Abstract
Bartonella are the only bacteria known to induce angioproliferative lesions of the human vasculature and liver during infection. Previous work from our lab suggests that GroEL participates in the mitogenic response observed in HUVEC cultures supplemented with the soluble fraction of Bartonella bacilliformis. Work in this study shows that exposure to high concentrations of the fraction is actually cytotoxic for HUVECs. To analyze this phenomenon, live B. bacillformis - HUVEC co-cultures were employed to study the effect of excess bacterial GroEL on the host cell during active infection. Four B. bacilliformis strains were generated to produce varying levels of GroEL. HUVEC co-cultures with LSS100, a strain that synthesizes markedly greater quantities of GroEL relative to others, significantly accelerates apoptosis of the co-cultured HUVECs relative to other strains. Acceleration of apoptosis can be inhibited by Z-VAD-FMK, a pan-caspase inhibitor. Time course data show that at 18 h of infection, both LSS100 and control strains significantly inhibit spontaneous apoptosis of co-cultured HUVECs, as previously reported for other Bartonella species. However, by 48 h LSS100 significantly increases apoptosis of the host cell. We hypothesize that intracellular Bartonella GroEL functions as an HSP60 analog, a eukaryotic orthologue known to accelerate procaspase 3 activation by enhancing its vulnerability to upstream activator caspases. These data suggest another strategy whereby Bartonella may regulate host cell growth.
INTRODUCTION
A frequent manifestation of bartonellosis in humans is the production of angioproliferative lesions of the skin (bacillary angiomatosis and verruga peruana) and liver (bacillary hepatis peliosis) (13). Such vascular lesions can be life-threatening to the patient, and data suggest they are induced by a consortium of stimuli, including Bartonella-derived mitogens for endothelial cells (5, 9, 15), inhibition of endothelial cell apoptosis by the bacterium (12, 21), plus induction of host cell cytokines, growth factors and a pro-inflammatory response (7, 11, 17).
Recent data from our laboratory suggest that GroEL participates in the increased in vitro proliferation of human umbilical vein endothelial cells (HUVECs) exposed to culture supplements consisting of the soluble fraction of Bartonella bacilliformis (16). Experiments showed that: 1) final HUVEC numbers correlated with GroEL levels in the culture supplement, 2) mitogenicity of the fraction can be increased if the Bartonella source culture is subjected to temperature upshift, and 3) mitogenicity of the fraction can be inhibited if it is pre-treated with anti- B. bacilliformis GroEL antiserum. Whether GroEL is a mitogen for HUVECs or serves as a chaperonin to the actual Bartonella mitogen(s) (or both) is unknown. Interestingly, B. bacilliformis GroEL was found to be actively secreted, and the chaperonin was detectable in both soluble and inner/outer membrane fractions of the bacterium (16).
In addition to serving as major antigens during bacterial infection (28), GroEL homologues from other pathogens have been reported to alter the growth rates of cultured human cells. For example, Chlamydia pneumoniae GroEL is mitogenic for human vascular smooth muscle cells in culture (19). Actinobacillus actinomyctemcomitans GroEL is mitogenic for cultured epithelial cells at low concentration but is cytotoxic at higher concentrations or upon prolonged exposure (10, 26, 27).
The purpose of this study was to examine the effect of B. bacilliformis on HUVEC co-cultures and to study the consequences of excess bacterial GroEL during an active infection in vitro.
MATERIALS AND METHODS
Bacterial strains, cell lines and tissue culture
Bacterial strains used in this study are listed in Table 1. B. bacilliformis was cultivated on heart infusion agar/blood plates (HIAB) as previously described (16). E. coli was grown overnight at 37° C in Luria Bertani medium supplemented with appropriate antibiotics as needed. Human umbilical vein endothelial cells (HUVECs) were purchased and routinely cultured in EGM at 37° C, 5% CO2 and 100% relative humidity, per the supplier’s instructions (Clonetics, Walkersville, Md.). HUVEC use was restricted to tissue culture passages 1–4 to minimize variability between experiments.
