Abstract
UDP-galactose reaches the Golgi lumen through the UDP-galactose transporter (UGT) and is used for the galactosylation of proteins and lipids. Ceramides and diglycerides are galactosylated within the endoplasmic reticulum by the UDP-galactose:ceramide galactosyltransferase. It is not known how UDP-galactose is transported from the cytosol into the endoplasmic reticulum. We transfected ceramide galactosyltransferase cDNA into CHOlec8 cells, which have a defective UGT and no endogenous ceramide galactosyltransferase. Cotransfection with the human UGT1 greatly stimulated synthesis of lactosylceramide in the Golgi and of galactosylceramide in the endoplasmic reticulum. UDP-galactose was directly imported into the endoplasmic reticulum because transfection with UGT significantly enhanced synthesis of galactosylceramide in endoplasmic reticulum membranes. Subcellular fractionation and double label immunofluorescence microscopy showed that a sizeable fraction of ectopically expressed UGT and ceramide galactosyltransferase resided in the endoplasmic reticulum of CHOlec8 cells. The same was observed when UGT was expressed in human intestinal cells that have an endogenous ceramide galactosyltransferase. In contrast, in CHOlec8 singly transfected with UGT 1, the transporter localized exclusively to the Golgi complex. UGT and ceramide galactosyltransferase were entirely detergent soluble and form a complex because they could be coimmunoprecipitated. We conclude that the ceramide galactosyltransferase ensures a supply of UDP-galactose in the endoplasmic reticulum lumen by retaining UGT in a molecular complex.
INTRODUCTION
Galactosylation of glycosphingolipids and proteins occurs in the Golgi lumen by galactosyltransferases that use UDP-galactose. Translocation of UDP-galactose from the cytosol into the lumen is mediated by an antiporter that transports UMP in the opposite direction (Kuhn and White, 1976; Hirschberg et al., 1998). In rat liver, UDP-galactose transport activity is exclusively localized to Golgi fractions and absent from endoplasmic reticulum (ER; Perez and Hirschberg, 1985). The cDNAs of two human UDP-galactose transporter isoforms, UGT1 and UGT2, have been cloned. The conceptual translation product of UGT2 differs from UGT1 in the C-terminal eight amino acids. UGT1 was localized to the Golgi by immunofluorescence and by activity (Ishida et al., 1996; Miura et al., 1996; Yoshioka et al., 1997). Ectopically expressed UGT2 distributed similarly to a Golgi 58-kDa protein in murine Had-1 cells (Kawakita, personal communication). In mouse and Schizosaccharomyces pombe, UGT also localized to the Golgi and restored UGT activity in UGT-deficient cells (Tabuchi et al., 1997; Ishida et al., 1999; Segawa et al., 1999). These and other studies demonstrate that UGT is involved in transport of UDP-galactose across the Golgi membrane. Its absence from the ER suggests that it does not translocate UDP-galactose into the ER lumen (for reviews, see Hirschberg et al., 1998; Kawakita et al., 1998).
Glycosphingolipids are synthesized in the Golgi, except for galactosylceramide (GalCer), which is made in the ER by the UDP-galactose:ceramide galactosyltransferase (GalT-1; nomenclature as in Basu et al., 1987). GalT-1 belongs to the family of the ER glucuronyltransferases, type I proteins possessing an ER retention signal in their cytosolic tail (Schulte and Stoffel, 1993; Stahl et al., 1994; Schaeren-Wiemers et al., 1995; Sprong et al., 1998). GalT-1 preferentially galactosylates hydroxy fatty acid-containing ceramides (Morell and Radin, 1969) but also uses nonhydroxy fatty acid ceramides and diglycerides (van der Bijl et al., 1996b). Knockout mice lacking GalT-1 do not make GalCer or galactosyldiglyceride, demonstrating that there is only one GalT-1 (Bosio et al., 1996; Coetzee et al., 1996; Fujimoto et al., 2000). The active center faces the ER lumen (Sprong et al., 1998). The precursor ceramide is synthesized in the ER membrane and is sufficiently hydrophobic to equilibrate rapidly between the two lipid leaflets, possibly facilitated by a translocator protein. In contrast, it is unclear how UDP-galactose reaches the ER lumen from the cytosol (Segawa et al., 1999).
When GalT-1-negative cells were transfected with GalT-1, they produced GalCer (Schaeren-Wiemers et al., 1995; van der Bijl et al., 1996b; Sprong et al., 1998), showing that they can import UDP-galactose into the ER lumen. MDCKII-RCAR cells defective in Golgi UDP-galactose import also contain very little GalCer (Brändli et al., 1988). Likewise, CHOlec8 cells defective in UDP-galactose import in the Golgi (Deutscher and Hirschberg, 1986; Taki et al., 1991; Oelmann et al., 2001) also displayed a defect in UDP-galactose transport into the ER (Sprong et al., 1998). In conclusion, various cell types import UDP-galactose into the ER lumen, and this ability is affected in two cell lines with a mutation in the Golgi UGT. Thus, either ER UDP-galactose import depends on the Golgi UGT, or MDCKII-RCAR and CHOlec8 cells have mutations in both an ER and a Golgi UDP-galactose transporter. We report that GalT-1 forms a complex with the Golgi UGT. Only in cells expressing GalT-1, the Golgi UGT is retained in the ER in a complex with GalT-1.
MATERIALS AND METHODS
[1-14C]Acetic acid (1.8 GBq/mmol) was from Amersham Biosciences UK (Little Chalfont, Buckinghamshire, United Kingdom). Bovine serum albumin (BSA, fraction V), UDP-galactose, UDP-glucose, and lipid standards were from Sigma-Aldrich (St. Louis, MO). Digitonin and octylglucoside were from Calbiochem (La Jolla, CA). C6-NBD-ceramide was from Molecular Probes (Eugene, OR). C6-NBD-glucosylceramide (GlcCer) was synthesized from NBD-hexanoic acid (Sigma-Aldrich) and 1-β-d-glucosylsphingosine (Sigma-Aldrich) as described previously (Kishimoto, 1975), purified by two-dimensional-thin layer chromatography (TLC) as described previously (Sprong et al., 2000), and quantified spectrophotometrically at Lex = 465 nm and Lem = 540 nm. Michel Bornens (Institute Curie, Paris, France) kindly provided the mouse monoclonal antibody (mAb) against the cis/medial-Golgi marker CTR433 (Jasmin et al., 1990). The mouse mAb 1D3 against protein-disulfide isomerase (PDI) was kindly provided by Stephen Fuller (European Molecular Biology Laboratory, Heidelberg, Germany). A rabbit polyclonal antiserum Y-11 against the hemagglutinin (HA)-epitope was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rat mAb 3F10 against the HA-epitope was from Roche Diagnostics (Mannheim, Germany). Rabbit antibodies against the N terminus of human invariant chain p33, ICN1 (Morton et al., 1995), were a kind gift of Monique Kleijmeer (Utrecht University, The Netherlands). The affinity-purified rabbit antiserum 635 recognizing the N terminus of GalT-1 has been described previously (Sprong et al., 1998). The anti-p62(yes) mouse monoclonal was from Transduction Laboratories (Lexington, KY), and rabbit anti-calnexin and anti-calreticulin antisera (Zhang et al., 1997) were a kind gift of Ineke Braakman (Utrecht University). The rabbit polyclonal A-14 against the c-myc epitope tag was from Santa Cruz Biotechnology. Fluorescein isothiocyanate (FITC)- and Texas Red-labeled goat anti-rabbit, goat anti-mouse, and goat anti-rat antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Goat anti-rabbit and rabbit anti-rat antibodies coupled to horseradish peroxidase were from DAKO (Glostrup, Denmark).
cDNAs and Plasmids
cDNA of rat GalT-1 was released with HindIII and XbaI from GalT-1-pcDNA3 (van der Bijl et al., 1996b) and inserted in pCB7 (Hansen and Casanova, 1994) cut with the same enzymes. Human invariant chain p33 and a chimera carrying the cytosolic tail of p33 and the lumenal domain of human UDP-galactose:protein(β1–4)galactosyltransferase (p33-PGalT) have been described previously (Nilsson et al., 1994). A plasmid with the human UGT1 bearing an influenza virus HA epitope at the C terminus (HA-hUGT1-pMKIT-neo) was described previously (Aoki et al., 1999). The myc-tagged sialyltransferase cDNA was from S. Munro (Medical Research Council, Cambridge, United Kingdom; Sprong et al., 2001).
