Abstract
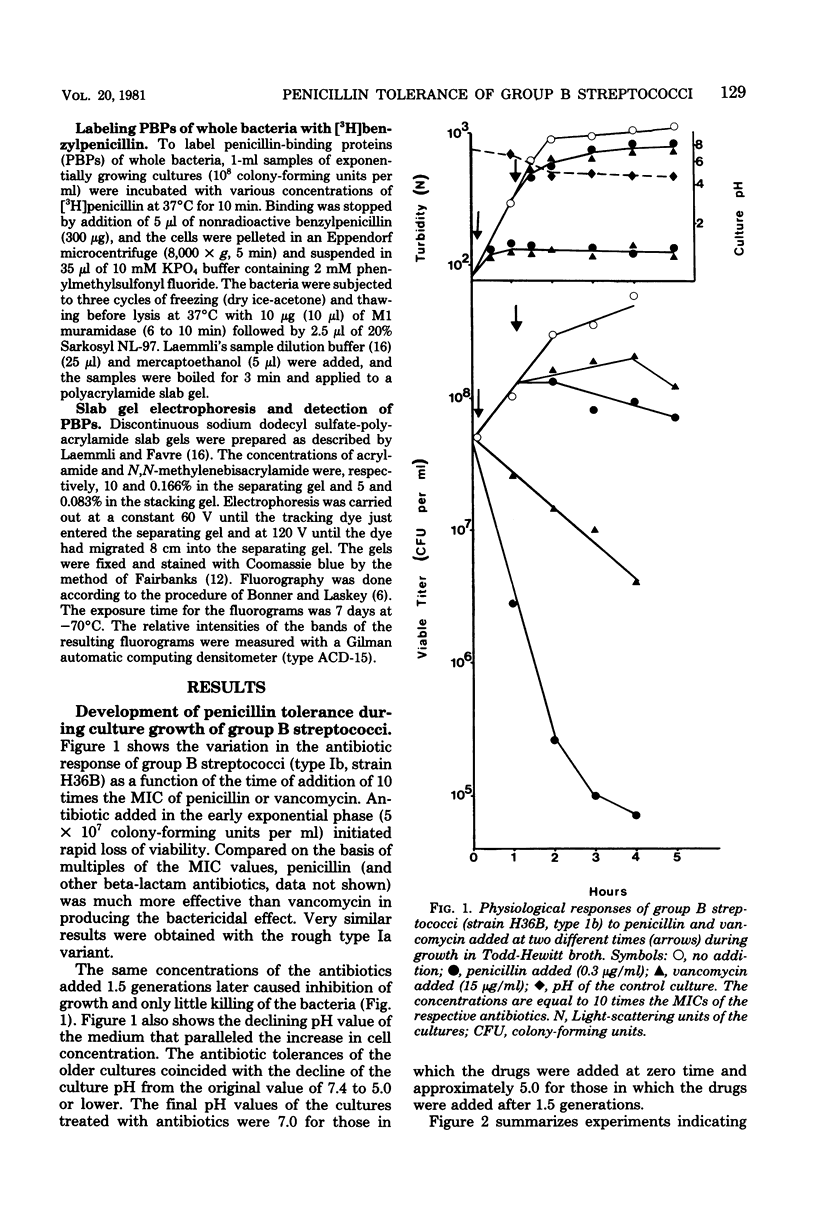
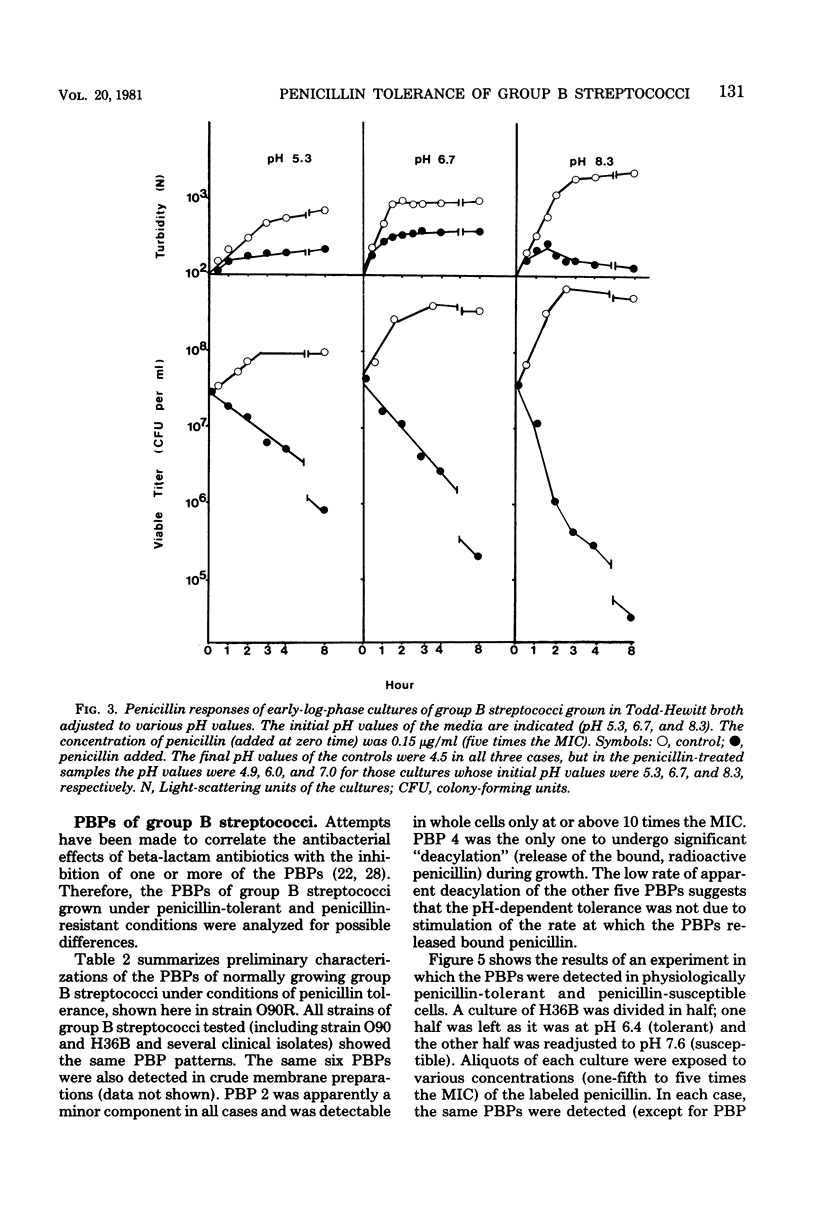
Group B streptococci lose viability without apparent lysis during treatment with beta-lactam antibiotics and vancomycin. Rapid loss of viability was observed in early-exponential-phase cultures. Cultures in the mid-exponential growth phase exhibited various degrees of resistance to the bactericidal effect of the antibiotics, whereas their susceptibilities to the growth-inhibitory effect remained unchanged. This growth-phase-dependent tolerance was caused by the gradual increase in acidity of the cultures as the cell concentration increased. Retitration of the pH to neutrality made the formerly tolerant bacteria again fully susceptible to the killing effect of penicillin. Conversely, lowering the pH value of the medium resulted in antibiotic tolerance throughout culture growth. The penicillin-binding proteins of whole bacteria and their labeling pattern were found to be independent of culture pH. It is suggested that the mechanism of Ph-dependent tolerance is indirect and may be mediated by an autolysin. The tolerance of group B streptococci for penicillin could be clinically relevant in view of the relatively low pH values known to prevail in the natural host environments colonized by these bacteria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. L., Sprunt K. Discrepancy between minimum inhibitory and minimum bactericidal concentrations of penicillin for group A and group B beta-hemolytic streptococci. J Pediatr. 1978 Jul;93(1):69–71. doi: 10.1016/s0022-3476(78)80603-9. [DOI] [PubMed] [Google Scholar]
