranes does not correlate with Golgi tubulation.
Figure 6.
β-COP is found on Golgi tubules in CI-976–treated cells. After experimental treatment, cells were processed for double-label immunofluorescence microscopy by using polyclonal anti-ManII (A, C, and E) and monoclonal anti-β-COP antibodies (B, D, and F). (A and B) Untreated cells. Cells treated with CI-976 (20 μM) for 10 (C and D) or 30 min (E and F). Arrows indicate tubules that stain for both ManII and β-COP.
We also examined the effect of CI-976 on other organelles and found that the ER-Golgi-intermediate compartment-53 staining and trans-Golgi network (TGN) (TGN38 staining) also formed tubules; however, endosomes and lysosomes did not (our unpublished data). Also, CI-976–stimulated membrane tubulation and retrograde transport of resident Golgi enzymes was temperature and energy dependent (sensitive to sodium azide and deoxyglucose). Finally, depolymerization of microtubules with nocodazole greatly reduced CI-976–stimulated Golgi tubulation (our unpublished data).
The CI-976 Effect Is Inhibited by PLA2 Antagonists
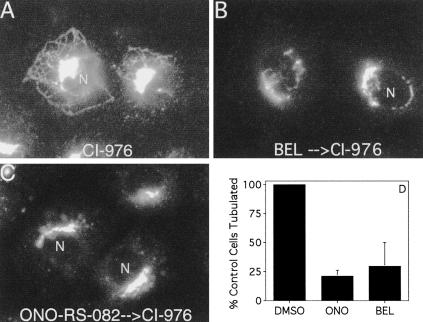
We hypothesize that CI-976–stimulated Golgi tubulation is due to the accumulation of LPL formed by a PLA2; therefore, inhibiting the formation of LPL from PL by PLA2 antagonists should reduce CI-976–induced tubulation. To test this hypothesis, we pretreated cells with antagonists (ONO-RS-082, BEL) that are selective for cytoplasmic Ca2+-independent PLA2 enzymes (Balsinde et al., 1999) for 10 min before adding CI-976. Golgi tubulation stimulated by CI-976 (Figure 7A) was substantially inhibited by both the PLA2 antagonists (Figure 7, B–D). These data strongly suggest that the action of a PLA2(s), i.e., the formation of LPL, is necessary for CI-976–stimulated Golgi tubulation.
Figure 7.
PLA2 antagonists inhibit CI-976–stimulated Golgi tubulation. Clone 9 cells were treated with CI-976 (20 μM) (A) for 15 min at 37°C, or pretreated with the PLA2 antagonists BEL (10 μM) (B) or ONO-RS-082 (10 μM) (C) for 10 min before the addition of CI-976 as in A. Cells were fixed and processed for ManII immunofluorescence, and the micrographs are representative of the cells examined for each condition. These results were quantified by determining the fraction of CI-976–treated cells that could produce tubules when pretreated with ONO-RS-082 (ONO) or BEL as described above (D). The results represent the mean + 1 SD from three experiments.
CI-976 Enhances Golgi Membrane Tubulation In Vitro and Increases LPL Content
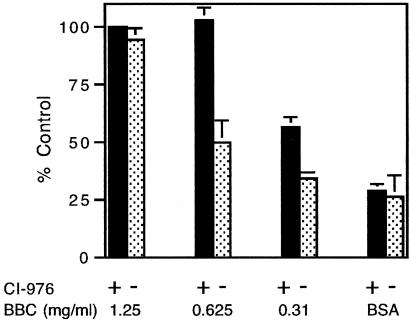
Because CI-976 stimulated the tubulation of the Golgi complex in vivo (Figure 2), we tested whether this compound would also cause tubulation of Golgi membranes in vitro. When CI-976 alone was added to isolated, intact Golgi stacks, no membrane tubulation occurred (our unpublished data), probably because the preparation lacked the PLA2 activity needed to produce LPLs, whose conversion back to PLs would be inhibited by CI-976. As we have previously shown, a highly fractionated extract of BBC contains a PLA2 antagonist-sensitive tubulation activity (Banta et al., 1995; de Figueiredo et al., 1999; Polizotto et al., 1999); therefore, we examined the ability of CI-976 to influence tubule formation in the presence of BBC. When increasing amounts of BBC were mixed with CI-976, and then added to Golgi membranes, the extent of Golgi membrane tubulation was enhanced, compared with that seen with BBC preincubated with DMSO as a control (Figure 8). We found that CI-976 maximally enhanced tubulation in the presence of 0.625 mg/ml BBC. This result is consistent with the idea that CI-976 enhancement of Golgi membrane tubulation requires a component in cytosol, such as a PLA2.