Cell Culture and Transfection
Chinese hamster ovary lec8 cells (CHOlec8 cells; American Type Culture Collection, Rockville, MD) were grown in DMEM containing 10% fetal calf serum and were maintained at 37°C with 5% CO2. CHOlec8 cells were transfected with GalT-1-pCB7, HA-hUGT1-pMKIT-neo, and the empty vectors pCB7 and pcDNA3 by using the calcium phosphate precipitation procedure (Graham and van der Eb, 1973). Transfectants were cultured in DMEM containing 10% fetal calf serum and 200 U/ml hygromycin B or 0.6 mg/ml geneticin. Stable cell lines were obtained by subcloning individual colonies. Positive clones were selected by immunofluorescence microscopy, and expression was subsequently tested by Western blotting (WB). Transient transfections were performed with 350 ng of plasmid DNA/cm2 tissue culture dish, by using calcium phosphate precipitation. Cells were assayed 2–3 d after transfection. Chinese hamster ovary (CHO) cells expressing a C-terminally hemagglutinin (HA)-tagged CMP-sialic acid transporter were gratefully obtained from Rita Gerardy-Schahn (Medizinische Hochschule, Hannover, Germany) and cultured as described previously (Oelmann et al., 2001). Protein expression was induced by 5 mM sodium butyrate (Fluka, Buchs, Germany) 14–16 h before all experiments.
Subcellular Fractionation and Determination of Enzyme Activities
The fractionation was performed below 4°C. Cells were washed, and allowed to swell in a low salt buffer (10 mM HEPES-NaOH, 15 mM KCl, 1.5 mM MgCl2, pH 7.2) for 15 min. Cells were scraped, pelleted, and homogenized in the same buffer by 15 passages through a 25-gauge 5/8 needle. A postnuclear supernatant (PNS) was obtained by a 10-min 300 gmax spin. The membrane fraction was prepared by ultracentrifugation in a SW41 rotor for 1 h at 265,000 gmax. For subcellular fractionation, a PNS was applied to the top of a linear sucrose gradient (0.7–1.5 M sucrose, in 1 mM EDTA, 10 mM HEPES-NaOH pH 7.2), and the 11-ml gradient was spun in a SW41 rotor for 3 h at 265,000 gmax (Burger et al., 1996). Fractions were collected from the bottom. Enzyme activities of GalT-1 and of UDP-glucose:ceramide glucosyltransferase were determined in 250 μl of each fraction in the presence of 10 nmol of C6-NBD-ceramide, 1% (wt/vol) BSA, 2 mM MnCl2, 2 mM UDP-glucose, and 2 mM UDP-galactose. To monitor the synthesis of lactosylceramide (LacCer), fractions were incubated in the presence of 10 nmol of C6-NBD-GlcCer, 1% (wt/vol) BSA, 2 mM MnCl2, and 2 mM UDP-galactose at 37°C in a total volume of 500 μl. When enzyme activities were determined directly on the PNS, samples were preincubated in the absence or presence of 0.5% (wt/vol) saponin for 30 min on ice. Activities were measured in the linear range of the assay. Lipids were extracted and separated as described previously (Burger et al., 1996) and quantified on a Storm 860 (Molecular Dynamics, Sunny-vale, CA) by using C6-NBD-phosphatidylcholine as a standard. Protein concentration was determined by the bicinchoninic acid method (Pierce Chemical, Rockford, IL). Esterase activity was used as an ER marker and was assayed as described previously (Beaufay et al., 1974). The distribution of proteins was determined by Western blotting, as described under “Immunoprecipitation.”
Immunofluorescence Microscopy
Cells were grown on coverslips to 30–50% confluence. The cells were fixed with 3% paraformaldehyde and quenched in phosphate-buffered saline (PBS) containing 50 mM NH4Cl. Cells were then permeabilized and blocked for 1 h in PBS, 0.5% bovine serum albumin, 0.1% saponin (blocking buffer), and subsequently labeled with mixtures of primary antibodies in blocking buffer. The coverslips were washed for 45 min in blocking buffer with three buffer changes. Coverslips were incubated with 10% goat serum in blocking buffer for 20 min and subsequently counterstained for 30 min with fluorescently labeled secondary antibodies at 1:50 dilutions in blocking buffer. The coverslips were then washed in blocking buffer for 45 min with three buffer changes, rinsed briefly in PBS and then water, and finally mounted in Mowiol 4-88 (Calbiochem, La Jolla, CA) containing 2.5% 1,4-diazabicyclo[2.2.2]octane (Sigma-Aldrich, St. Louis, MO). The cells were examined with a confocal microscope (Leica, Heidelberg, Germany) by using separate filters for each fluorochrome viewed (FITC: Lex = 488 nm and Lem = 515 long pass filter; Texas Red: Lex = 568 nm and Lem = 585 LP). Single-labeled cells with each primary/secondary antibody combination were examined, which showed that no bleed-through nor cross-reactivity occurred for the given confocal conditions.
Metabolic Labeling of Cellular Lipids
Subconfluent cells on 3-cm dishes were transfected with GalT-1 or empty vector and were incubated with 1.5-ml culture medium containing [1-14C]acetic acid (37 kBq/ml) for 3 d. Cells were washed three times with ice-cold PBS. Lipids were extracted and separated by two-dimensional TLC as described previously (Sprong et al., 2000), but by using chloroform/methanol/25% NH4OH [65:25:4 (vol/vol)] as the first running solvent. Radiolabeled spots were detected by exposure of phosphorimaging screens (Amersham Biosciences UK) and read out on a Storm PhosphorImager (Molecular Dynamics). Spots were identified by comparison with standards and quantified using standard software.
Immunoprecipitation and Detergent-resistant Membranes
The complete experiment was performed below 4°C. UGT-CHOlec8 cells transiently transfected with GalT-1, p33-PGalT, or empty vector were washed, scraped, and pelleted in low salt buffer. Cell pellets were resuspended in lysis buffer [50 mM HEPES-NaOH, pH 7.2, 100 mM NaCl, 1 μg/ml protease inhibitors (aprotinin, chymostatin, leupeptin, and pepstatin A, and either 1% (wt/vol) digitonin, octylglucoside or Triton X (TX)-100] and centrifuged at 20,000 × g for 5 min. A fraction of each detergent lysate was used to determine relative amounts of UGT by Western blotting with the anti-HA antiserum. The remainder was incubated with anti-GalT-1 antiserum or anti-p33-PGalT antiserum, adsorbed to protein A-Sepharose CL-4B for 1 h, and washed four times with corresponding lysis buffer. Washed immunoprecipitates were resuspended in reducing Laemmli sample buffer, incubated 10 min at room temperature and 30 min at 50°C, and subjected to SDS-PAGE and Western blotting for the HA-tagged UGT by using the anti-HA monoclonal, as described previously (Sprong et al., 1998). In the test for the presence of disulfide-bonded oligomers, the whole procedure was performed both in the presence and in absence of 20 mM N-ethylmaleimide, an alkylating agent that prevents artificial disulfide bond formation. For the preparation of detergent-resistant membranes a TX-100 lysate was adjusted to 1.2 ml 40% Optiprep (Nycomed, Oslo, Norway), overlaid with 2.1 ml 30% and 0.9 ml 5% Optiprep in TX-100 lysis buffer and spun at 40,000 rpm for 4 h in a SW60 rotor (Beckman Coulter, Palo Alto, CA). Seven 600-μl fractions were collected from the top.