Table 1.
Bacterial strains and plasmids used in this study
| Strain/Plasmid | Relevant Genotype/Description | Source/Reference |
|---|---|---|
| B. bacilliformis | ||
| KC583 | Neotype strain | ATCC (3) |
| JB584 | Transformation-competent strain | (2) |
| LSS100 | JB584 containing pGRO1 | (16) |
| LSS001 | JB584 containing pBBR1-MCS2 | (16) |
| LSS200 | JB584 containing pGRO1Δ | This study |
| E. coli | ||
| DH5α | Host for plasmid propagation | Gibco-BRL, Grand Island, N.Y. |
| Plasmids | ||
| pBBR1MCS-2 | B. bacilliformis shuttle vector, Kmr | (2, 14) |
| pGRO1 | pBBR1MCS-2 with cloned B. bacilliformis groESL operon | (16) |
| pGRO1Δ | pGRO1 with 1002-bp deletion in groEL and four intervening XhoI linkers | This study |
B. bacilliformis - HUVEC tissue co-cultures
The effect of B. bacilliformis infection and over-expression of GroEL on HUVEC growth and morphology was examined in co-cultures. Gelatin-coated, 96-well plates containing EGM were seeded with ~2000 HUVECs per well, and plates were incubated for 4 hours to allow for adherence. Cultures were infected by adding 1.6 x 108 bacteria suspended in EGM to each well and incubating for three hours. Unbound bacteria were subsequently removed by thoroughly rinsing three times with M199 containing 20% fetal calf serum (M199-FCS). The wells were brought to a final volume of 100 μl M199-FCS. HUVECs were cultured and assayed for cell numbers at 96 h post-infection. Digital micrographs were obtained by staining cells with Hema-Quik Stain (Fisher Scientific, Pittsburgh, Penn.) and visualizing with a Zeiss Axioskop photomicroscope (Zeiss, Thornwood, Calif.) interfaced with Image-Pro Plus 3.0 software (Media Cybernetics, Silver Spring, Md.).
Growth and apoptosis assays
B. bacilliformis cell lysates were prepared by harvesting bacteria into ice-cold phosphate-buffered saline (PBS; pH 7.4) containing 0.1 mm glass beads and lysing for 3 min with a Fastprep FP120 disruptor (Thermo Savant, Holbrook, N.Y.). Particulates were removed from the lysate by centrifuging at 16,000 x g for 5 min followed by ultracentrifugation at 100,000 x g for 60 min. HUVECs were typically seeded at 1,000 cells per well in EGM or M199-FCS media with lysate supplements of 0 – 300 μg protein /ml. Heat-treated lysates were prepared by treating for 7 min at 100° C. HUVEC numbers were measured using an acid phosphatase assay (6) calibrated with linear standard curves of enzyme activity as a function of viable, adherent HUVEC numbers (0–36,000 cells by hemocytometer) as previously described (16). Standard curves for the assays encompassed all OD405 values reported in this study.