Figure 8.
Treatment of Golgi membranes with CI-976 enhances BBC-dependent membrane tubulation. Increasing amounts of BBC, or 2.5 mg/ml BSA, previously incubated in reaction buffer + CI-976 (closed bars) or DMSO (stippled bars), were added to Golgi membranes, and the mixture was incubated at 37°C for 15 min. Membranes were then pipetted onto copper grids, negatively stained, and examined in the electron microscope. The results represent the mean + 1 SD from three experiments.
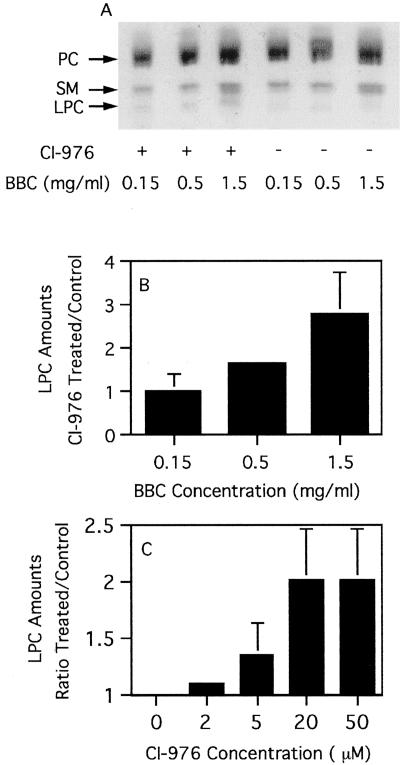
Isolated Golgi complexes treated with BBC were also examined to see whether CI-976 caused an increase in the levels of LPLs. In agreement with previous studies (Keenan and Morre, 1970; Fleischer et al., 1974), we found that LPCs constitute a very small fraction (∼0.4%) of total Golgi lipid. Other LPLs were undetectable in our system. When added at a fixed concentration (50 μM), CI-976 caused a maximal two to threefold increase in the levels of LPC over those produced by BBC alone (Figure 9, A and B). Likewise, when BBC was kept at a constant concentration, increasing amounts of CI-976 caused an increase in the levels of LPC (Figure 9C). These results are consistent with the idea that CI-976 enhancement of BBC-dependent tubulation of isolated Golgi membranes may be caused by accumulation of LPLs, specifically LPC.
Figure 9.
CI-976 treatment of isolated Golgi complexes causes an increase in LPC levels. Isolated Golgi membranes and differing amounts of BBC were incubated with CI-976 (50 μM final concentration) under conditions that normally stimulate tubulation (as in Figure 8). At the end of the experiment, membranes were extracted and lipids analyzed by HPTLC. (A) A region of a representative TLC plate that contains the LPC band. Other lipids include PC and sphingomyelin (SM). (B) Quantitative results from several experiments as in A. The results are expressed as the ratio of LPC in CI-976–treated samples:control for each BBC concentration. (C) Isolated Golgi membranes, a fixed concentration of BBC (1.5 mg/ml final concentration), and different amounts of CI-976 were incubated under tubulation conditions and then lipids were extracted and analyzed by HPTLC. The results are expressed as the ratio of LPC in CI-976–treated samples:control (no CI-976). The results in B and C represent the mean + 1 SD from three experiments.