RESULTS
Expression of the UDP-Galactose Transporter and GalT-1 in CHOlec8 Cells
To identify the mechanism by which UDP-galactose reaches the ER lumen, we used as an assay the activity of GalT-1, the enzyme that uses UDP-galactose in the lumen of the ER for the synthesis of GalCer. As an ideal background for our study, we chose CHOlec8 cells, which do not express endogenous GalT-1 (Sprong et al., 1998) and which display impaired UDP-galactose import into the Golgi apparatus (Deutscher and Hirschberg, 1986; Oelmann et al., 2001). We generated stable CHOlec8 lines expressing either rat GalT-1 or the HA-tagged human UGT1 (UGT) by transfection. GalT-1/CHOlec8 cells expressed a protein with an apparent molecular mass of 54 kDa that was recognized by anti-GalT-1 antiserum 635 (Sprong et al., 1998) on Western blots and that was not found in the mock-transfected CHOlec8 and in the UGT-CHOlec8 cells (Figure 1A). In a previous study, we identified the 54-kDa band as GalT-1 (Sprong et al., 1998). UGT-CHOlec8 cells expressed the HA-tagged UGT as a protein with an apparent molecular mass of ∼36 kDa that was specifically recognized by anti-HA antiserum Y-11 (Figure 1A), corroborating previous findings (Aoki et al., 1999). Because stable double transfectants were not viable, we transiently transfected CHOlec8 and UGT-CHOlec8 cells with GalT-1, and 2 d after transfection comparable levels of GalT-1 were detected by Western blotting in both cell lines (Figure 1A). The expression level of UGT was not affected by transient transfection with GalT-1.
Figure 1.
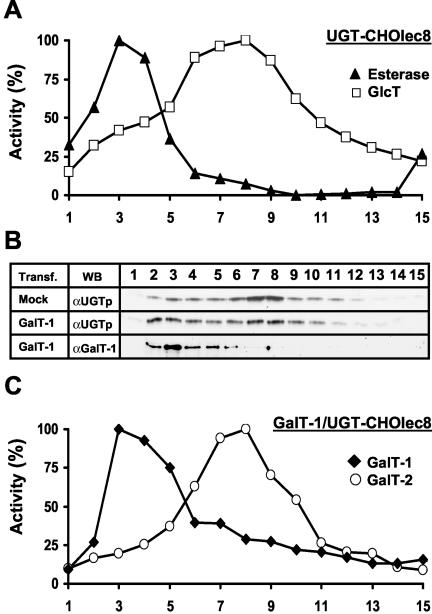
Functional expression of UGT and GalT-1 in CHOlec8 cells. (A) CHOlec8 cells were stably (St) transfected with UGT (UGT) or empty vector (–). In addition, the cells were stably (St) or transiently (Tr) transfected with GalT-1 or empty vector (–). Twenty micrograms of protein of the membrane fraction of a PNS of the cells were resolved on 10% SDS-polyacrylamide gels followed by Western blotting with antisera (αUGT and αGalT-1). Molecular weight standards (in kilodaltons) are indicated on the right. (B) CHOlec8 and UGT-CHOlec8 cells were transiently transfected with GalT-1 and labeled with [14C]acetate for 3 d. Lipids were extracted, separated by two-dimensional-TLC, and imaged by a phosphorimaging screen as described in text. Typical TLC images are shown. Numbers indicate sphingolipid spots described in C. O, origin. (1) Sphingomyelin (SM) consists of two species carrying different fatty acids. (2) Similarly, glucosylceramide (GlcCer) runs as two spots along the diagonal. (3) GM3 was identified by its retention factor. (4) Galactosylceramide (GalCer) runs as three spots, whereas only one spot (5) is observed for galactosyldiacylglycerol. The identity of these spots was verified by labeling with [14C]galactose (van der Bijl et al., 1996B). (C) Radiolabeled lipid spots were quantitated by phosphorimaging and expressed as a percentage of total radiolabeled lipids. Data are means of two independent experiments (n = 4). SD was <1% of total radiolabeled lipids.
UDP-Galactose Import in ER and Golgi
As a measure of UDP-galactose import into the lumen of the ER, we assayed the synthesis of GalCer by GalT-1, which occurs in the lumen of the ER, and not in the Golgi (Sprong et al., 1998). For UDP-galactose import in the Golgi, we analyzed the synthesis of sialyllactosylceramide (GM3). In the Golgi lumen, GlcCer is converted to LacCer by the UDP-galactose:glucosylceramide(β1–4)galactosyltransferase (GalT-2) (Burger et al., 1996; Lannert et al., 1998). LacCer is then rapidly converted to GM3 by the CMP-sialic acid:LacCer(α2–3)sialyltransferase. Transfection of CHOlec8 cells with UGT increased GM3 synthesis from [14C]acetate two- to threefold, indicating that UDP-galactose import in the Golgi was restored. This was independent of the presence of GalT-1 (Figure 1, B and C). CHOlec8 cells transiently transfected with GalT-1 synthesized only minor amounts of GalCer. Strikingly, when UGT-CHOlec8 cells were transfected with GalT-1, they synthesized 35 times more GalCer and galactosyldiglycerides than CHOlec8 cells that were transiently transfected with GalT-1 alone (Figure 1, B and C). Efficient synthesis of GalCer in the lumen of the ER required the Golgi UDP-galactose transporter.
At least two mechanisms could explain these results. Either UGT could import UDP-galactose directly into the ER of GalT-1/UGT-CHOlec8 cells, or alternatively, import might occur in the Golgi and UDP-galactose could reach the ER lumen by retrograde vesicular transport. To discriminate between these possibilities, the activities of GalT-1 and GalT-2 were measured in a postnuclear supernatant, which does not support vesicular traffic (Spang and Schekman, 1998). When cellular membranes were permeabilized with saponin, the specific activities of GalT-1 and GalT-2 were comparable between cells with and without UGT, indicating that the cells expressed equal amounts of the transferases (Table 1). In intact membranes, the apparent GalT-2 activity in membranes from CHOlec8 and GalT-1/CHOlec8 cells was threefold lower than from the corresponding cells expressing UGT, indicating that UGT restored UDP-galactose import in the Golgi. Similarly, the apparent activity of GalT-1 in intact membranes from GalT-1/CHOlec8 cells was fourfold lower than from the same cells expressing UGT. These results show that expression of UGT relieved the block in UDP-galactose import in the ER vesicles without the necessity for vesicular traffic, suggesting that UGT is present in the ER membrane of these cells.
Table 1.
Lipid galactosylation in postnuclear supernatants from exogenous UDP-galactose
|
Enzyme activity (pmol/mg protein
h)a
|
||||
|---|---|---|---|---|
| Transferases | CHOlec8 | GalT-1 CHOlec8 | UGT-CHOlec8 | GalT-1 UGT-CHOlec8 |
| GalT-1 + saponin | ND | 9 | ND | 8b |
| GalT-1 | ND | 7 | ND | 28 |
| GalT-2 + saponin | 26 | 28 | 29 | 27 |
| GalT-2 | 11 | 9 | 35 | 33 |
ND, not detectable: a fluorescent signal corresponding to less than 0.5 pmol/mg protein h.
