Abstract
In nonneuronal cells, several plasma membrane proteins such as exofacial enzymes, receptors, and ion channels recycle between their intracellular compartment(s) and the cell surface via an endosomal pathway. In neurons, however, this pathway has not been extensively characterized. In particular, it remains unclear whether or not it is related to the recycling of small synaptic vesicles, the major pathway of membrane traffic in nerve terminals. To approach this problem, we purified and studied a vesicular fraction from rat brain synaptosomes. Two distinct populations of vesicles with different buoyant densities and sedimentation coefficients were detected in this fraction by sucrose gradient centrifugation and Western blot analysis of the individual proteins. Both populations contain proteins that are markers of synaptic vesicles, namely, SV2, synaptotagmin, synaptophysin, secretory carrier membrane proteins (SCAMPs), synaptobrevin, and rab3a. A striking difference between the two populations is the presence of arginine aminopeptidase activity (a previously suggested marker for the regulated endosomal recycling pathway) exclusively in the lighter less-dense vesicles. The same two vesicular populations were also detected in the preparation of clathrin-coated vesicles isolated from whole rat brain or purified synaptosomes after removal of their clathrin coats by incubation at pH 8.5. We conclude, therefore, that both types of vesicles recycle in synaptosomes via a clathrin-mediated pathway. These data present experimental evidence for biochemical heterogeneity of synaptic-like vesicles in rat brain.
Membrane traffic in nerve terminals and, in particular, the release of neurotransmitters via regulated exocytosis, is currently the subject of intensive research. Chemical neurotransmitters such as acetylcholine, glutamate, glycine, γ-aminobutyric acid, and biogenic amines are released from specialized secretory organelles, synaptic vesicles, that are continuously regenerated in nerve terminals by exo–endocytic recycling (1–6). Synaptic vesicle exo- and endocytosis has emerged as a model system for studying the protein–protein interactions (7, 8) that are involved in regulated (9–13) and constitutive (14–16) membrane trafficking.
In addition to the highly specialized and regulated recycling of synaptic vesicles, neurons, like any other cell type, have a pathway of delivery of newly synthesized plasma membrane proteins to their functioning sites and a mechanism for maintaining them at the cell surface. Such mechanisms must be especially effective in neurons in comparison to nonneuronal cells, because the integrity of the plasma membrane is maintained in spite of the immense “mixing” effect caused by the repeated cycles of synaptic vesicle exo- and endocytosis.
In nonneuronal cells that lack this highly specialized pathway of synaptic vesicle recycling, the homeostasis of the plasma membrane composition is maintained by constitutive or regulated endosomal recycling. Although it was proposed that nerve terminals may possess “housekeeping endosomes” that are different from “specialized endosomes” producing “classical” small synaptic vesicles (17, 18), these structures have not been purified and characterized. Moreover, transport vesicles that may originate from “housekeeping endosomes” have never been identified in synaptosomes, and it remains unclear to what extent they are different from small synaptic vesicles.
An additional pathway has been found in several cell types that is different from both the neurotransmitter release and constitutive “housekeeping” pathways and allows the transient modification of the cell surface by recruitment of membrane proteins from an intracellular storage pool to the cell surface (17, 18). In particular, this pathway (regulated endosomal recycling) is present in insulin-sensitive fat and skeletal muscle cells, where several plasma membrane proteins including glucose transporter isoform 4 (GLUT4) are colocalized in intracellular vesicles and are coordinately translocated to the cell surface in response to insulin (19, 20). It is likely that translocation of aquaporin-containing vesicles in collecting ductules of the kidney in response to antidiuretic hormone represents an analogous pathway (21). It was suggested that regulated endosomal recycling pathway(s) may also exist in neurons (17), although the membrane structures of this pathway have not yet been purified and the constituent proteins are still unknown.
Both GLUT4-containing vesicles from fat and skeletal muscle cells and water-channel-containing vesicles from renal papilla possess a high level of aminopeptidase activity (21, 22). In adipose cells, an isoform of this easily detectable enzyme (insulin responsive aminopeptidase) is completely colocalized with GLUT4 in the endosomal recycling vesicles and is not present in any other subcellular compartment (23, 24). These data establish aminopeptidase activity as an appropriate marker for the regulated endosomal recycling pathway. Therefore, we decided to use this marker (which has never been reported to be present in classical small synaptic vesicles) to explore the organization of membrane traffic in synaptosomes. Our results provide evidence that there are at least two biochemically distinct vesicular populations in what is commonly identified as a homogeneous pool of synaptic vesicles from rat brain, both of which recycle via a clathrin-mediated pathway.