Characterization of the CI-976–sensitive, Golgi-associated LPAT Activity
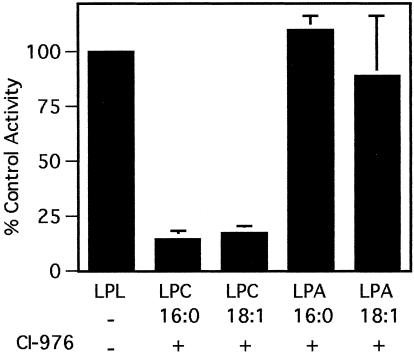
To determine whether the CI-976-sensitive Golgi LPAT is similar to other acyltransferases known to affect Golgi membranes, e.g., CtBP/BARS (Weigert et al., 1999), we have examined the substrate preference and other characteristics of this enzyme. The Golgi-associated LPAT displayed significant CI-976–sensitive activity when using either LPC (16:0) or LPC (18:1) as an acyl chain acceptor. However, the Golgi-associated LPAT was not sensitive to CI-976 (up to 50 μM) when LPA (16:0) was used as a substrate and only slightly sensitive when LPA (18:1) was used as a substrate (Figure 10). These results demonstrate that, unlike CtBP/BARS, the CI-976 target enzyme greatly prefers LPC over LPA as a substrate. In other studies, we found that the CI-976–sensitive, Golgi-associated LPAT could not be washed from membranes with 1 M salt, and it had little preference for long or short acyl chain donors (our unpublished data). Finally, CI-976 also inhibited the transfer of exogenous fatty acids to endogenous LPC in Golgi membranes (our unpublished data).
Figure 10.
Golgi-associated, CI-976-sensitive LPAT activity uses LPC, but not LPA, as the acyl-chain acceptor. The conversion of LPL to PL was monitored by incubating isolated Golgi membranes with various LPA or LPC substrates, CI-976 (50 μM), and [14C]palmitoyl-CoA at 37°C for 5 min. Total lipids were extracted, separated by TLC and the radioactivity of PA- or PC-containing spots was measured by phosphorimaging. The enzyme activity is reported as the percentage of the control (mean plus 1 SD from three experiments).
DISCUSSION
The striking ability of CI-976 to induce tubule formation from Golgi membranes in vivo most likely results from inhibition of LPAT, not ACAT, activity. First, more potent ACAT inhibitors (DuP-128 and PKF058035) did not inhibit Golgi LPAT activity or stimulate tubule formation. Second, the effects of CI-976 on both Golgi tubulation and LPAT activity occurred in the same micromolar range. And, third, inhibiting the formation of LPL by pretreating cells with PLA2 antagonists prevented the formation of CI-976–induced Golgi tubules. Based on its structure (Figure 1C), CI-976 may be acting as a substrate analog of fatty acids.
CI-976 is a unique pharmacological tool, similar in some ways to BFA (Lippincott-Schwartz et al., 1991; Wood et al., 1991). However, unlike BFA (Donaldson et al., 1990; Klausner et al., 1992), CI-976 did not cause the rapid loss of β-COP from Golgi membranes, and, in fact, β-COP could be found coating the tubules stimulated in response to CI-976. Although it is possible that the membrane associated β-COP (and by extension the coatomer complex) is nonfunctional, the simplest explanation is that tubule formation from the Golgi complex is not dependent on coatomer dissociation, contrary to previously proposed mechanisms for BFA-stimulated tubulation (Scheel et al., 1997). Consistent with this idea, a number of studies have now demonstrated that membrane tubules can form from the Golgi complex or TGN under a variety of conditions without dissociation of coatomer or Adaptor protein-1 (AP-1) clathrin complexes, respectively (de Figueiredo and Brown, 1995, 1999; Kano etal., 2000). In addition, overexpression of BIG2, a BFA-inhibited guanine nucleotide exchange factor for ADP-ribosylation factor (ARF1), prevented BFA-induced redistribution of AP-1 complexes and ARF1 from TGN membranes, but still allowed BFA-stimulated tubule formation (Shinotsuka et al., 2002). Together, these studies strongly suggest that tubulation of Golgi membranes is not absolutely dependent on ARF or coat protein dissociation, and, at least in the case of CI-976–induced tubules, is more likely related to the accumulation of LPLs.
Similar to BFA, after Golgi markers had completely redistributed to the ER in CI-976–treated cells, β-COP was localized to small, punctate structures throughout the cytoplasm that could be Golgi remnants (Hendricks et al., 1992) and/or vesicular-tubular clusters at ER exit sites (Allan and Balch, 1999; Lippincott-Schwartz and Hirschberg, 2000). In contrast to BFA, however, CI-976 stimulation of tubulation and retrograde trafficking had a longer lag phase, ∼5 versus 10–15 min (Lippincott-Schwartz et al., 1989, 1990). This difference could be related to the time required for CI-976 to penetrate cells, bind its target enzyme, and cause a sufficient accumulation of LPL in Golgi membranes.