Postnuclear supernatants prepared from CHOlec8 and UGT-CHOlec8 cells transiently transfected with GalT-1 and empty vector were analyzed for GalT-1 and GalT-2 activity on exogenous C6-NBD-Cer and C6-NBD-GlcCer, respectively, in the presence of UDP-galactose and in the presence or absence of saponin. The addition of UDP-glucose to the GalT-1 assay allowed the simultaneous measurement of the activity of the UDP-glucose: ceramideglucosyltransferase. C6-NBD-GlcCer synthesis (on the cytosolic surface; Burger et al., 1996) was similar for all cell lines, 380 ± 15 pmol/mg protein h, corroborating that the same amounts of the lysates were used. Activities were determined in at least two independent experiments with similar results. A representative experiment is shown (n = 2, range is <20% of signal).
The reduction in GalT-1 activity by saponin in GalT-1 UGT-CHOlec8 cells shows that saponin partially inhibited GalT-1 activity. When corrected for this inhibition, the apparent activity of GalT-1 CHOlec8 cells was enhanced four-fold by opening the membranes by saponin.
Cellular Localization of UGT and GalT-1
UGT has been localized to the Golgi (Yoshioka et al., 1997) and GalT-1 to the ER (Sprong et al., 1998) of transfected cells. Whether UGT was present in the ER of UGT-CHOlec8 cells transfected with GalT-1 was investigated directly by subcellular fractionation and by immunofluorescence microscopy. Subcellular fractionation on sucrose gradients clearly discriminates ER proteins from Golgi proteins (Figure 2). In UGT-CHOlec8 cells, esterase and UDP-glucose:ceramide glucosyltransferase activity as markers for ER and Golgi, respectively (Perez and Hirschberg, 1985; Burger et al., 1996; Yoshioka et al., 1997; Lannert et al., 1998), showed the ER at a higher density than the bulk of the Golgi (Figure 2A). A Western blot with an anti-calreticulin antibody (ER) and GalT-2 activity (Golgi; Burger et al., 1996; Lannert et al., 1998) confirmed these locations (our unpublished data). In the cells, that were mock transfected for GalT-1, UGT clearly located to Golgi fractions (Figure 2B, Mock). In UGT-CHOlec8 cells transfected with GalT-1, GalT-1 and its activity located to ER fractions (Figure 2, B and C), which were further identified by the ER markers esterase and calreticulin (our unpublished data), whereas GalT-2 activity displayed a Golgi pattern (Figure 2C) and colocalized with the activity of the Golgi marker UDP-glucose:ceramide glucosyltransferase (our unpublished data). Remarkably, transfection with GalT-1 shifted a substantial fraction of UGT from Golgi to ER fractions (Figure 2B).
Figure 2.
Subcellular fractionation of UGT-CHOlec8 cells. (A) Postnuclear supernatants were fractionated on 0.7–1.5 M linear sucrose gradients. Fractions (bottom to top) were analyzed for enzyme activities (percentage of maximum activity) as described under MATERIALS AND METHODS. The position of the ER was determined by esterase activity (closed triangles). The position of the Golgi was determined by the activity of UDP-glucose:ceramide glucosyltransferase (open squares). The curves represent a typical experiment. Enzyme profiles were determined in at least two independent experiments with identical results. (B) The localization of UGT and GalT-1 was determined in UGT-CHOlec8 cells transiently transfected with empty vector (Mock) or GalT-1 (GalT-1) by WB with the anti-UGT and anti-GalT-1 antisera. Transfection with GalT-1 shifts part of the UGT to the heavy ER fractions. (C) The subcellular localization of GalT-1 (closed diamonds) and GalT-2 (open circles) activities was determined in UGT-CHOlec8 transiently transfected with GalT-1.
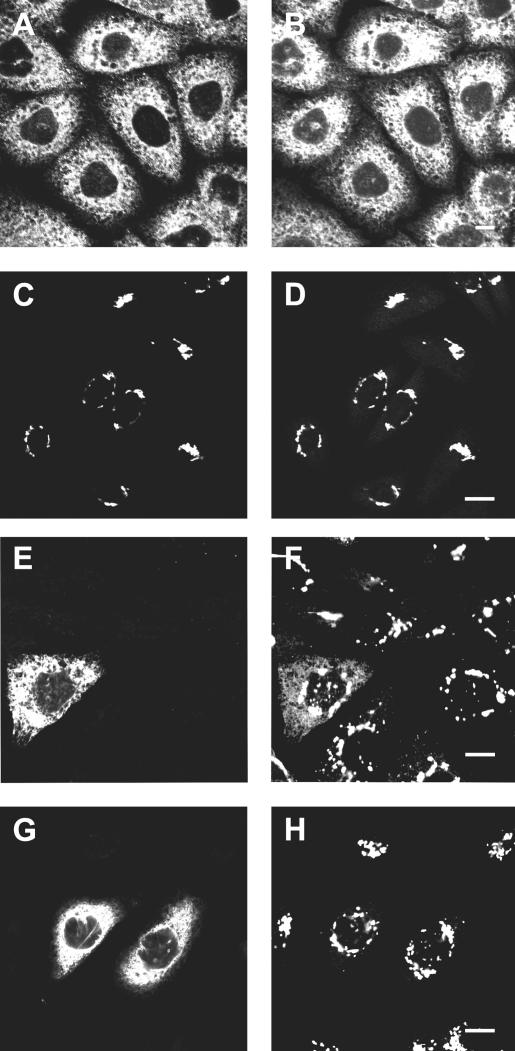
Independently, UGT was localized by immunofluorescence microscopy. In GalT-1/CHOlec8 cells, the staining pattern of GalT-1 overlapped with the diffuse reticular ER and nuclear envelope staining of PDI, a resident protein of the lumen of the ER and intermediate compartment (Edman et al., 1985) (Figure 3, A and B). The staining pattern of UGT in UGT-CHOlec8 cells overlapped with the distribution of the cis/medial-Golgi marker CTR433 (Jasmin et al., 1990), and essentially no UGT signal was found outside the Golgi area (Figure 3, C and D). In UGT-CHOlec8 cells transfected with GalT-1, the staining pattern of GalT-1 was the same as in GalT-1/CHOlec8 cells. Strikingly, only in the UGT-CHOlec8 cells that expressed GalT-1 the distribution of UGT was different: A significant portion of UGT was found outside the Golgi area and had a diffuse reticular staining pattern that overlapped with the GalT-1 signal (Figure 3, E and F). In neighboring cells that did not express GalT-1, UGT was restricted to the Golgi.
Figure 3.
Cellular localization of UGT and GalT-1. To determine the cellular localization of GalT-1, GalT-1/CHOlec8 cells were fixed, permeabilized, and labeled with anti-GalT-1 antiserum (A) (Sprong et al., 1998) and an anti-PDI monoclonal as an ER marker (B). To determine the localization of UGT, UGT-CHOlec8 cells were labeled with anti-HA antiserum to label UGT (C) and the anti-CTR433 antibody as a Golgi marker (D). To determine the effect of GalT-1 expression on the localization of UGT, UGT-CHOlec8 cells transiently transfected with GalT-1 were labeled with anti-GalT-1 antiserum (E) and the anti-HA monoclonal to label UGT (F). In the cell expressing CGalT-1 (E), a part of the UGT is present in the ER (F). As a control, UGT-CHOlec8 cells expressing an HA-tagged CMP-sialic acid transporter were transiently transfected with GalT-1. Cells labeled by the anti-GalT-1 antiserum (G) did not display any ER localization of the CMP-sialic acid transporter as labeled by the anti-HA monoclonal (H). Cells were counterstained with the appropriate FITC- (A, C, E, and G) and Texas Red-labeled (B, D, F, and H) secondary antibodies, and analyzed in a confocal laser scanning fluorescence microscope. The antibodies used to localize UGT did not cross-react with GalT-1 or with any reagent used to localize GalT-1 (our unpublished data). Bar, 10 μm.