MATERIALS AND METHODS
Antibodies.
Antibodies to SV2 and synaptotagmin were gifts from K. Buckley (Harvard Medical School). Antibodies to synaptophysin and Rab 3a were gifts from R. Jahn (Yale University School of Medicine). Anti-vacuolar proton pump antibody was a gift from G. Bowman (University of California, Santa Cruz). Antibodies to clathrin heavy chain were from ICN and to the light chain were from S. Puszkin (Columbia University).
Isolation and Fractionation of Synaptic Vesicles from Rat Brain.
Synaptic vesicles were prepared from rat brains by the procedure of Huttner et al. (25). Briefly, whole rat brains (or dissected gray or white matter) were washed in buffered sucrose (320 mM sucrose/4 mM Hepes, pH 7.4) and homogenized in the same buffer with 12 up and down strokes in a Teflon–glass homogenizer. The homogenate was centrifuged for 10 min at 1,100 × g. The resulting supernatant was centrifuged for 15 min at 9,200 × g, and the pellet was resuspended in ≈10 ml of buffer per brain and centrifuged for 15 min at 10,500 × g. The resulting pellet (synaptosomes) was resuspended in buffered sucrose, then diluted with 9 vol of ice-cold H2O (hypotonic lysis of synaptosomes to release synaptic vesicles), and immediately homogenized with three strokes in a Dounce homogenizer. To this homogenate, 1 M Hepes (pH 7.4) was added to a final concentration of 7.5 mM Hepes and incubated on ice for 30 min. The homogenate was centrifuged for 20 min at 25,500 × g, and the pellet (synaptosomal membrane) was discarded. The supernatant was centrifuged for 2 h at 48,000 rpm, in a Ti60 rotor, and the resulting pellet was resuspended in 1 ml of 30 mM sucrose/4 mM Hepes, pH 7.4, and homogenized by passage through a 25-gauge needle five times back and forth. This material was loaded on a continuous gradient of 50–800 mM sucrose/4 mM Hepes, pH 7.4, and the gradient was centrifuged for 5 h at 22,000 rpm in an AH627 rotor. Fractions containing synaptic vesicle marker proteins were pooled, pelleted by centrifugation at 48,000 rpm for 2 h, resuspended in buffer A (150 mM NaCl/10 mM Hepes, pH 7.4/1 mM EGTA/0.1 mM MgCl2), then loaded onto an equilibrium density sucrose gradient [10–50% (wt/vol) in buffer A], and centrifuged at 48,000 rpm for 18 h in an SW50.1 rotor (26). With this procedure, two populations of vesicles were obtained from the equilibrium density gradient. These two protein peaks were pooled separately and centrifuged on a 10–30% sucrose velocity gradient for 55 min at 48,000 rpm. After each centrifugation, fractions were collected starting from the bottom of the gradient and analyzed for the total protein content, presence of specific proteins by Western blotting, and aminopeptidase activity.
Purification of Clathrin-Coated Vesicles from Rat Brain.
Clathrin-coated vesicles were purified from rat brain essentially by the procedure of Maycox et al. (27). Briefly, rat brains were isolated, washed, and homogenized (10 strokes at 1,500 rpm) in Mes buffer (pH 6.5; 0.1 M Mes/1 mM EGTA/0.5 mM MgCl2). The homogenate was then centrifuged at 20,000 × g for 20 min, and the resulting supernatant was collected and centrifuged at 55,000 × g for 1 h. The resulting pellet was resuspended in Mes buffer, followed by homogenization (3 strokes at 2,000 rpm) and dispersion through a 27-gauge needle. The suspension was then mixed with an equal volume of Mes buffer containing 12.5% (wt/vol) Ficoll and 12.5% (wt/vol) sucrose and centrifuged for 40 min at 40,000 × g. The supernatant was removed, diluted 1:5 with Mes buffer, and centrifuged for 1 h at 100,000 × g to pellet coated vesicles. The pellet was resuspended in Mes buffer and cleared by centrifugation at 20,000 × g for 20 min, and the supernatant was layered on top of Mes buffer containing 8% sucrose and centrifuged for 2 h at 27,000 rpm in an AH627 rotor. The final pellet containing purified coated vesicles was resuspended in 0.2 ml of Mes buffer. Clathrin-coated vesicles were stripped of their clathrin coats by incubation in 5 mM Tris, pH 8.5/150 mM sucrose/0.5 mM EGTA at 23°C for 1 h, followed by centrifugation for 1 h at 100,000 × g, as described by Cantley et al. (28).