How could the inhibition of a Golgi-associated LPAT activity cause membrane tubulation? Our model suggests that membrane tubulation is initiated by the direct action of phospholipid-modifying enzymes on the cytoplasmic leaflet of a lipid bilayer. This model is supported by our previous findings that Golgi tubule formation and retrograde trafficking were inhibited by PLA2 antagonists and enhanced by PLA2 agonists (de Figueiredo et al., 1998, 1999, 2000; Drecktrah and Brown, 1999; Polizotto et al., 1999). PLA2 antagonists also prevented the tubule-mediated assembly and maintenance of intact Golgi ribbons (de Figueiredo et al., 1999). Mechanistically, production of LPL from PL in one membrane leaflet by PLA2 enzymes could result in membrane bending due to the change between cylindrically shaped PLs and inverted cone-shaped LPLs (Scales and Scheller, 1999). This change in the LPL/PL ratio in one membrane leaflet would cause the bilayer to curve into buds and tubules, as experimentally demonstrated to occur on the surfaces of erythrocytes and liposomes (Fujii and Tamura, 1979; Mui et al., 1995). We propose that CI-976 produces its effects by inhibiting a Golgi-associated LPAT enzyme(s), thus resulting in a local LPL increase that leads to membrane curvature and finally tubule formation. In this case, the ability of CI-976 to induce tubulation would depend on the prior activity of an LPL-generating PLA2, and three results were consistent with this idea. First, pretreatment of cells with PLA2 antagonists inhibited the LPAT effect. Second, CI-976 alone was incapable of inducing tubulation in the in vitro reconstitution system; however, CI-976 was capable of enhancing in vitro Golgi tubulation in the presence of low amounts of BBC, which contain BEL- and ONO-RS-082–sensitive PLA2 enzymes (de Figueiredo et al., 1999; Polizotto et al., 1999). And, third, we found that Golgi membranes had increased amounts of LPC after CI-976 enhancement of cytosol-dependent tubulation.
The idea that membrane shape changes directly influence trafficking events, by modulating the balance between PLs and LPLs within a single leaflet of a bilayer, received significant support by the recent discovery that CtBP/BARS and endophilin A1 are LPA-specific acyltransferases (LPA-AT) (Schmidt et al., 1999; Weigert et al., 1999). Interestingly, CtBP/BARS stimulates the fission of Golgi membrane tubules (Weigert et al., 1999) and is inhibited by BFA-stimulated ADP-ribosylation (Spano et al., 1999). Thus, inhibition of CtBP/BARS by BFA might lead to exaggerated tubulation. The ability of the LPAT inhibitor CI-976 to induce Golgi tubulation might suggest that CtBP/BARS is its target; however, several pieces of data strongly argue against this idea. First, CtBP/BARS is specific for LPA, whereas the Golgi-associated, CI-976–sensitive LPAT is very active by using LPC but not LPA as a substrate. Second, CtBP/BARS is a cytosolic enzyme, whereas the CI-976–sensitive LPAT is very tightly associated with Golgi membranes. And third, CtBP-BARS prefers to transfer long chain fatty acids (Weigert et al., 1999), whereas the CI-976–sensitive Golgi LPAT was active with a broad range of acyl chain lengths (our unpublished data). Therefore, our results point to another LPAT that controls Golgi morphology, perhaps one similar to a previously described activity (Lawrence et al., 1994).
Interestingly, the LPA-AT endophilin A1 and a related protein, endophilin B, have been shown to induce tubule formation when directly bound to artificial liposomes (Farsad et al., 2001). However, endophilin A1 is able to induce tubulation in the apparent absence of any LPAT substrate. Thus, its tubule-inducing activity seems to be independent of its LPA-AT activity. Instead, endophilin forms membrane tubules by virtue of its ability to self-assemble into ring- or spring-like structures on the outer surface of liposomes, which causes the membrane to deform into a tubule. This self-assembly is very similar to that displayed by the dynamins and the amphiphysins, both of which can also induce membrane tubulation (Sever et al., 2000; Huttner and Schmidt, 2002). Indeed, a muscle-specific form of amphiphysin, M-amphiphysin 2, seems to be involved in the formation of T-tubules in skeletal muscle, and its overexpression in fibroblasts induces tubule formation from the plasma membrane (Lee et al., 2002). It is unclear at present whether any of the “self-assembling” proteins, such as endophilin, contribute to the formation of CI-976–induced tubules shown herein.