To determine whether redistribution of UGT by GalT-1 was specific, we studied another Golgi nucleotide sugar transporter, the CMP-sialic acid transporter (Oelmann et al., 2001). In CHO cells stably expressing an HA-tagged CMP-sialic acid transporter, this transporter displayed a Golgi localization (Figure 3H). In cells that were transiently transfected with GalT-1, GalT-1 localized to the ER (Figure 3G), but no Golgi-to-ER shift of the transporter was observed (Figure 3H), showing that the shift by GalT-1 was specific for the UGT.
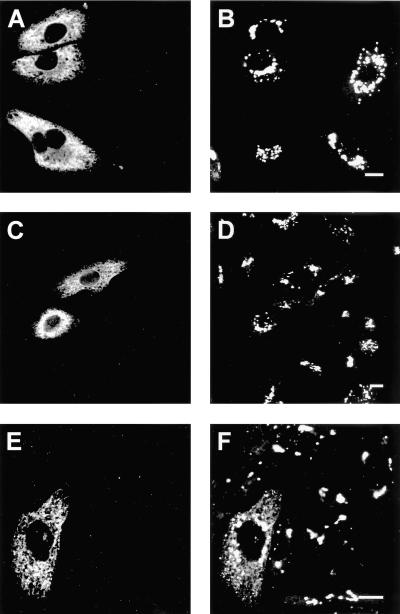
To extend these findings to cells that naturally express GalT-1, we compared the localization of UGT-1 in the human hepatocyte cell line HepG2, which does not express GalT-1 activity (Burger et al., 1996), to that in the human Caco-2 intestinal cell line, which does (van der Bijl et al., 1996a). Because antibodies to study the native UGT were not available, both cell lines were transiently transfected with the HA-tagged human UGT construct. In the human liver cells UGT selectively localized to the Golgi (Figure 4B) as marked by CTR433 (Figure 4A), in line with studies on transport activity (Perez and Hirschberg, 1985). However, in the human intestinal cells, which have endogenous GalT-1, a significant fraction of the transfected UGT displayed the typical ER labeling pattern (Figure 4D). To confirm that retention of transfected UGT in the ER was specific, we transfected Caco-2 cells with myc-tagged sialyltransferase, another Golgi membrane protein (Sprong et al., 2001). Immunofluorescence experiments documented that this protein was localized to the Golgi complex and not retained aspecifically in the ER of transfected Caco-2 cells (Figure 4F).
Figure 4.
Cellular localization of UGT in human cells expressing native GalT-1. Human liver-derived HepG2 cells, which lack GalT-1, were transfected with the UGT construct. UGT-1 labeled by the anti-HA antiserum (B) colocalized with the anti-CTR433 antibody used as a Golgi marker (A, C, and E). In contrast, when UGT was transfected into Caco-2 cells, which express GalT-1, part of the UGT labeled by the anti-HA antiserum displayed a typical ER pattern (D). No labeling of the ER was observed when Caco-2 cells were transfected with the Golgi marker myc-tagged sialyltransferase, which was labeled by an anti-myc antiserum (F). Cells were counterstained with the appropriate FITC- (A, C, and E) and Texas Red-labeled (B, D, and F) secondary antibodies. Bar, 10 μm.
Interaction between UGT and GalT-1
To test whether UGT is retained in the ER of GalT-1–expressing cells by a specific interaction with GalT-1, we assayed oligomerization of the two proteins. For this, we assessed whether UGT was coimmunoprecipitated with GalT-1 from TX-100 lysates. After precipitation of GalT-1 from GalT-1–transfected UGT-CHOlec8 cells, the immunoprecipitates were separated by SDS-PAGE and blotted with anti-HA monoclonal to detect UGT. As shown in Figure 5A, UGT was indeed coimmunoprecipitated by anti-GalT-1 antibodies from UGT-CHOlec8 cells that were transfected with GalT-1, but not from mock-transfected cells. This shows that UGT is associated with GalT-1 in the TX-100 lysate.
Figure 5.
Coimmunoprecipitation of UGT and galactosyltransferases. Two days after transfection with GalT-1 (A–C) or p33-PGalT (B), UGT-CHOlec8 cells were lysed in lysis buffer containing different detergents [1% (wt/vol)]. A fraction of each detergent lysate was used to determine the relative amounts of UGT by Western blotting with the anti-HA polyclonal antiserum (WB, αUGTp). From the remainder, GalT-1 and p33-PGalT were adsorbed to protein A-Sepharose with antisera against GalT-1 (IP, αGalT-1) and invariant chain (IP, αp33-PGalT), respectively. Immunoprecipitates were subjected to SDS-PAGE and Western blotting. Coimmunoprecipitated UGT was detected using the anti-HA mAb (WB, αUGTm). Molecular weight standards (in kilodaltons) are indicated on the right. In C, the detergent lysates were adjusted to 40% Optiprep, overlaid with 30 and 5% Optiprep, and centrifuged. The distribution of p62(yes) (Yes), calnexin, UGT (UGT), and GalT-1 over the seven fractions was determined by Western blotting as described under MATERIALS AND METHODS.
To rule out that the coprecipitation of UGT and GalT-1 was caused by their presence in ER membrane domains that were insoluble in TX-100 in the cold (Bagnat et al., 2000), we first analyzed fractionation of both proteins with detergent-resistant membranes (1% TX-100) by a standard flotation protocol (Bagnat et al., 2000), under the conditions where UGT was coimmunoprecipitated by anti-GalT-1 antibodies (Figure 5A). p62(yes) floated in the gradient and was used as a positive control (McCabe and Berthiaume, 2001), whereas the ER membrane protein calnexin served as a negative control. We found no evidence for cofractionation of UGT with p62(yes) on the gradient in the absence nor in the presence of GalT-1 (Figure 5C). We also performed the co-immunoprecipitations on cell lysates prepared with octylglucoside, a detergent that dissolves TX-100–insoluble domains (Brown and Rose, 1992). Because UGT was still immunoprecipitated with GalT-1 from octylglucoside detergent lysates (Figure 5A), we conclude that GalT-1 and UGT associated via protein–protein interactions. The association between UGT and GalT-1 was probably not due to intermolecular disulfide bridges, because we did not detect high molecular weight complexes on nonreducing SDS-PAA gels (our unpublished data).
Interaction between UGT and UDP-galactose:protein(β1–4)galactosyltransferase
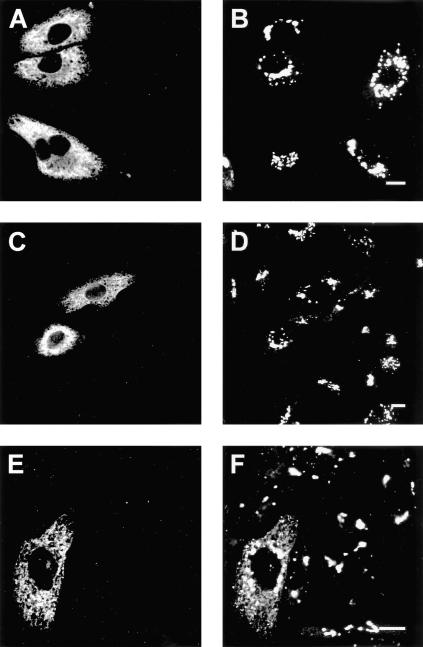
The finding that UGT is retained in the ER by a protein–protein interaction with GalT-1 suggested that its normal localization in the Golgi may be caused by interactions of UGT with Golgi galactosyltransferases. We therefore studied whether UGT interacts with the ubiquitous UDP-galactose:protein(β1–4)galactosyltransferase (PGalT) by testing whether artificial retention of this Golgi transferase in the ER would induce ER retention of UGT. The human PGalT was retained in the ER by exchanging its cytoplasmic tail for the cytoplasmic tail of the human invariant chain p33 (p33-PGalT; Nilsson et al., 1994). Western blot analysis showed that p33-PGalT was expressed as a 50-kDa protein and that it did not affect the expression level of UGT in UGT-CHOlec8 cells (our unpublished data). In UGT-CHOlec8 cells transiently transfected with the invariant chain p33 or with p33-PGalT, p33 and p33-PGalT displayed an ER staining pattern (Figure 6, A and C), which overlapped with the ER marker PDI (our unpublished data), confirming previous findings (Nilsson et al., 1994). In these cells, UGT remained fully localized to the Golgi (Figure 6, B and D), whereas in a parallel experiment transfection of cells with GalT-1 relocalized part of the UGT to the ER (Figure 6, E and F). In addition, UGT could not be coimmunoprecipitated with p33-PGalT under conditions where coimmunoprecipitation with GalT-1 did occur (Figure 5B). Thus, the interaction of UGT with the ER enzyme GalT-1 is specific. It explains the retention of UGT in the ER of GalT-1–expressing cells and provides the cell with a unique mechanism to supply GalT-1 with its UDP-galactose substrate.