Aminopeptidase Activity.
This activity was measured by the fluorometric method of Little et al. (29). Fractions (10 μl) from the gradient were mixed in the presence of 1% Triton X-100, with 1 mM (final concentration) arginine β-naphthylamide (0.5 ml) in a total volume of 1.5 ml of buffer A. The mixture was incubated for 30 min at 37°C, and fluorescence in the fractions was measured at 410 nm. The excitation wavelength was 340 nm.
Glutamate Accumulation into Synaptic Vesicles.
Fractions from the equilibrium density gradient were assayed for ATP-dependent glutamate uptake by the method of Kish et al. (30). Equal volumes of each fraction were preincubated at 30°C for 5 min in incubation medium (4 mM MgCl2/20 mM Hepes, pH 7.4). After preincubation the glutamate mixture (50 μM glutamate/2 μCi of [3H]glutamate/4 mM KCl in incubation medium, with or without 2 mM ATP; 1 Ci = 37 GBq) was added, mixed, and incubated for an additional 1.5 min. Glutamate uptake was terminated by addition of ice-cold 150 mM KCl, followed by immediate filtration through Millipore HAWP filters (0.45-μm pore size). The filters were washed four times, and radioactivity retained on the filters was determined in a scintillation counter (LKB).
Substance P Radioimmunoassay.
Substance P content in fractions from the velocity sucrose gradient and the equilibrium density gradient (50- and 100-μl aliquots) was determined by radioimmunoassay as described (31). In these experiments, substance P-specific antibody (1:80,000 dilution) was incubated with the samples and 125I-labeled substance P tracer for 72 h.
Gel Electrophoresis and Immunoblotting.
Proteins were separated by SDS/PAGE according to Laemmli (32) and transferred to Immobilon-P membrane in 25 mM Tris/192 mM glycine. After transfer, the membrane was blocked with 10% nonfat dry milk in PBS for 1 h at 37°C and probed with specific antibodies.
Protein Determination.
Protein content was determined with the BCA kit (Pierce) according to the manufacturer’s instructions.
RESULTS
Heterogeneity of Synaptic-Like Vesicles.
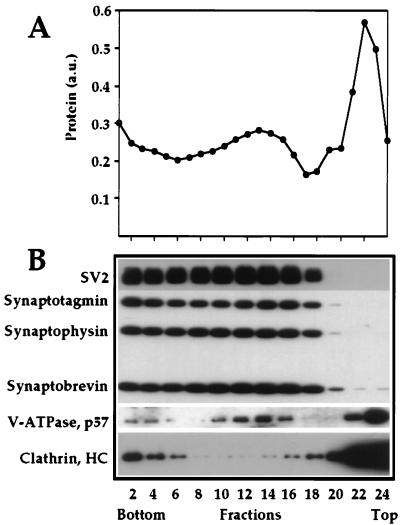
Synaptosomes (presynaptic terminals) were prepared either from whole rat brain or from gray or white matter separately, and synaptic vesicles were purified from their interior after osmotic rupture of the synaptosomal membrane. After removal of the synaptosomal membrane by centrifugation, synaptic vesicles were purified in a 50–800 mM sucrose velocity gradient (Fig. 1). Fractions enriched in synaptic vesicle marker proteins (Fig. 1, fractions 6–17) were pooled and pelleted at 150,000 × g for 2 h. Electron microscopy analysis demonstrated that at this step we have a highly enriched preparation of virtually uniform synaptic vesicles (results not shown). Noteworthy, this fraction lacks any substance P-containing material that was recovered mainly in the fraction of synaptosomal plasma membranes and in the pellet of the sucrose gradient centrifugation (data not shown). We conclude, therefore, that after this centrifugation step, we have separated small synaptic vesicles from peptide-containing large dense core particles.
Figure 1.
Isolation of synaptic vesicles in sucrose velocity gradient. A crude preparation of synaptic vesicles was centrifuged in a 50–800 mM sucrose velocity gradient. (A) Total protein profile of fractions collected from the gradient. (B) Western blot analysis of equal volume aliquots of every even fraction.