- Anthony B. F., Concepcion N. F. Group B streptococcus in a general hospital. J Infect Dis. 1975 Nov;132(5):561–567. doi: 10.1093/infdis/132.5.561. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F., Gordon R. C., Yow M. D. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973 Apr;82(4):724–729. doi: 10.1016/s0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Microcapsule of type III strains of group B Streptococcus: production and morphology. Infect Immun. 1976 Jan;13(1):189–194. doi: 10.1128/iai.13.1.189-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Barrett F. F. Antimicrobial susceptibility of group B streptococci isolated from a variety of clinical sources. Antimicrob Agents Chemother. 1976 Jul;10(1):128–131. doi: 10.1128/aac.10.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Broughton D. D., Mitchell W. G., Grossman M., Hadley W. K., Cohen M. S. Recurrence of group B streptococcal infection. J Pediatr. 1976 Aug;89(2):182–185. doi: 10.1016/s0022-3476(76)80441-6. [DOI] [PubMed] [Google Scholar]
- Dorand R. D., Adams G. Relapse during penicillin treatment of group B streptococcal meningitis. J Pediatr. 1976 Aug;89(2):188–190. doi: 10.1016/s0022-3476(76)80444-1. [DOI] [PubMed] [Google Scholar]
- Durfee M. A., Forsyth P. S., Hale J. A., Holmes K. K. Ineffectiveness of erythromycin for treatment of Haemophilus vaginalis-associated vaginitis: possible relationship to acidity of vaginal secretions. Antimicrob Agents Chemother. 1979 Nov;16(5):635–637. doi: 10.1128/aac.16.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franciosi R. A., Knostman J. D., Zimmerman R. A. Group B streptococcal neonatal and infant infections. J Pediatr. 1973 Apr;82(4):707–718. doi: 10.1016/s0022-3476(73)80604-3. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Lethal effect of a heterologous murein hydrolase on penicillin-treated Streptococcus sanguis. Antimicrob Agents Chemother. 