Figure 6.
Cellular localization of UGT and an ER construct of the PGalT. UGT-CHOlec8 cells were transiently transfected with invariant chain p33 (A and B), p33-PGalT (C and D), and GalT-1 (E and F); fixed; and labeled with anti-p33 antiserum (A and C), anti-GalT-1 antiserum (E), and with the anti-HA mAb to detect UGT (B, D, and F). Cells were counterstained with FITC-labeled goat anti-rabbit (A, C, and E) or Texas Red-labeled goat anti-rat (B, D, and F). UGT is retained in the ER in cells expressing GalT-1 (F), but not in cells expressing p33 (B) or p33-PGalT (D). Bar, 10 μm.
DISCUSSION
Expression and Cellular Localization of Galactosyltransferases
Galactosylation of newly synthesized proteins occurs exclusively in the lumen of the mammalian Golgi by PGalT (Strous, 1986). Likewise, the simple glycosphingolipid GlcCer is converted to LacCer in the Golgi lumen by GalT-2 (Lannert et al., 1994, 1998; Burger et al., 1996). Both transferases are type II membrane proteins. Further modification to complex glycosphingolipids involves other lipid galactosyltransferases that are cell type specific but always restricted to the Golgi lumen. Glycosylation of lipids and proteins occurs in a highly regulated manner (Varki, 1998). Most enzymes involved in the synthesis of complex N-glycans have been precisely localized and show distinct, but overlapping, distributions in the Golgi that are consistent with their order in the glycoprotein biosynthetic pathway (Rabouille et al., 1995). The galactosyltransferases PGalT, GalT-2, GalT-3, and GalT-4 have been located to late Golgi by immunofluorescence (PGalT; Roth and Berger, 1982) and cell fractionation (Holmes, 1989; Trinchera et al., 1990; Lannert et al., 1998; Giraudo et al., 2001). GalT-1 is the only enzyme known to use UDP-galactose in the ER lumen. It produces GalCer from ceramide on the lumenal aspect of the ER (Sprong et al., 1998). GalT-1 is only expressed in specialized cells such as myelinating cells, spermatogonia, and, depending on the mammalian species, in various epithelial cell types (Schulte and Stoffel, 1993; Stahl et al., 1994; Schaeren-Wiemers et al., 1995; Burger et al., 1996; Fujimoto et al., 2000). Only cells expressing GalT-1 require UDP-galactose import in the ER.
Expression and Cellular Localization of UGTs
Consistent with a housekeeping role for UGT in glycoconjugate biosynthesis, its mRNA is ubiquitously expressed. Two isoforms of the human UGT, UGT1 and UGT2, derived from the same gene by alternative splicing, have been identified (Ishida et al., 1996). Whether they differ in function or tissue expression and whether other mammals also have more than one isoform, remains to be determined. Studies on UDP-galactose import have generally used cells that did not express GalT-1 and found UGT and its activity restricted to the Golgi (Perez and Hirschberg, 1985; Ishida et al., 1996, 1999; Miura et al., 1996; Tabuchi et al., 1997; Yoshioka et al., 1997; Segawa et al., 1999). UGT2 has a putative ER retrieval sequence identical to that of hamster UGT. However, hamster UGT located to the Golgi of CHOlec8 cells (Oelmann et al., 2001). The precise localization of UGT in the Golgi has not been resolved yet. The mechanisms that determine the steady-state distribution of Golgi transferases and nucleotide-sugar transporters are not fully understood (Colley, 1997; Fullekrug and Nilsson, 1998; Munro, 1998), but the physical association between transferases in the Golgi apparatus can play a role (Giraudo et al., 2001). The fact that many cells do not express GalT-1 allowed us to study the role of GalT-1 in the intracellular localization of UGT.
UDP-Galactose Import in ER and Golgi
To distinguish between UDP-galactose import in the ER and Golgi, we measured the synthesis of GalCer and that of GM3 as a measure of LacCer synthesis. Although the GalT-1 yielding GalCer exclusively acts in the ER lumen (Sprong et al., 1998), GalT-2 and CMP-sialic acid:LacCer(α2–3)sialyltransferase are oriented toward the lumen of the Golgi (Lannert et al., 1994, 1998; Burger et al., 1996). UDP-galactose import was measured indirectly, because the galactose incorporation into lipids depended on the availability of the precursor lipids, ceramide for GalCer and GlcCer for LacCer and GM3. Cells synthesizing high levels of GalCer synthesized less GlcCer, because both use ceramide as a substrate, which led to an underestimate of the subsequent synthesis of LacCer and GM3 (Figure 1C). However, the effect of the presence of UGT on galactose incorporation was sufficiently large to allow the unequivocal conclusion that the presence of UGT was required for the synthesis of GalCer (Figure 1C). In vitro experiments on cells transfected with GalT-1 had already shown that CHOlec8 cells, in addition to a defect in Golgi UDP-galactose import, have impaired UDP-galactose import in the ER compared with CHO cells (Sprong et al., 1998). Herein, we show that transfection with UGT restored the UDP-galactose import in the ER (Table 1).
Interaction between UGT and Galactosyltransferases
In the present study, we have discovered that UGT oligomerizes with GalT-1 (Figure 5) and that this results in the presence of a fraction of the UGT in the ER (Figures 3F, 4D, and 6F). Whether this is due to the retention of the UGT by an ER-resident GalT-1 or to recycling through the cis-Golgi and continuous retrieval is unclear at present. The nature and stoichiometry of their interaction are unclear. No disulfide bridges between the two proteins were found, and although both proteins have a leucine zipper, current data indicate that the leucine zipper of UGT is oriented to the cytosol, whereas the leucine zipper of GalT-1 is in the ER lumen. One striking difference between GalT-1 and all other galactosyltransferases is that GalT-1 is a type I membrane protein. The proteins may interact via their transmembrane domains, or indirectly, via a third protein. GalT-1 is a member of the large family of the glucuronosyltransferases (Schulte and Stoffel, 1993; Stahl et al., 1994). A UDP-glucuronic acid transporter has been identified and located to the ER (Muraoka et al., 2001). It has no recognizable ER retrieval sequence. In line with our present findings, it may require binding to an ER transferase for retention.