Apoptosis was examined using 96-h co-cultures infected with B. bacilliformis LSS001 or LSS100, in the presence or absence of the pan-caspase inhibitor, Z-VAD-FMK, at a final concentration of 50 μM. Negative control cultures were treated with equal volumes of solvent (100% ethanol) minus the inhibitor. Briefly, HUVECS were incubated for 3 h at 37° C with B. bacilliformis and 50 μM Z-VAD-FMK. Co-cultures were subsequently rinsed three times with M199-FCS and cultured an additional 96 h in M199-FCS containing Z-VAD-FMK (50 μM). Final HUVEC numbers were quantified using an acid phosphatase assay as described above. Apoptosis was also quantified by assaying DNA fragmentation in B. bacilliformis/HUVEC co-cultures incubated for 18 h, 48 h and 96 h post-infection using a Cell Death Detection ELISA per the manufacturer’s instructions (Roche Applied Science, Indianapolis, Ind.). Supernatants were also assayed to assess the extent of secondary necrosis in the cultures.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis
Protein concentrations of samples were determined with a BCA assay kit (Pierce, Rockford, Il.) and a bovine serum albumin standard. SDS-PAGE was done by standard protocol (1) using protein samples solubilized in Laemmli sample buffer and polyacrylamide gels (12.5% acrylamide; w/v). SDS-PAGE gels were fixed and stained with Coomassie brilliant blue by standard protocol (1) or were immediately transferred to nitrocellulose (0.45 μm pore size; Osmonics, Minnetonka, Minn.) for immunoblot analysis (22). Immunoblots were developed as previously described (16), using rabbit anti-B. bacilliformis GroEL antiserum (1:1000 dilution), horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2000 dilution) (Sigma Chemical, St. Louis, Mo.), H202 as a substrate and 4-chloronapthol as the chromogen. Images were digitized with a Scanmaker 9800XL and ScanWizard Pro software (Microtek, Carson, Calif.).
DNA cloning, manipulation and gene expression
Plasmids used in this study are listed in Table 1. The pGRO1 plasmid contains a functional B. bacilliformis groESL operon (16) and was mutagenized by digestion with PmlI to remove an internal 1002-bp fragment of groEL. The resulting DNA was purified from crystal violet-stained agarose gels (1% agarose; w/v) with a GeneClean II kit (QBiogene, Vista, Calif.), and then ligated to 10-mer XhoI linkers (Promega, Madison, Wisc.) using T4 DNA ligase and standard protocol (1). The resulting plasmid, pGRO1Δ, was sequenced using an ABI 3100 automated sequencer (Applied Biosystems, Foster City, Calif.) as previously described (4) and was found to contain an intact groES gene, 36 residual bases at the 5′ end of groEL, a 1002-bp deletion and four copies of the XhoI linker, resulting in a nonsense mutation immediately downstream. Transformation of B. bacilliformis was done by electroporation as previously described (2).
Graphical and statistical analyses
Graphs were generated with Sigmaplot 8.0 software (SPSS, Chicago, Il.). Statistical significance was determined by a Student’s t test or one way analysis of variance using Instat 3.0 software (GraphPad, San Diego, Calif.). A P value of <0.05 was considered significant. Mean values ± standard deviation (SD) are reported in this study.
RESULTS AND DISCUSSION
In a previous study, we discovered that HUVEC numbers in 96-h cultures correlated with the quantity of B. bacilliformis lysate initially added to the tissue culture, with maximal numbers resulting from a supplement of 10 μg lysate protein/ml medium (16). However, assays in this study did not examine lysate doses greater than 50 μg/ml. In the same study, we found that B. henselae cell lysates possessed significantly less mitogenicity for HUVECs than B. bacilliformis lysates at a 10 μg/ml dose (16). Subsequent studies with B. henselae lysates actually showed a dose-dependent inhibition of HUVEC growth in response to the supplement (Smitherman and Minnick, unpublished data). To determine if B. bacilliformis lysates are also inhibitory at higher doses, we assayed HUVEC growth in EGM medium (Clonetics) supplemented with a broad range of lysate concentrations. As before, resulting data show that a B. bacilliformis lysate is significantly mitogenic (p ≤ 0.001) at doses ranging from 1–10 μg lysate/ml culture, resulting in a 2- to 3-fold increase in HUVEC numbers relative to heat-treated controls. A dosage of 100 μg/ml was neither mitogenic nor inhibitory for HUVECs, and treatment with heat-treated or raw lysates at this concentration produced indistinguishable results. Further analysis showed that the lysate significantly inhibited (p ≤ 0.006) HUVEC growth when used at 200 and 300 μg/ml doses, with 83% and 92% reductions relative to their respective heat-treated controls. Data also suggest that a heat-resistant mitogen, perhaps LPS, may be active at very high doses (200–300 μg/ml; see Fig. 1).