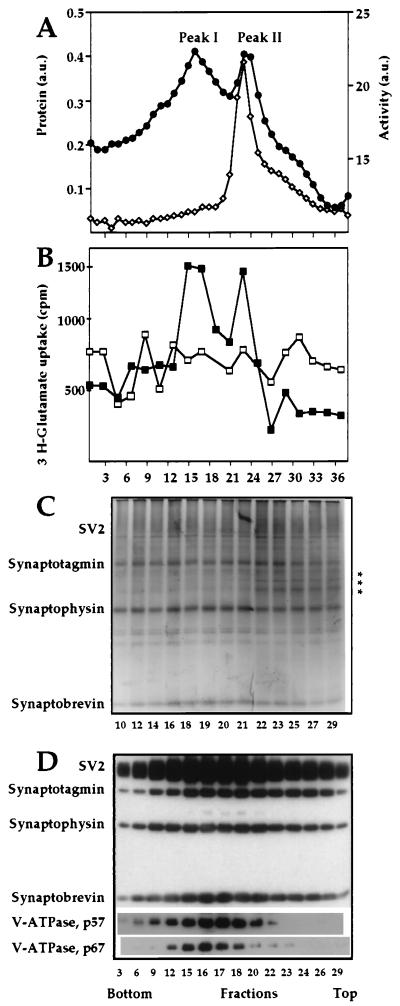
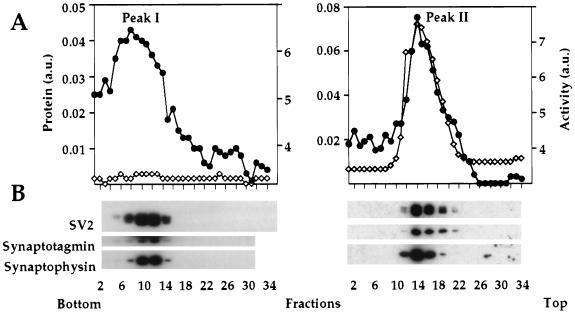
Centrifugation of this crude synaptic vesicle preparation in a 10–50% continuous equilibrium sucrose gradient resulted in separation of vesicles into two distinct protein peaks, peaks I and II (Fig. 2A). To demonstrate that vesicles from both peaks have properties of synaptic vesicles such as neurotransmitter uptake, we determined ATP-dependent glutamate uptake into vesicles from each fraction of the equilibrium density gradient (30). Fig. 2B demonstrates that both peaks I and II represent vesicles that can import glutamate in an ATP-dependent fashion. None of these vesicles, however, contained any measurable amount of substance P (data not shown).
Figure 2.
Fractionation of synaptic vesicles in an equilibrium density gradient. Fractions containing synaptic vesicle proteins from Fig. 1 were pooled, pelleted, and then loaded on an equilibrium density sucrose gradient (10–50%). Even fractions were analyzed for total protein (•) and aminopeptidase activity (◊) (A) and [3H]glutamate uptake was determined in the presence (▪) or absence (□) of ATP (B). (C) Silver staining of the two synaptic vesicle populations. The asterisks indicate the location of bands that are present preferentially in peak II. (D) Individual synaptic vesicle proteins analyzed by Western blotting. Equal-volume aliquots from fractions of the equilibrium density gradient were subjected to SDS/PAGE and Western blot analysis.
The separation of vesicles into two subpopulations is rather difficult to detect by either silver staining or Western blotting, due to the semiquantitative nature of these types of analyses. On the other hand, the accurate measurements of the total protein (Fig. 2A) and, especially, aminopeptidase activity (see below) provide very obvious proof for vesicle heterogeneity.
Both silver staining and Western blotting of fractions from the equilibrium density gradient allow the direct visualization of several major marker proteins of synaptic vesicles, including SV2 (seen as a diffuse band), synaptotagmin, synaptophysin, and synaptobrevin (Fig. 2 C and D), and show that all these proteins are present in roughly equivalent amounts in both peaks. Fig. 2C also shows that the total polypeptide composition of the two vesicle subpopulations is very similar with the exception of a few minor protein bands in the molecular mass range of 40–50 kDa, which are present specifically in peak II. More differences in the protein content of the two vesicle subpopulations are revealed by Western blot analysis, which demonstrates that the V-type H+-ATPase is somewhat enriched in peak I (Fig. 2D). We have not detected any obvious correlation between the vacuolar proton pump content and ATP-dependent glutamate uptake into the different vesicles populations (Fig. 2B). This may be explained by the fact that H+-ATPase activity in synaptic vesicles is regulated by a protein factor (33) that has an unknown distribution between the two subpopulations. We believe, however, that the different H+-ATPase content in vesicles from peaks I and II may be an important indication of their specific biological functions.