Numerous studies have established that membrane tubules emanate from nearly every region of the Golgi complex, including the TGN (Cooper et al., 1990; Hirschberg et al., 1998; Presley et al., 1998; Polishchuk et al., 2000; Toomre et al., 2000; Liljedahl et al., 2001) and that these tubules are involved in intracellular trafficking, e.g., retrograde trafficking from the Golgi to the ER and anterograde from the TGN to the plasma membrane. Our studies here with CI-976 are consistent with this view and provide additional, independent evidence that tubules are involved in Golgi-to-ER retrograde trafficking. Although tubules seem to mediate certain trafficking events on their own, they may also provide a mechanism, by virtue of their high surface-to-volume ratio, for increasing the efficiency of sorting receptor-ligand complexes into coated vesicles that subsequently bud along the shaft or at the tip of a tubule (Futter et al., 1998; Palokangas et al., 1998). In addition to lipid remodeling, other factors may contribute to the initiation and/or maintenance of membrane tubules, such as the binding of as yet unidentified peripheral proteins (Weidman et al., 1993; Aridor et al., 2001). Also, it is clear that in vivo, microtubules facilitate the formation of long membrane tubules from the Golgi complex (Lippincott-Schwartz, 1998) and ER (Aridor et al., 2001). Nevertheless, stimulation of Golgi tubulation by CI-976, and its inhibition by PLA2 antagonists, clearly suggests the involvement of PL metabolism in directly initiating and mediating tubule formation.
Acknowledgments
We thank Drs. Kelley Moremen (anti-ManII), Jaakko Saraste (anti-p58), and William Balch (anti-β-COP) for providing antibodies, and Dr. Jennifer Lippincott-Schwartz for providing DNA constructs (GalT-GFP). We also want to thank Marian Strang for expert assistance with the electron microscopy. This work was supported by National Institutes of Health grant DK 51596 (to W.J.B.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-11-0711. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0711.
References
- Allan, B.B., and Balch, W.E. (1999). Protein sorting by directed maturation of Golgi compartments. Science 285, 63–68. [DOI] [PubMed] [Google Scholar]
- Aridor, M., Fish, K.N., Bannykh, S.I., Weissman, J., Roberts, T.H., Lippincott-Schwartz, J., and Balch, W.E. (2001). The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde, J., Balboa, M.A., Insel, P.A., and Dennis, E.A. (1999). Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 39, 175–189. [DOI] [PubMed] [Google Scholar]
- Banta, M., Polizotto, R.S., Wood, S.A., de Figueiredo, P., and Brown, W.J. (1995). Characterization of a cytosolic activity that induces the formation of Golgi membrane tubules in a cell-free reconstitution system. Biochemistry 34, 13359–13366. [DOI] [PubMed] [Google Scholar]
- Bligh, F.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Brown, W.J., and Farquhar, M.G. (1987). The distribution of the 215 kD mannose 6-phosphate receptors within cis (heavy) and trans (light) Golgi subfractions varies in different cell types. Proc. Natl. Acad. Sci. USA 84, 9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson, A., Kuypers, F.A., Roelofsen, B., Op den Kamp, J.A., and van Deenen, L.L. (1985). Lipid molecular shape affects erythrocyte morphology: a study involving replacement of native phosphatidylcholine with different species followed by treatment of cells with sphingomyelinase C or phospholipase A2. J. Cell Biol. 101, 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett, E.B., and Brown, W.J. (1992). Adhesion of Golgi cisternae by proteinaceous interactions - intercisternal bridges as putative adhesive structures. J. Cell Sci. 103, 773–784. [DOI] [PubMed] [Google Scholar]
