Abstract
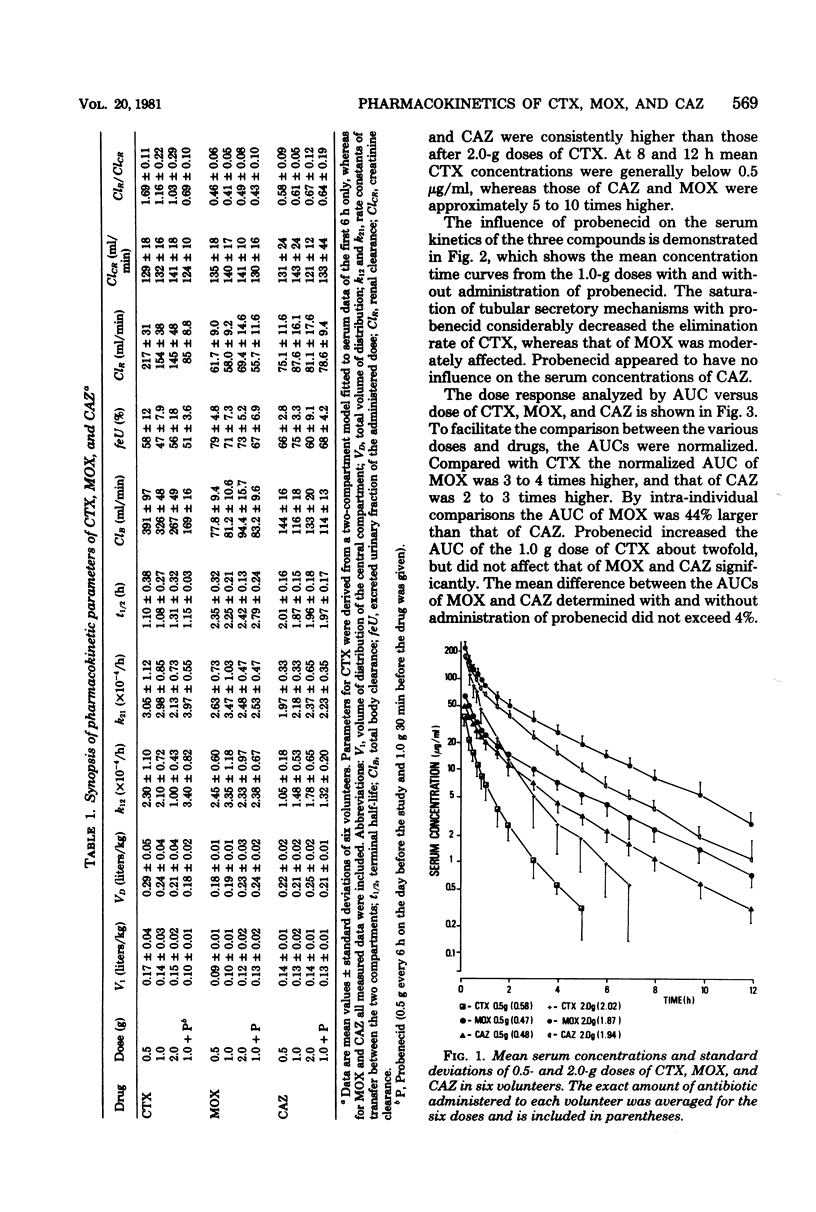
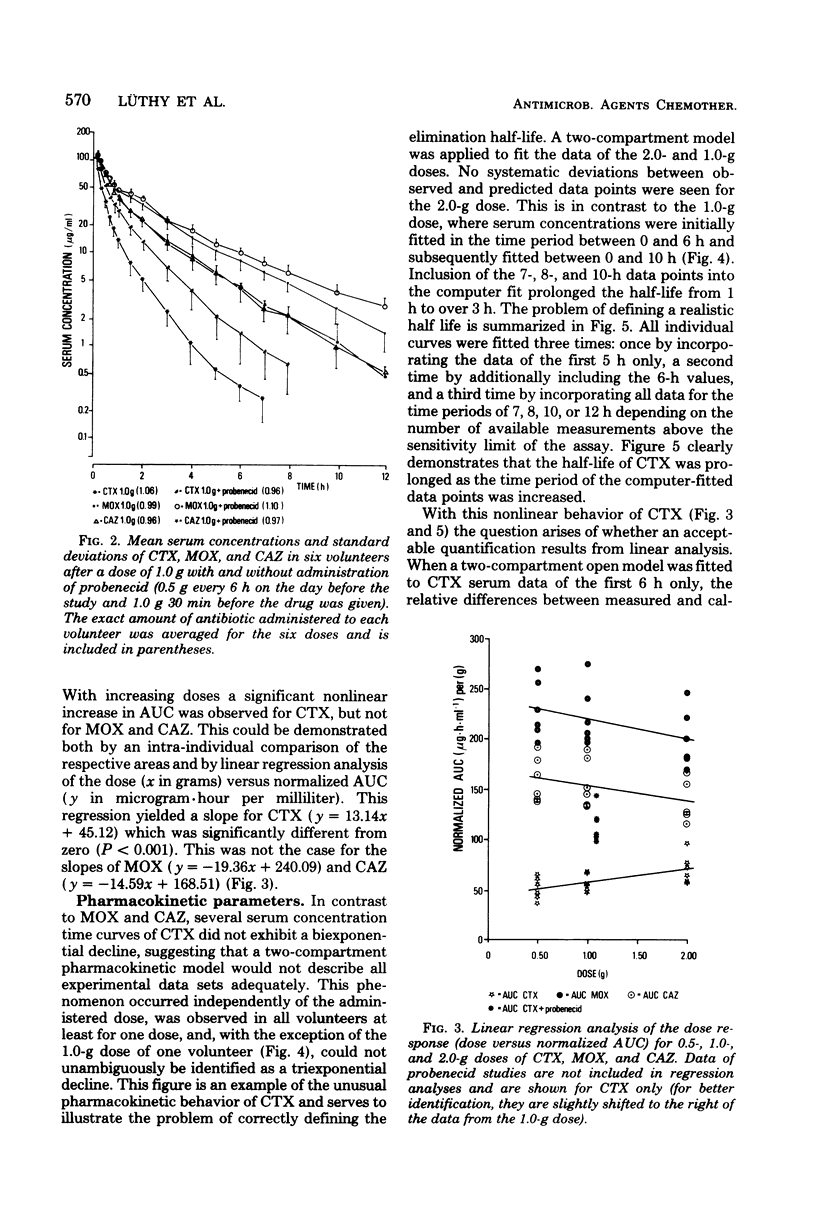
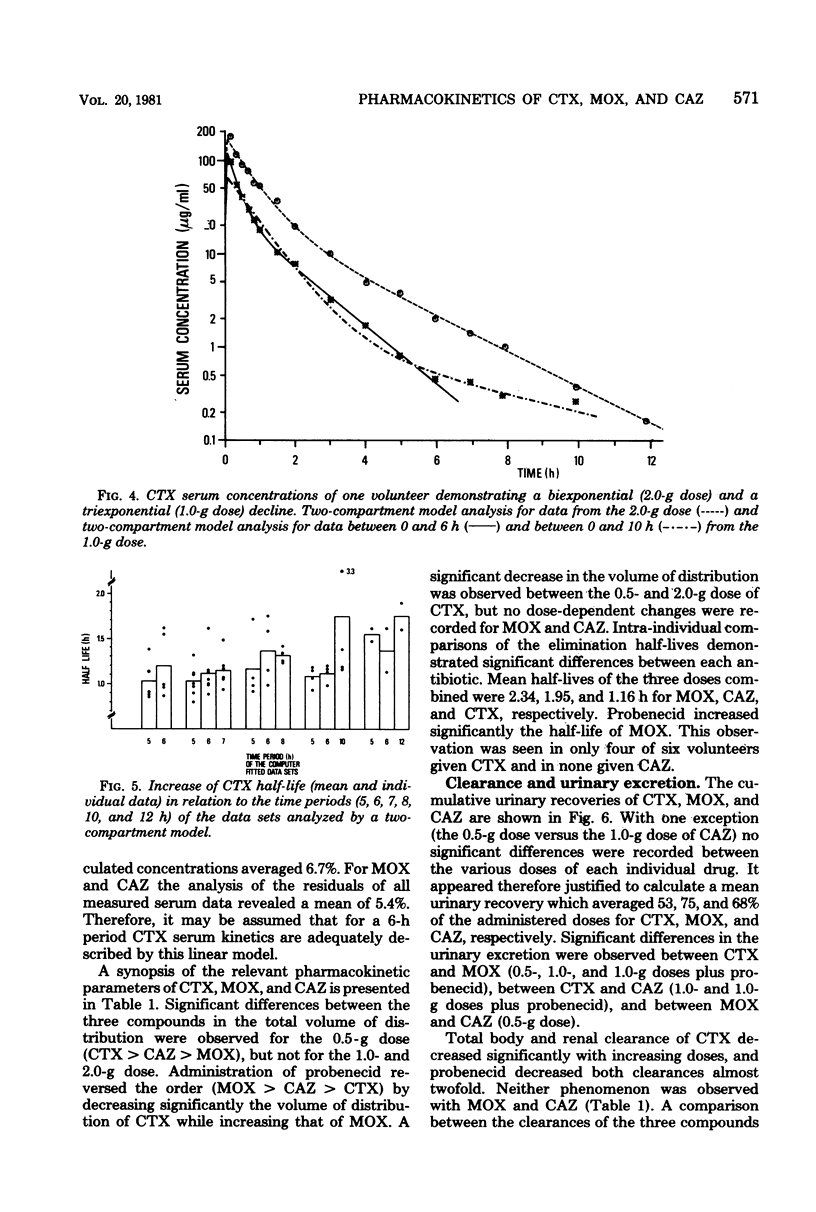
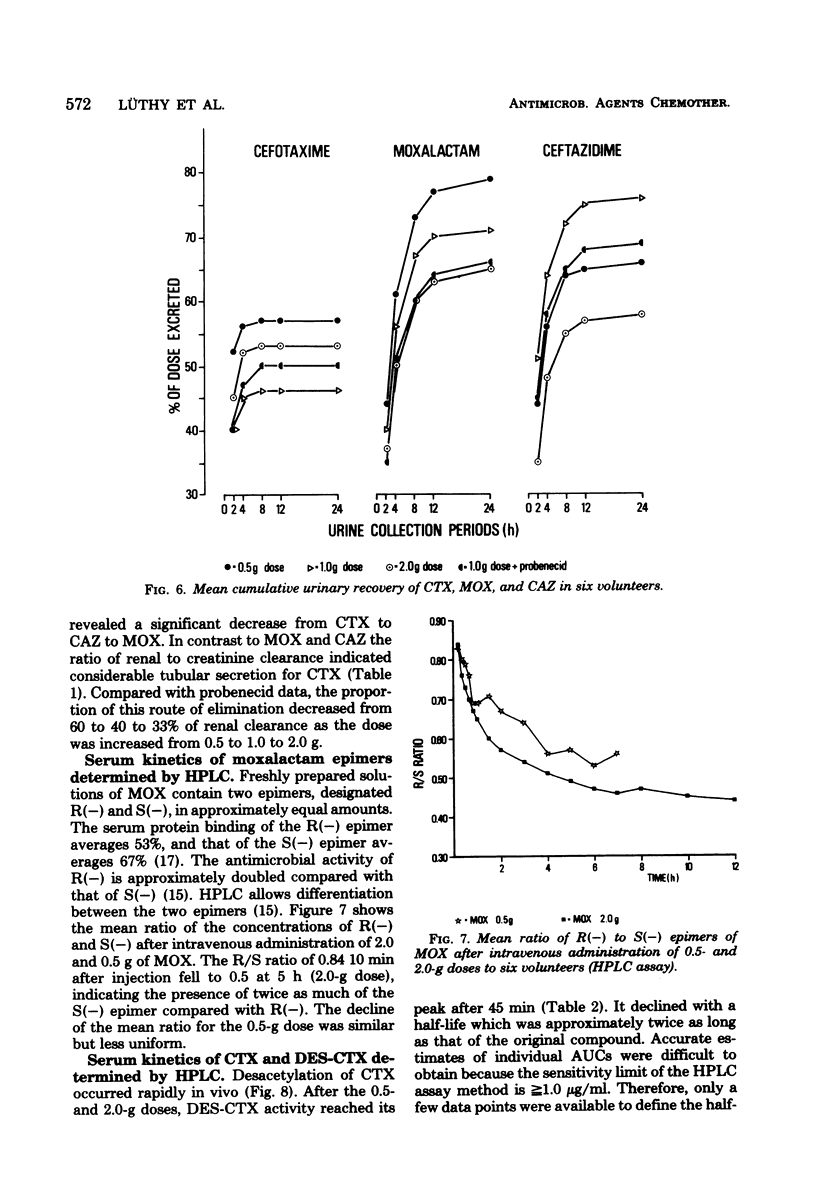
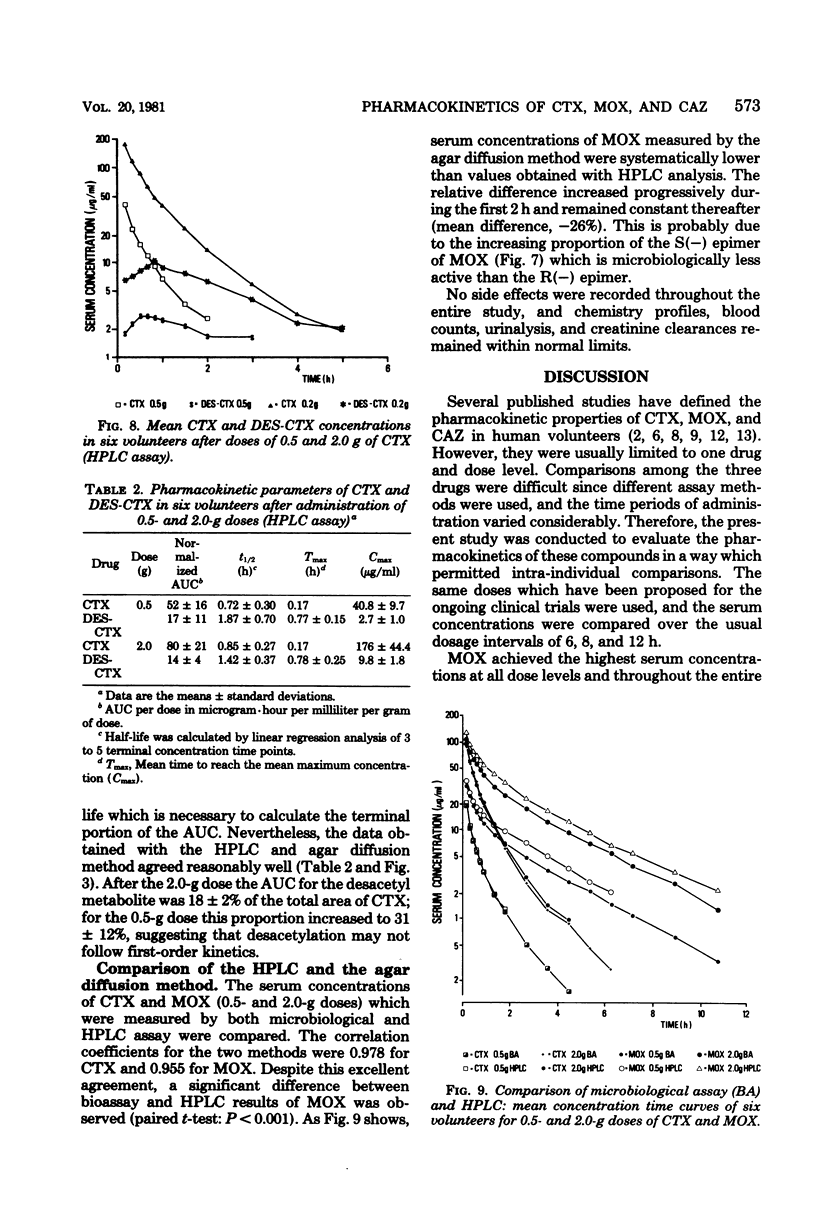
The pharmacokinetics of cefotaxime, moxalactam, and ceftazidime were investigated in six human volunteers who received in a crossover fashion doses of 0.5, 1.0, and 2.0 g of each drug by a 5-min infusion. Doses of 1.0 g were repeated after the administration of probenecid. Serum and urine concentrations were assayed with an agar diffusion method. Serum concentrations of moxalactam exceeded those of ceftazidime at all times and were distinctly higher than those of cefotaxime. The normalized area under the concentration time curve (defined as the ratio of the area under the curve per dose) reflects this relationship: compared with cefotaxime the normalized area under the curve of moxalactam was 3 to 4 times higher, and that of ceftazidime was 2 to 3 times higher. By intra-individual comparisons, the area under the curve of moxalactam was 44% larger than that of ceftazidime. With