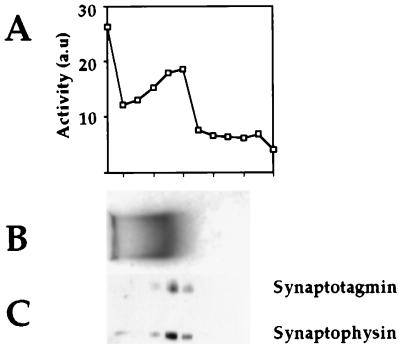
A striking difference between the two populations of vesicles is the presence of aminopeptidase activity specifically in peak II (Fig. 2A). This enzyme is capable of hydrolyzing several β-naphthylamide esters with the following specificity: Arg > Lys ≫ Ala (data not shown). As an additional control for assessing the association of aminopeptidase activity with a subpopulation of synaptic vesicles, we carried out agarose gel electrophoresis (34). As is seen in Fig. 3, this activity indeed comigrates with synaptic vesicles, as determined by Coomassie staining (Fig. 3B) and Western blot analysis (Fig. 3C). We also see a certain amount of the material at the starting point of the agarose gel that most probably results from vesicle aggregation.
Figure 3.
Agarose gel electrophoresis of peak II vesicle membranes. Peak I and II membranes were pelleted and electrophoresed in a 0.15% agarose gel in 50 mM Mes buffer (pH 6.5) for 24 h at 0.75 V/cm while recirculating the Mes electrophoresis buffer at 4°C (23). The lane containing peak I was stained with Coomassie Blue for direct visualization (B), and the peak II-containing lane was cut into fractions and incubated with synthetic substrate to localize the aminopeptidase activity (A). An aliquot from each fraction was electrophoresed in a 10% SDS/PAGE gel, and Western blot analysis was performed with anti-synaptotagmin and anti-synaptophysin antibodies (C).
Several experiments lead us to believe that the detected aminopeptidase most probably represents an integral membrane protein with the catalytic center facing the lumen of the vesicle. (i) Aminopeptidase activity cosediments with vesicle membranes after their treatment with NaHCO3 at pH 11 (data not shown). (ii) We can detect aminopeptidase activity in synaptic vesicles only in the presence of Triton X-100 or other nonionic detergents (data not shown). Interestingly, an in-gel reconstitution assay of the aminopeptidase activity shows the presence of the enzyme in the 40- to 50-kDa zone of the gel (data not shown), which suggests that at least one of the peak II-specific bands visible by silver staining in the molecular mass range of 45 kDa (Fig. 2C), may represent the detected enzyme. Our preliminary characterization of this enzyme in vitro suggests that this may be a distinct type of aminopeptidase, and we are currently in the process of purifying it for sequencing analysis.
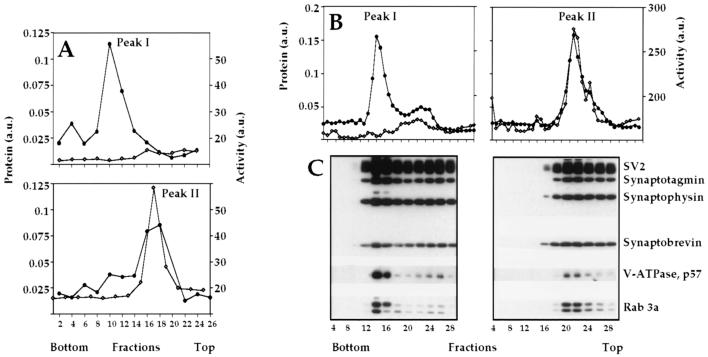
To verify the results of Fig. 2A, we pooled the fractions from the equilibrium density gradient containing peak I (fractions 12–18) and peak II (fractions 22–27) separately and recentrifuged each in equilibrium density (Fig. 4A) and velocity sucrose gradients (Fig. 4B). As expected, under conditions of equilibrium density centrifugation, we obtained two independent protein peaks (Fig. 4A). The protein peak constituting the less dense membrane population also contained the aminopeptidase activity, but the higher density population did not. The results of velocity centrifugation confirm the existence of the two subpopulations of synaptic vesicles and demonstrate that, in addition to different buoyant densities, the two vesicle populations also have different sedimentation coefficients (Fig. 4B).