FIG. 1.
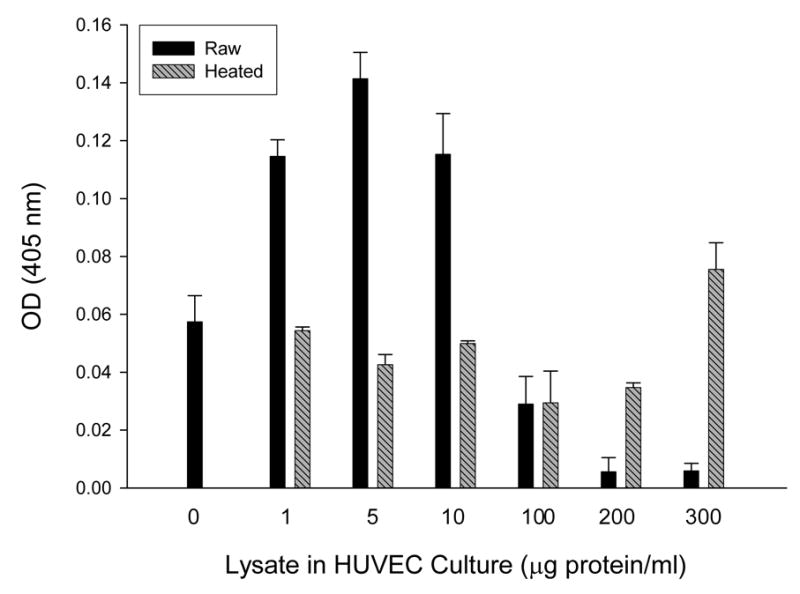
Dose-response of HUVECs cultured 96 h in EGM supplemented with a range of B. bacilliformis KC583 cell lysate concentrations as indicated, showing mitogenic activity at 1–10 μg/ml and inhibition of growth at 100–300 μg/ml dosages. Data represent the average of three experiments ± SD. (Raw, raw lysate; heated, lysate pretreated at 100° C for 7 min).
Since the results in Fig. 1 were similar to those observed with A. actinomyctemcomitans, a bacterium whose GroEL is mitogenic for cultured epithelial cells at low doses but cytotoxic at higher doses (10, 26), we explored the possibility that B. bacilliformis GroEL might be involved in inhibiting HUVEC growth at the higher lysate dosages. To that end, we generated four B. bacilliformis strains that produce different levels of GroEL. JB584 was chosen as the parental strain because of its amenability to transformation and genetic manipulation (2), and it was electroporated with pBBR1MCS-2 shuttle vector to produce a negative control strain, designated LSS001. JB584 was also transformed with pGRO1, a high copy-number plasmid containing the B. bacilliformis groESL operon, to generate strain LSS100. In order to examine the respective effect of GroES and GroEL on HUVEC growth, the groEL gene of pGRO1 was deleted to create pGRO1Δ, a construct transformed into JB584 to produce strain LSS200. SDS-PAGE analysis of the total protein profiles of the four resulting strains grown on HIAB plates, plus the corresponding immunoblot developed with rabbit anti-B. bacilliformis GroEL are shown in Fig. 2. These data clearly show that B. bacilliformis LSS100 produces substantially more GroEL in vitro, and densitometry analysis of immunoblots containing total protein profiles (Fig 2B) reveals approximately a two-fold increase in the protein relative to the other three strains.
FIG. 2.