increasing doses, cefotaxime exhibited a nonlinear increase of the area under the curve. The half-lives of moxalactam, ceftazidime, and cefotaxime were 2.34, 1.95, and 1.16 h, respectively. The volume of distribution averaged 0.20 ± 0.03, 0.23 ± 0.02, and 0.25 ± 0.04 liters per kg, and the mean total body clearance was 84, 131, and 328 ml/min for moxalactam, ceftazidime, and cefotaxime, respectively. The 24-h urinary recovery was highest for moxalactam (75 ± 4%) followed by ceftazidime (68 ± 11%) and cefotaxime (53 ± 6%). The influence of probenecid on serum concentrations, half-life, area under the curve, and clearance was most apparent with cefotaxime, whereas the pharmacokinetics of moxalactam and ceftazidime were only slightly affected. After the 0.5− and 2.0− g doses of cefotaxime, desacetyl-cefotaxime activity (determined by high-pressure liquid chromatography) reached a peak of 2.7 and 9.9 μg/ml and declined with a half-life of 1.9 and 1.4 h. The ratio of the R(−) and S(−) epimers of moxalactam, which could be differentiated by high-pressure liquid chromatography, fell rapidly from 0.81 at 0.17 h to 0.5 at 5 h, indicating the presence of twice as much of the microbiologically less active S(−) epimer. From a pharmacokinetic standpoint it appears reasonable to conclude that moxalactam and possibly ceftazidime could be administered twice daily and that cefotaxime could be administered three or even four times daily.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Aswapokee P., Ho I., Matthijssen C., Neu H. C. Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother. 1979 Nov;16(5):592–597. doi: 10.1128/aac.16.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Crawford S. A., Alexander G. A. In vitro activities of moxalactam and cefotaxime against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Jun;17(6):937–942. doi: 10.1128/aac.17.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., Münch R., Blaser J., Bhend H., Siegenthaler W. Human pharmacology of cefotaxime (HR 756), a new cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):127–133. doi: 10.1128/aac.16.