Figure 4.
Characterization of the two synaptic vesicle subpopulations. (A) Fractionation of synaptic vesicles in an equilibrium density sucrose gradient. Separately pooled fractions from the first equilibrium density gradient (Fig. 2) containing peaks I and II were recentrifuged under the same conditions. Total protein (•) and aminopeptidase activity (◊) are shown. (B) Fractionation of the two vesicle populations in a sucrose velocity gradient. The two populations of vesicles (peaks I and II), purified after the equilibrium density gradient (Fig. 2), were pooled separately and centrifuged on a 10–30% sucrose velocity gradient. Fractions were analyzed for total protein (•) and aminopeptidase activity (◊). (C) Individual proteins were identified by Western blotting.
We reinvestigated the degree of similarity between these highly purified vesicle populations to determine whether both these populations contained membrane proteins previously shown to be specific for synaptic vesicles (6, 8). As is seen in Fig. 4C, peaks I and II contain equivalent amounts of known synaptic vesicle membrane proteins such as SV2, synaptotagmin, synaptophysin, synaptobrevin, and rab3a. However, in agreement with the data as shown in Fig. 2D, the vacuolar proton pump is present preferentially in peak I, and aminopeptidase activity is highly specific to peak II and is virtually undetectable in peak I. It is seen in Fig. 4B that the sedimentational distribution of the material from peak I is not very uniform. Along with the major peak (fractions 14–16), we often see a minor component or a shoulder (fractions 24–26) in Fig. 4B. We initially thought that this minor peak may represent a contamination of the preparation with the material from peak II. However, because the position of the component represented by fractions 24–26 does not necessarily overlap with peak II, this phenomenon may be an indication for the further heterogeneity of synaptic vesicles.
We also checked the two vesicle subpopulations for the presence of several peripheral membrane proteins and t-SNAREs known to be associated with synaptic vesicles (35). In agreement with the published data (35), we detect the presence of syntaxin, SNAP-25, adaptor complex AP2, dynamin, and clathrin in both vesicle populations; however, the ratio of several proteins varies to some degree between peaks I and II (data not shown).
Heterogeneity of Clathrin-Coated Vesicles Stripped in Vitro.
Clathrin is highly concentrated in nerve terminals. Synaptic vesicle proteins represent the main cargo of brain clathrin-coated vesicles, establishing that the recycling of synaptic vesicles in nerve terminals is mediated by the clathrin-coated vesicle pathway (27, 36). Therefore, because clathrin-coated vesicles from brain represent endocytosed synaptic vesicles still carrying clathrin coats, we reasoned that the identification of heterogeneity in clathrin-coated vesicles, as is the case in synaptic vesicles, would provide independent proof for our findings and may also suggest a mechanism for the recycling of the two synaptic vesicle populations.
With this goal in mind, we purified clathrin-coated vesicles from either whole rat brain or from synaptosomes. Because the buoyant density of clathrin-coated vesicles is higher than that of synaptic vesicles, we used a 20–65% continuous sucrose gradient for the equilibrium density centrifugation. Under these conditions however, clathrin-coated vesicles have a rather narrow distribution and do not show any obvious heterogeneity when assayed for total protein (results not shown), suggesting that the presence of clathrin coats may somehow mask the small differences in the buoyant density of the two vesicular subpopulations. Therefore, we decided to remove the clathrin coats from the purified clathrin-coated vesicles and to analyze the distribution of the uncoated vesicles on an equilibrium density gradient.
The clathrin coats were stripped from the coated vesicles, resulting in the removal of >90% of clathrin from vesicles as controlled by Western blot analysis (results not shown). The uncoated vesicles were then pelleted and recentrifuged to equilibrium in a 10–50% sucrose gradient. We then pooled aminopeptidase positive and negative fractions separately as we did in the case of synaptic vesicles. Pooled material was pelleted and recentrifuged in a 10–30% velocity sucrose gradient (Fig. 5). In these experiments, we clearly obtained individual subpopulation of vesicles virtually identical by their sedimentational properties to those in peaks I and II (Fig. 4B). This suggests that the two populations of vesicles exist in nerve terminals in the endocytic and exocytic pathways.
Figure 5.