- Cooper, M.S., Cornell-Bell, A.H., Chemjavsky, A., Dani, J.W., and Smith, S.J. (1990). Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-Golgi elements into a reticulum. Cell 6, 135–145. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., and Brown, W.J. (1995). A role for calmodulin in organelle membrane tubulation. Mol. Biol. Cell 6, 871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo, P., and Brown, W.J. (1999). Clofibrate inhibits membrane trafficking to the Golgi complex and induces its retrograde movement to the endoplasmic reticulum. Cell Biol Toxicol 15, 311–323. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., Doody, A., Polizotto, R.S., Drecktrah, D., Wood, S., Banta, M., Strang, M., and Brown, W.J. (2001). Inhibition of transferrin recycling and endosome tubulation by phospholipase A2 antagonists. J. Biol. Chem. 276, 47361–47370. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., Drecktrah, D., Katzenellenbogen, J.A., Strang, M., and Brown, W.J. (1998). Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl. Acad. Sci. USA 95, 8642–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo, P., Drecktrah, D., Polizotto, R.S., Cole, N.B., Lippincott-Schwartz, J., and Brown, W.J. (2000). Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic 1, 504–511. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., Polizotto, R.S., Drecktrah, D., and Brown, W.J. (1999). Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol. Biol. Cell 10, 1763–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J.G., Lippincott-Schwartz, J., Bloom, G.S., Kreis, T.E., and Klausner, R.D. (1990). Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J. Cell Biol. 111, 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah, D., and Brown, W.J. (1999). Phospholipase A2 antagonists inhibit nocodazole-induced Golgi ministack formation: evidence of an ER intermediate and constitutive cycling. Mol. Biol. Cell 10, 4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad, K., Ringstad, N., Takei, K., Floyd, S.R., Rose, K., and De Camilli, P. (2001). Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 155, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer, R., Zambrano, F., and Fleischer, S. (1974). Biochemical characterization of the Golgi complex of mammalian cells. J. Supramol. Struct. 2, 737–750. [DOI] [PubMed] [Google Scholar]
- Fujii, T., and Tamura, A. (1979). Asymmetric manipulation of the membrane lipid bilayer of intact human erythrocytes with phospholipase A, C, or D induces a change in cell shape. J. Biochem. 86, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Futter, C.E., Gibson, A., Allchin, E.H., Maxwell, S., Ruddock, L.J., Odorizzi, G., Domingo, D., Trowbridge, I.S., and Hopkins, C.R. (1998). In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 141, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte, R.A., Yeaman, S.J., Jackson, B., and Suckling, K.E. (1995). Effect of membrane environment on inhibition of acyl-CoA:cholesterol acyltransferase by a range of synthetic inhibitors. Biochim. Biophys. Acta 1258, 241–250. [DOI] [PubMed] [Google Scholar]
- Hendricks, L.C., Mcclanahan, S.L., Mccaffery, M., Palade, G.E., and Farquhar, M.G. (1992). Golgi proteins persist in the tubulovesicular remnants found in brefeldin-A-treated pancreatic acinar cells. Eur J. Cell Biol. 58, 202–213. [PubMed] [Google Scholar]
- Hirschberg, K., Miller, C.M., Ellenberg, J., Presley, J.F., Siggia, E.D., Phair, R.D., and Lippincott-Schwartz, J. (1998). Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner, W.B., and Schmidt, A.A. (2002). Membrane curvature: a case of endofeelin'. Trends Cell Biol. 12, 155–158. [DOI] [PubMed] [Google Scholar]
- Kano, F., Sako, Y., Tagaya, M., Yanagida, T., and Murata, M. (2000). Reconstitution of brefeldin A-induced Golgi tubulation and fusion with the endoplasmic reticulum in semi-intact Chinese hamster ovary cells. Mol. Biol. Cell 11, 3073–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, T.W., and Morre, D.J. (1970). Phospholipid class and fatty acid composition of Golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry 9, 19–25. [DOI] [PubMed] [Google Scholar]
- Kerkhoff, C., G. L., Habben, K., Resch, K., Kaever, V. (1996). Identification of two different lysophosphatidylcholine:acyl-CoA acyltransferases (LAT) in pig spleen with putative distinct topological localization. Biochim. Biophys. Acta 1302, 249–256. [DOI] [PubMed] [Google Scholar]
- Klausner, R.D., Donaldson, J.G., and Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, J.B., Moreau, P., Cassagne, C., and Morre, D.J. (1994). Acyl transfer reactions associated with cis Golgi apparatus of rat liver. Biochim. Biophys. Acta 1210, 146–150. [DOI] [PubMed] [Google Scholar]
- Lee, E., Marcucci, M., Daniell, L., Pypaert, M., Weisz, O.A., Ochoa, G.C., Farsad, K., Wenk, M.R., and De Camilli, P. (2002). Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297, 1193–1196. [DOI] [PubMed] [Google Scholar]
- Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J., and Malhotra, V. (2001). Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104, 409–420. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J. (1998). Cytoskeletal proteins and Golgi dynamics. Curr. Opin. Cell Biol. 10, 52–59. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Donaldson, J.G., Schweizer, A., Berger, E.G., Hauri, H.P., Yuan, L.C., and Klausner, R.D. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60, 821–836. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., R. T., Hirschberg, K. (2000). Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell. Dev. Biol. 16, 557–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L., Tipper, C., Amherdt, M., Orci, L., and Klausner, R.D. (1991). Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67, 601–616. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L.C., Bonifacino, J.S., and Klausner, R.D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macala, L.J., Yu, R.K., and Ando, S. (1983). Analysis of brain lipids by high performance thin layer chromatography and densitometry. J. Lipid Res. 24, 1243–1250. [PubMed] [Google Scholar]
- Mui, B.L., Dobereiner, H.G., Madden, T.D., and Cullis, P.R. (1995). Influence of transbilayer area asymmetry on the morphology of large unilamellar vesicles. Biophys. J. 69, 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palokangas, H., Ying, M., Vaananen, K., and Saraste, J. (1998). Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell 9, 3561–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar, S.J., and Jurs, P.C. (2000). Prediction of IC50 values for ACAT inhibitors from molecular structure. J. Chem. Inf. Comput. Sci. 40, 706–723. [DOI] [PubMed] [Google Scholar]
- Polishchuk, R.S., Polishchuk, E.V., Marra, P., Alberti, S., Buccione, R., Luini, A., and Mironov, A.A. (2000). Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J. Cell Biol. 148, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizotto, R.S., de Figueiredo, P., and Brown, W.J. (1999). Stimulation of Golgi membrane tubulation and retrograde trafficking to the ER by phospholipase A2 activating protein (PLAP) peptide. J Cell Biochem. 74, 670–683. [PubMed] [Google Scholar]
- Presley, J.F., Smith, C., Hirschberg, K., Miller, C., Cole, N.B., Zaal, K.J., and Lippincott-Schwartz, J. (1998). Golgi membrane dynamics. Mol. Biol. Cell 9, 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, S.J., and Scheller, R.H. (1999). Lipid membranes shape up. Nature 401, 123–124. [DOI] [PubMed] [Google Scholar]
- Scheel, J., Pepperkok, R., Lowe, M., Griffiths, G., and Kreis, T.E. (1997). Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J. Cell Biol. 137, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., Wolde, M., Thiele, C., Fest, W., Kratzin, H., Podtelejnikov, A.V., Witke, W., Huttner, W.B., and Soling, H.D. (1999). Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 401, 133–141. [DOI] [PubMed] [Google Scholar]
- Sever, S., Damke, H., and Schmid, S.L. (2000). Garrotes, springs, ratchets, and whips: putting dynamin models to the test. Traffic 1, 385–892. [DOI] [PubMed] [Google Scholar]
- Shinotsuka, C., Yoshida, Y., Kawamoto, K., Takatsu, H., and Nakayama, K. (2002). Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J. Biol. Chem. 277, 9468–9473. [DOI] [PubMed] [Google Scholar]
- Spano, S., et al. (1999). Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate. A novel protein involved in the maintenance of the Golgi structure. J. Biol. Chem. 274, 17705–17710. [DOI] [PubMed] [Google Scholar]
- Toomre, D., Steyer, J.A., Keller, P., Almers, W., and Simons, K. (2000). Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy. J. Cell Biol. 149, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman, P., Roth, R., and Heuser, J. (1993). Golgi membrane dynamics imaged by freeze-etch electron microscopy: views of different membrane coatings involved in tubulation versus vesiculation. Cell 75, 123–133. [PubMed] [Google Scholar]
- Weigert, R., et al. (1999). CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402, 429–433. [DOI] [PubMed] [Google Scholar]
- Wood, S.A., Park, J.E., and Brown, W.J. (1991). Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell 67, 591–600. [DOI] [PubMed] [Google Scholar]