Abstract
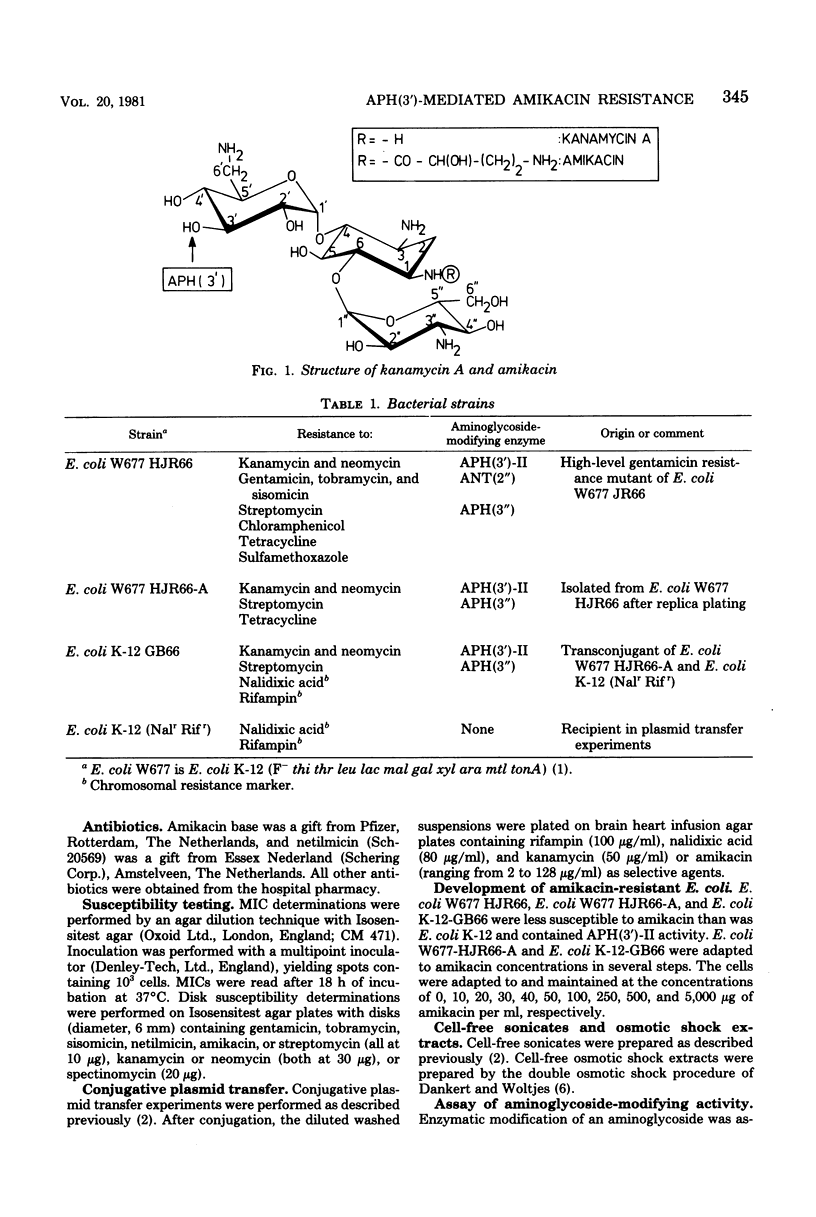
An Escherichia coli strain with a plasmidic amikacin resistance has been selected for which the evidence strongly indicates that resistance is mediated by aminoglycoside phosphotransferase [APH(3')-II]: (i) this resistance was coupled with resistance against kanamycin and neomycin; (ii) partially purified APH(3')-II[APH(3") free] modified amikacin by phosphorylation; (iii) the product of the APH(3')-II mediated reaction (i.e., 3'-O-phosphoryl-amikacin) lost its antibacterial activity; and (iv) the amikacin-modifying APH(3')-II activity increased 5- to 10-fold after adaptation of the cells to higher concentrations of amikacin. The substrate spectrum of this enzyme showed a low activity against amikacin as compared with neomycin. It is argued that the enzyme level rather than its substrate spectrum is important for enzyme-mediated resistance. The increase in enzyme levels was found to be correlated with an increase in copy number of a 110-Megadalton plasmid (pBN66) which coded for the APH(3')-II and the APH(3") activity. The increase in copy number was irreversible, and therefore this phenomenon is ascribed to a mutation of a gene which affects the copy number. In transconjugants, the original low copy number was present, and therefore the mutation must be located on the chromosome and not on the plasmid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Bongaerts G. P., Bruggeman-Ogle K. M. Effect of beta-lactamase and salt on mecillinam susceptibility of enterobacterial strains. Antimicrob Agents Chemother. 1980 Nov;18(5):680–686. doi: 10.1128/aac.18.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska M., Davies J. Two enzymes which phosphorylate neomycin and kanamycin in Escherichia coli strains carrying R factors. Antimicrob Agents Chemother. 1973 Feb;3(2):266–269. doi: 10.1128/aac.3.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert J., Woltjes J. Double-osmotic-shock procedure to prepare gentamicin adenine mononucleotide transferase for the enzymatic assay of gentamicin. Antimicrob Agents Chemother. 1977 Jun;11(6):1074–1076. doi: 10.1128/aac.11.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martel A., Moreau N., Capmau M. L., Soussy C. J., Duval J. 2"-O-phosphorylation of gentamicin components by a Staphylococcus aureus strain carrying a plasmid. Antimicrob Agents Chemother. 1977 Jul;12(1):26–30. doi: 10.1128/aac.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Amikacin resistance associated with a plasmid-borne aminoglycoside phosphotransferase in Escherichia coli. Antimicrob Agents Chemother. 1979 Nov;16(5):598–604. doi: 10.1128/aac.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., DeFuria M. D., Pursiano T. A. Amikacin, an aminoglycoside with marked activity against antibiotic-resistant clinical isolates. J Infect Dis. 1976 Nov;134(Suppl):S249–S261. doi: 10.1093/infdis/135.supplement_2.s249. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H. Gentamicin:adenine mononucleotide transferase: partial purification, characterization, and use in the clinical quantitation of gentamicin. J Infect Dis. 1974 Apr;129(4):391–401. doi: 10.1093/infdis/129.4.391. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Kondo S. Ion-exchange chromatography of aminoglycoside antibodies. Methods Enzymol. 1975;43:263–278. doi: 10.1016/0076-6879(75)43087-7. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]