Two populations of in vitro-uncoated clathrin-coated vesicles. Clathrin-coated vesicles were uncoated in vitro and repurified in a 10–50% sucrose equilibrium density gradient. Aminopeptidase-containing and non-aminopeptidase-containing fractions were combined separately and centrifuged in a continuous 10–30% sucrose gradient. Fractions were analyzed for total protein (•) and aminopeptidase activity (◊) (A) and individual synaptic vesicle proteins by Western blotting (B).
DISCUSSION
We have purified the total fraction of synaptic vesicles from rat synaptosomes and found that this preparation represents a mixture of at least two individual vesicular populations, only one of which contains aminopeptidase activity. This observation of two populations of synaptic vesicle has previously been detected both in mammalian brain (37) and in the electric organ of Torpedo fish (for review, see ref. 38) but not extensively characterized. Vesicles from these two pools differ in buoyant density and sedimentation coefficient, yet they have a very similar protein composition. In particular, both populations are found to contain roughly equivalent amounts of the synaptic vesicle proteins SV2, synaptotagmin, synaptophysin, secretory carrier membrane proteins (SCAMPs), synaptobrevin, and rab3a. In addition, both types of vesicles are able to transport glutamate into their interior in an ATP-dependent manner and, therefore, fit the definition of small synaptic vesicles. In fact, the very similar protein composition and physical properties of the two recycling vesicles may explain why this heterogeneity has not been detected before.
Clathrin-coated vesicles from rat brain uncoated in vitro also fractionate into two vesicular populations with and without aminopeptidase activity. These distinct types of vesicles, therefore, can be detected not only at the exocytic but also at the endocytic stage of their recycling. The two populations are unlikely to represent vesicles from different neuron classes located in different brain regions, because we have demonstrated that synapses from a single class of neuron, retinal ganglion cells, also contain the same vesicular pools (unpublished observations).
The presence of aminopeptidase in only one type of vesicle population suggests that the two populations originate from different endosomes and recycle separately in brain synapses. The nature of these recycling pathways is currently under investigation. One hypothesis is that aminopeptidase-containing vesicles represent a regulated endosomal recycling pathway and, thus, are analogous to GLUT4-containing vesicles from fat and muscle cells and water- channel-containing vesicles from renal papilla. The existence of such a compartment in neuronal cells has previously been postulated by Kelly and associates (17). However, to make a more definitive conclusion for the existence of an endosomal recycling pathway in neurons, additional biochemical markers are needed that are specifically present in one or another vesicular population. We have data showing that a neuron-specific isoform of glucose transporter protein, GLUT3, is preferentially present in the aminopeptidase-containing vesicles (unpublished results). This observation emphasizes the similarity between glucose-transporter-containing vesicles in neurons and nonneuronal cells and provides indirect evidence for the endosomal origin of the material in peak II.
The total polypeptide composition of both types of vesicles is very similar (Fig. 2C). We believe, therefore, that the likely explanation for the heterogeneity is that both vesicle populations are active in regulated neurotransmitter release and that one subpopulation, namely that in peak II, also carries some plasma membrane proteins (aminopeptidase, GLUT3) in addition to its neurotransmitter cargo. The presence of these extra proteins may be compensated by a decrease in the specific content of certain nonregulatory components of synaptic vesicles, such as the proton pump (Fig. 2D). So, the population of vesicles from peak II may represent a hybrid of small synaptic vesicles and the compartment responsible for the regulated endosomal recycling, just as neurosecretory vesicles represent a hybrid of small synaptic vesicles and secretory granules (39).
Yet another possibility is that one of the populations may represent mature synaptic vesicles and the other may be de novo assembled (or maturing) synaptic vesicles on their first round of cycling to the synaptic membrane and, possibly, not yet carrying neurotransmitter cargo (40). Thus, to understand the functional significance of vesicle heterogeneity it should be very interesting to compare the neurotransmitter content of the two populations and to determine whether or not both are exocytosed in response to an action potential.
Acknowledgments
We thank Dr. Paul F. Pilch for financial support and helpful discussions and Eric Berg for determination of substance P. This work was supported by Grant R01DK52057 from the National Institutes of Health, by a research grant from the American Diabetes Association, and by research Grant 197029 from the Juvenile Diabetes Foundation (K.V.K.), Grant R37 AG-05894, a Merit Award grant from the Department of Veterans Affairs, and Pilot Research Grant IIRG-95-049 from the Alzheimer’s Association/F. M. Kirby Foundation to R.E.F., and by Grants DK30425 and DK44269 to P. F. Pilch.