1980 Feb;17(2):235–246. doi: 10.1128/aac.17.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory J. H., Au-Yeung Y. B., Sugg V. M., Chiu T. T., Garrison R. D. Recurrent group B streptococcal infection in an infant: ventriculitis complicating type Ib meningitis. J Pediatr. 1978 Feb;92(2):231–233. doi: 10.1016/s0022-3476(78)80011-0. [DOI] [PubMed] [Google Scholar]
- Paredes A., Wong P., Yow M. D. Failure of penicillin to eradicate the carrier state of group B Streptococcus in infants. J Pediatr. 1976 Aug;89(2):191–193. doi: 10.1016/s0022-3476(76)80445-3. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf V., Deveikis A., Riff L., Serota A. Antibiotic-killing kinetics of group B streptococci. J Pediatr. 1976 Aug;89(2):194–198. doi: 10.1016/s0022-3476(76)80446-5. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Aber R. C., Warford L. R., Murphy K. E., Feeley J. C., Hayes P. S., Wilkinson H. W., Facklam R. R. Possible nosocomial transmission of group B streptococci in a newborn nursery. J Pediatr. 1975 Nov;87(5):784–787. doi: 10.1016/s0022-3476(75)80311-8. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Truog W. E., Davis R. F., Ray C. G. Recurrence of group B streptococcal infection. J Pediatr. 1976 Aug;89(2):185–186. doi: 10.1016/s0022-3476(76)80442-8. [DOI] [PubMed] [Google Scholar]
- Walker S. H., Santos A. Q., Quintero B. A. Recurrence of group B III streptococcal meningitis. J Pediatr. 1976 Aug;89(2):187–188. doi: 10.1016/s0022-3476(76)80443-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W. Analysis of group B streptococcal types associated with disease in human infants and adults. J Clin Microbiol. 1978 Feb;7(2):176–179. doi: 10.1128/jcm.7.2.176-179.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]