In cells expressing GalT-1, the shift of UGT to the ER was partial, indicating that not all UGT associated with GalT-1. It will be interesting to see whether conditions can be found where all of the UGT is retained in the ER, and, if so, how this affects protein galactosylation. Now, what would be the mechanism that normally drives UGT to the Golgi? UGT may contain an intrinsic Golgi retention signal or it may interact with Golgi proteins. We detected no interaction between UGT and PGalT tagged with p33, but we cannot exclude oligomerization of UGT with endogenous PGalT in the Golgi. Kin recognition was originally proposed as a basis for the localization of the N-acetylglucosaminyltransferase-1 and mannosidase II to the medial-Golgi (Nilsson et al., 1994), and complex formation between different membrane proteins may be one general principle of cis- and medial-Golgi localization (Jungmann and Munro, 1998). In contrast, the late Golgi glycosyltransferases PGalT and α1,2-fucosyltransferase were found to occur as monomers and dimers, respectively (Opat et al., 2000). Finally, a physical association has been established between the Golgi glycolipid N-acetylgalactosaminyltransferase and the next galactosyltransferase in the pathway (Giraudo et al., 2001), whereas the N-acetylgalactosaminyltransferase can also be in a complex with the sialyltransferase II and its substrate GM3 (Bieberich et al., 2002), and it has been proposed that these associations improve the efficiency of synthesis by a channeling of substrates.
To our knowledge, we describe for the first time an association between a nucleotide-sugar transporter and one of its transferases. Future work will be required to fully explore the molecular details of this novel interaction. It will be interesting to see what determines the Golgi localization of UGT, and whether UGTs interact with PGalT or one of the other galactosyltransferases in the Golgi complex.
Acknowledgments
We are grateful to Hans Bakker, Michel Bornens, Ineke Braakman, Stephen Fuller, Rita Gerardy-Schahn, Monique Kleijmeer, Sean Munro, and Hiroaki Segawa for sharing reagents and to Gerrit Jan Romeyn for performing the esterase activity assay. We are grateful to Rita Gerardy-Schahn for suggesting the CMP-sialic acid transporter experiment. This work was supported by grants from the Netherlands Foundations for Chemical Research (NWO-CW), Life Sciences (NWO-ALW), and Medical Sciences (ZonMW), with financial aid from the Netherlands Organization for Scientific Research to P.v.d.S. and G.v.M., and from the Mizutani Foundation for Glycoscience (to G.v.M.), and Institut National de la Santé et de la Recherche Médicale to S.D.
Abbreviations used: C6-NBD-, N-6(7-nitro-2,1,3-benzoxadiazol-4-yl)-aminohexanoyl-; CHO, Chinese hamster ovary; GalCer, galactosylceramide; GalT-1, UDP-galactose:ceramide galactosyltransferase; GalT-2, UDP-galactose:glucosylceramide(β1–4)galactosyltransferase; GlcCer, glucosylceramide; GM3, sialyllactosylceramide; HA, hemagglutinin epitope; LacCer, lactosylceramide; PDI, protein-disulfide isomerase; p33-PGalT, UDP-galactose:protein(β1–4)galactosyltransferase with the cytoplasmic domain of invariant chain p33; PNS, postnuclear supernatant; UGT, UDP-galactose transporter.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-03-0130. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-03-0130.
References
- Aoki, K., Sun-Wada, G.H., Segawa, H., Yoshioka, S., Ishida, N., and Kawakita, M. (1999). Expression and activity of chimeric molecules between human UDP-galactose transporter and CMP-sialic acid transporter. J. Biochem. 126, 940–950. [DOI] [PubMed] [Google Scholar]
- Bagnat, M., Keranen, S., Shevchenko, A., and Simons, K. (2000). Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97, 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, M., De, T., Das, K.K., Kyle, J.W., Chon, H.-C., Schaeper, R.J., and Basu, S. (1987). Glycolipids. Methods Enzymol. 138, 575–607. [DOI] [PubMed] [Google Scholar]
- Beaufay, H., Amar-Costesec, A., Feytmans, E., Thines-Sempoux, D., Wibo, M., Robbi, M., and Berthet, J. (1974). Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J. Cell Biol. 61, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich, E., MacKinnon, S., Silva, J., Li, D.D., Tencomnao, T., Irwin, L., Kapitonov, D., and Yu, R.K. (2002). Regulation of ganglioside biosynthesis by enzyme complex formation of glycosyltransferases. Biochemistry 41, 11479–11487. [DOI] [PubMed] [Google Scholar]
- Bosio, A., Binczek, E., and Stoffel, W. (1996). Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA 93, 13280–13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändli, A.W., Hansson, G.C., Rodriguez-Boulan, E., and Simons, K. (1988). A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J. Biol. Chem. 263, 16283–16290. [PubMed] [Google Scholar]
- Brown, D.A., and Rose, J.K. (1992). Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Burger, K.N.J., van der Bijl, P., and van Meer, G. (1996). Topology of sphingolipid galactosyltransferases in ER and Golgi: transbilayer movement of monohexosyl sphingolipids is required for higher glycosphingolipid biosynthesis. J. Cell Biol. 133, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee, T., Fujita, N., Dupree, J., Shi, R., Blight, A., Suzuki, K., Suzuki, K., and Popko, B. (1996). Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86, 209–219. [DOI] [PubMed] [Google Scholar]
- Colley, K.J. (1997). Golgi localization of glycosyltransferases: more questions than answers. Glycobiology 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher, S.L., and Hirschberg, C.B. (1986). Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J. Biol. Chem. 261, 96–100. [PubMed] [Google Scholar]
- Edman, J.C., Ellis, L., Blacher, R.W., Roth, R.A., and Rutter, W.J. (1985). Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature 317, 267–270. [DOI] [PubMed] [Google Scholar]
- Fujimoto, H., Tadano-Aritomi, K., Tokumasu, A., Ito, K., Hikita, T., Suzuki, K., and Ishizuka, I. (2000). Requirement of seminolipid in spermatogenesis revealed by UDP-galactose:ceramide galactosyltransferase-deficient mice. J. Biol. Chem. 275, 22623–22626. [DOI] [PubMed] [Google Scholar]
- Fullekrug, J., and Nilsson, T. (1998). Protein sorting in the Golgi complex. Biochim. Biophys. Acta 1404, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo, C.G., Daniotti, J.L., and Maccioni, H.J. (2001). Physical and functional association of glycolipid N-acetyl-galactosaminyl and galactosyl transferases in the Golgi apparatus. Proc. Natl. Acad. Sci. USA 98, 1625–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, F.L., and van der Eb, A.J. (1973). A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52, 456–467. [DOI] [PubMed] [Google Scholar]
- Hansen, S.H., and Casanova, J.E. (1994). GSα stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J. Cell Biol. 126, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, C.B., Robbins, P.W., and Abeijon, C. (1998). Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 67, 49–69. [DOI] [PubMed] [Google Scholar]
- Holmes, E.H. (1989). Characterization and membrane organization of β1℘3- and β1℘4-galactosyltransferases from human colonic adenocarcinoma cell lines Colo 205 and SW403: basis for preferential synthesis of type 1 chain lacto-series carbohydrate structures. Arch. Biochem. Biophys. 270, 630–646. [DOI] [PubMed] [Google Scholar]
- Ishida, N., Miura, N., Yoshioka, S., and Kawakita, M. (1996). Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter, and of related complementary DNAs belonging to the nucleotide-sugar transporter gene family. J. Biochem. 120, 1074–1078. [DOI] [PubMed] [Google Scholar]