References
- 1.De Camilli P. FEBS Lett. 1995;369:3–12. doi: 10.1016/0014-5793(95)00739-v. [DOI] [PubMed] [Google Scholar]
- 2.De Camilli P, Takei K. Neuron. 1996;16:481–486. doi: 10.1016/s0896-6273(00)80068-9. [DOI] [PubMed] [Google Scholar]
- 3.Calakos N, Scheller R H. Physiol Rev. 1996;76:1–25. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Bajjalieh S M, Scheller R H. J Biol Chem. 1995;270:1971–1974. doi: 10.1074/jbc.270.5.1971. [DOI] [PubMed] [Google Scholar]
- 5.Jahn R, Südhof T C. Annu Rev Neurosci. 1993;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- 6.Südhof T C, Jahn R. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- 7.Südhof T C, De Camilli P, Niemann H, Jahn R. Cell. 1993;75:1–4. [PubMed] [Google Scholar]
- 8.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 9.McMahon H T, Südhof T C. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 10.Calakos N, Scheller R H. J Biol Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- 11.Pevsner J, Hsu S C, Braun J E, Calakos N, Ting A E, Bennet M K, Scheller R H. Neuron. 1991;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 12.Pevsner J, Hsu S C, Scheller R H. Proc Natl Acad Sci USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calakos N, Bennet M K, Peterson K E. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 14.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Germanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 15.Söllner T, Bennet M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 16.Bennet M K, Scheller R H. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman G A, Bonzelius F, Cieutat A, Kelly R B. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly R B. Nature (London) 1993;364:487–488. doi: 10.1038/364487a0. [DOI] [PubMed] [Google Scholar]
- 19.Kandror K V, Pilch P F. Semin Cell Dev Biol. 1996;7:269–278. [Google Scholar]
- 20.Kandror K V, Pilch P F. Am J Physiol. 1996;271:E1–E14. doi: 10.1152/ajpendo.1996.271.1.E1. [DOI] [PubMed] [Google Scholar]
- 21.Harris H W, Ziedel M L, Jo I, Hammond T G. J Biol Chem. 1994;269:11993–12000. [PubMed] [Google Scholar]
- 22.Kandror K V, Coderre L, Pushkin A V, Pilch P F. Biochem J. 1995;307:383–390. doi: 10.1042/bj3070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandror K V, Pilch P F. Proc Natl Acad Sci USA. 1994;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross S A, Scott H M, Morris N J, Leung W-Y, Mao F, Lienhard G E, Keller S R. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- 25.Huttner W B, Schiebler P, Greengard P, De Camilli P. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clift-O’Grady L, Linstedt A D, Lowe A W, Grote E, Kelly R B. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maycox P R, Link E, Reetz A, Morris S A, Jahn R. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forgac M, Cantley L. J Biol Chem. 1984;259:8101–8105. [PubMed] [Google Scholar]
- 29.Little G H, Starnes W L, Behal F J. Methods Enzymol. 1976;45:495–503. doi: 10.1016/s0076-6879(76)45044-9. [DOI] [PubMed] [Google Scholar]
- 30.Kish P E, Ueda T. Methods Enzymol. 1989;174:9–25. doi: 10.1016/0076-6879(89)74005-2. [DOI] [PubMed] [Google Scholar]
- 31.Mroz E A, Leeman S E. In: Methods of Radioimmunoassay. Jaffe R M, Behrman H R, editors. New York: Academic; 1978. p. 121. [Google Scholar]
- 32.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Ozkan E D, Lee F S, Ueda T. Proc Natl Acad Sci USA. 1997;94:4137–4142. doi: 10.1073/pnas.94.8.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein J L R, Fine R E, Luskey B D, Rothman J E. J Cell Biol. 1981;89:357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walch-Solimena C, Blasi J, Edelmann L, von Mollard G F, Jahn R. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takei K, Mundigl O, Daniell L, De Camilli P. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floor E, Schaeffer S F, Feist B E, Leeman S E. J Neurochem. 1988;50:1588–1596. doi: 10.1111/j.1471-4159.1988.tb03048.x. [DOI] [PubMed] [Google Scholar]
- 38.Whittaker V P. The Cholinergic Neuron and its Target. Berlin: Springer; 1992. [Google Scholar]
- 39.Bauerfeind R, Jelinek R, Hellwig A, Huttner W B. Proc Natl Acad Sci USA. 1995;92:7342–7346. doi: 10.1073/pnas.92.16.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly, R. B. (1993) Cell 72,/Neuron 10, 43–53.