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. N., Romano J. M., Levison M. E. Pharmacology of a new 1-oxa-beta-lactam (LY127935) in normal volunteers. Antimicrob Agents Chemother. 1980 Feb;17(2):226–228. doi: 10.1128/aac.17.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., Holt A., Bywater M. J., Wise R., Logan M. N., Andrews J. M., Broughall J. M. Comparison of sensititre dried microtitration trays with a standard agar method for determination of minimum inhibitory concentrations of antimicrobial agents. Antimicrob Agents Chemother. 1980 Dec;18(6):844–852. doi: 10.1128/aac.18.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., White L. O., Holt H. A., Bahari D., Bywater M. J., Bax R. P. Human metabolism of cefotaxime. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):93–101. doi: 10.1093/jac/6.suppl_a.93. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Bedford K. A. Comparison of in vitro activity of GR 20263, a novel cephalosporin derivative, with activities of other beta-lactam compounds. Antimicrob Agents Chemother. 1980 May;17(5):884–889. doi: 10.1128/aac.17.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S., Livingston R. Comparison of cefotaxime and moxalactam pharmacokinetics and tissue levels. Antimicrob Agents Chemother. 1980 Sep;18(3):369–371. doi: 10.1128/aac.18.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S., Wright N., Livingston R. The pharmacokinetics of LY127935, a broad spectrum oxa-beta-lactam. J Antimicrob Chemother. 1980 May;6(3):319–322. doi: 10.1093/jac/6.3.319. [DOI] [PubMed] [Google Scholar]
- Wise R., Wills P. J., Bedford K. A. Epimers of moxalactam: in vitro comparison of activity and stability. Antimicrob Agents Chemother. 1981 Jul;20(1):30–32. doi: 10.1128/aac.20.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Wright N., Wills P. J. Pharmacology of cefotaxime and its desacetyl metabolite in renal and hepatic disease. Antimicrob Agents Chemother. 1981 Apr;19(4):526–531. doi: 10.1128/aac.19.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Ichihashi T., Hirano K., Kinoshita H. Plasma protein binding and urinary excretion of R- and S-epimers of an arylmalonylamino 1-oxacephem. I: In humans. J Pharm Sci. 1981 Jan;70(1):112–113. doi: 10.1002/jps.2600700130. [DOI] [PubMed] [Google Scholar]