- Ishida, N., Yoshioka, S., Iida, M., Sudo, K., Miura, N., Aoki, K., and Kawakita, M. (1999). Indispensability of transmembrane domains of Golgi UDP-galactose transporter as revealed by analysis of genetic defects in UDP-galactose transporter-deficient murine had-1 mutant cell lines and construction of deletion mutants. J. Biochem. 126, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Jasmin, B.J., Cartaud, A., Ludosky, M.A., Changeux, J.P., and Cartaud, J. (1990). Asymmetric distribution of dystrophin in developing and adult Torpedo marmorata electrocyte: evidence for its association with the acetylcholine receptor-rich membrane. Proc. Natl. Acad. Sci. USA 87, 3938–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann, J., and Munro, S. (1998). Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with alpha-1,6-mannosyltransferase activity. EMBO J. 17, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita, M., Ishida, N., Miura, N., Sun-Wada, G.H., and Yoshioka, S. (1998). Nucleotide sugar transporters: elucidation of their molecular identity and its implication for future studies. J. Biochem. 123, 777–785. [DOI] [PubMed] [Google Scholar]
- Kishimoto, Y. (1975). A facile synthesis of ceramides. Chem. Phys. Lipids 15,: 33–36. [DOI] [PubMed] [Google Scholar]
- Kuhn, N.J., and White, A. (1976). Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem. J. 154, 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannert, H., Bünning, C., Jeckel, D., and Wieland, F.T. (1994). Lactosylceramide is synthesized in the lumen of the Golgi apparatus. FEBS Lett. 342, 91–96. [DOI] [PubMed] [Google Scholar]
- Lannert, H., Gorgas, K., Meissner, I., Wieland, F.T., and Jeckel, D. (1998). Functional organization of the Golgi apparatus in glycosphingolipid biosynthesis. Lactosylceramide and subsequent glycosphingolipids are formed in the lumen of the late Golgi. J. Biol. Chem. 273, 2939–2946. [DOI] [PubMed] [Google Scholar]
- McCabe, J.B., and Berthiaume, L.G. (2001). N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol. Biol. Cell 12, 3601–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, N., Ishida, N., Hoshino, M., Yamauchi, M., Hara, T., Ayusawa, D., and Kawakita, M. (1996). Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. 120, 236–241. [DOI] [PubMed] [Google Scholar]
- Morell, P., and Radin, N.S. (1969). Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry 8, 506–512. [DOI] [PubMed] [Google Scholar]
- Morton, P.A., Zacheis, M.L., Giacoletto, K.S., Manning, J.A., and Schwartz, B.D. (1995). Delivery of nascent MHC class II-invariant chain complexes to lysosomal compartments and proteolysis of invariant chain by cysteine proteases precedes peptide binding in B-lymphoblastoid cells. J. Immunol. 154, 137–150. [PubMed] [Google Scholar]
- Munro, S. (1998). Localization of proteins to the Golgi apparatus. Trends Cell Biol. 8, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka, M., Kawakita, M., and Ishida, N. (2001). Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 495, 87–93. [DOI] [PubMed] [Google Scholar]
- Nilsson, T., Hoe, M.H., Slusarewicz, P., Rabouille, C., Watson, R., Hunte, F., Watzele, G., Berger, E.G., and Warren, G. (1994). Kin recognition between medial Golgi enzymes in HeLa cells. EMBO J. 13, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmann, S., Stanley, P., and Gerardy-Schahn, R. (2001). Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 276, 26291–26300. [DOI] [PubMed] [Google Scholar]
- Opat, A.S., Houghton, F., and Gleeson, P.A. (2000). Medial Golgi but not late Golgi glycosyltransferases exist as high molecular weight complexes. Role of luminal domain in complex formation and localization. J. Biol. Chem. 275, 11836–11845. [DOI] [PubMed] [Google Scholar]
- Perez, M., and Hirschberg, C.B. (1985). Translocation of UDP-N-acetylglucosamine into vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 260, 4671–4678. [PubMed] [Google Scholar]
- Rabouille, C., Hui, N., Hunte, F., Kieckbusch, R., Berger, E.G., Warren, G., and Nilsson, T. (1995). Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 108, 1617–1627. [DOI] [PubMed] [Google Scholar]
- Roth, J., and Berger, E.G. (1982). Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J. Cell Biol. 93, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers, N., van der Bijl, P., and Schwab, M.E. (1995). The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. J. Neurochem. 65, 2267–2278. [DOI] [PubMed] [Google Scholar]
- Schulte, S., and Stoffel, W. (1993). Ceramide UDPgalactosyltransferase from myelinating rat brain: purification, cloning, and expression. Proc. Natl. Acad. Sci. USA 90, 10265–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa, H., Ishida, N., Takegawa, K., and Kawakita, M. (1999). Schizosaccharomyces pombe UDP-galactose transporter: identification of its functional form through cDNA cloning and expression in mammalian cells. FEBS Lett. 451, 295–298. [DOI] [PubMed] [Google Scholar]
- Spang, A., and Schekman, R. (1998). Reconstitution of retrograde transport from the Golgi to the ER in vitro. J. Cell Biol. 143, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong, H., Degroote, S., Claessens, T., van Drunen, J., Oorschot, V., Westerink, B.H., Hirabayashi, Y., Klumperman, J., van der Sluijs, P., and van Meer, G. (2001). Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J. Cell Biol. 155, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong, H., Kruithof, B., Leijendekker, R., Slot, J.W., van Meer, G., and van der Sluijs, P. (1998). UDP-galactose:ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J. Biol. Chem. 273, 25880–25888. [DOI] [PubMed] [Google Scholar]
- Sprong, H., van Meer, G., and van der Sluijs, P. (2000). Analysis of galactolipids and UDP-galactose:ceramide galactosyltransferase. Methods Enzymol. 311, 59–73. [DOI] [PubMed] [Google Scholar]
- Stahl, N., Jurevics, H., Morell, P., Suzuki, K., and Popko, B. (1994). Isolation, characterization, and expression of cDNA clones that encode rat UDP-galactose:ceramide galactosyltransferase. J. Neurosci. Res. 38, 234–242. [DOI] [PubMed] [Google Scholar]
- Strous, G.J. (1986). Golgi and secreted galactosyltransferase. CRC Crit. Rev. Biochem. 21, 119–151. [DOI] [PubMed] [Google Scholar]
- Tabuchi, M., Tanaka, N., Iwahara, S., and Takegawa, K. (1997). The Schizosaccharomyces pombe gms1+ gene encodes an UDP-galactose transporter homologue required for protein galactosylation. Biochem. Biophys. Res. Commun. 232, 121–125. [DOI] [PubMed] [Google Scholar]
- Taki, T., Ogura, K., Rokukawa, C., Hara, T., Kawakita, M., Endo, T., Kobata, A., and Handa, S. (1991). Had-1, a uridine 5′-diphosphogalactose transport-defective mutant of mouse mammary tumor cell FM3A: composition of glycolipids, cell growth inhibition by lactosylceramide, and loss of tumorigenicity. Cancer Res. 51, 1701–1707. [PubMed] [Google Scholar]
- Trinchera, M., Pirovano, B., and Ghidoni, R. (1990). Sub-Golgi distribution in rat liver of CMP-NeuAc GM3- and CMP-NeuAc: GT1bα2℘8 sialyltransferases and comparison with the distribution of the other glycosyltransferase activities involved in ganglioside biosynthesis. J. Biol. Chem. 265, 18242–18247. [PubMed] [Google Scholar]
- van der Bijl, P., Lopes-Cardozo, M., and van Meer, G. (1996a). Sorting of newly synthesized galactosphingolipids to the two surface domains of epithelial cells. J. Cell Biol. 132, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bijl, P., Strous, G.J., Lopes-Cardozo, M., Thomas-Oates, J., and van Meer, G. (1996b). Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase. Biochem. J. 317, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki, A. (1998). Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol. 8, 34–40. [DOI] [PubMed] [Google Scholar]
- Yoshioka, S., Sun-Wada, G.H., Ishida, N., and Kawakita, M. (1997). Expression of the human UDP-galactose transporter in the Golgi membranes of murine Had-1 cells that lack the endogenous transporter. J. Biochem. 122, 691–695. [DOI] [PubMed] [Google Scholar]
- Zhang, J.X., Braakman, I., Matlack, K.E., and Helenius, A. (1997). Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol. Biol. Cell 8, 1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]