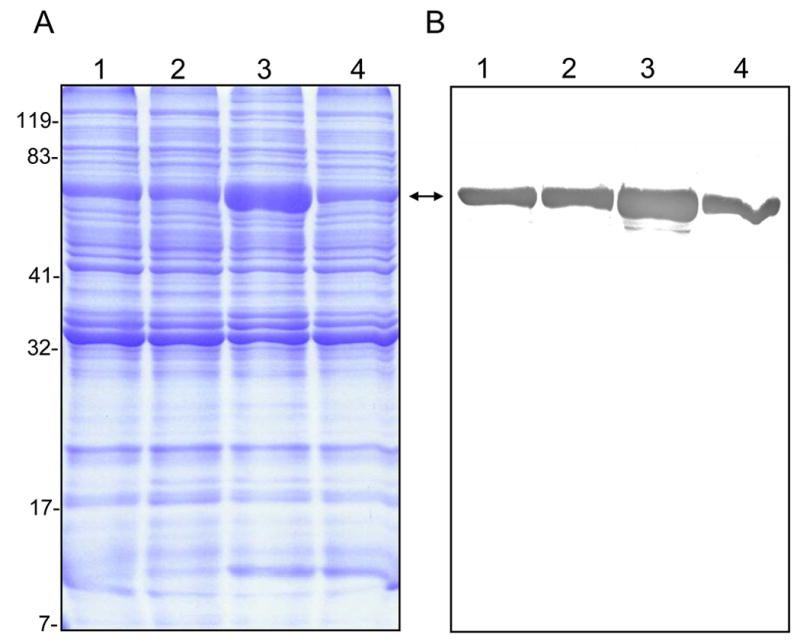
Differential GroEL synthesis in four B. bacilliformis strains. A) Coomassie blue-stained SDS-PAGE analysis of the total protein profiles for strains JB584, LSS001, LSS100, and LSS200 (lanes 1–4, respectively), and B) the corresponding immunoblot developed with rabbit anti-B. bacilliformis GroEL antiserum. All lanes contain 30 μg total protein. GroEL is indicated by a double arrow. Molecular mass values from protein standards are given to the left in kDa.
The effect of these four B. bacilliformis strains on the growth of HUVECs in co-culture was examined at 96 h post-infection. Data show a significant increase in cell numbers for HUVECs co-cultured with strains JB584 (31% increase; p<0.0001) or LSS001 (34% increase; p<0.0001) compared to uninfected controls (Fig. 3). However, strain LSS100, producing roughly twice the amount of GroEL as compared to the other strains (Fig. 2), causes a marked and significant reduction in HUVEC numbers (79% less than JB584-infected co-cultures and 72% less than uninfected HUVECs; p<0.0001 for both) (Fig. 3).
FIG. 3.
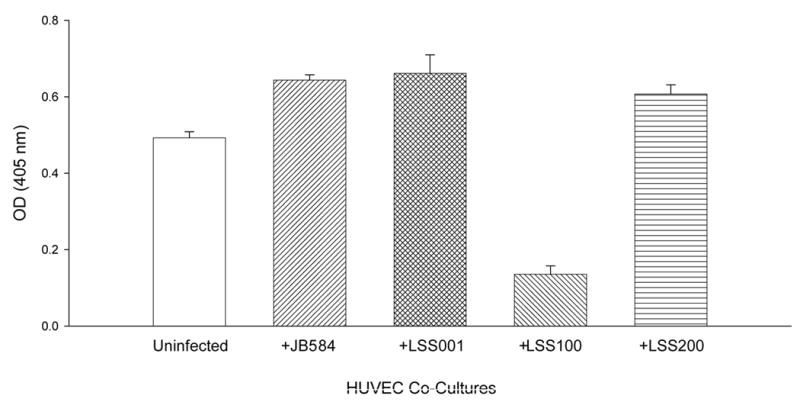
Number of HUVECs in M199-FCS medium following a 96-h co-culture with B. bacilliformis strains JB584, LSS001, LSS100 or LSS200. Data represent the average of six experiments ± SD.
To ensure that GroEL levels in LSS100 were also elevated during tissue culture infections, large-scale HUVEC co-cultures (5 ml) were prepared and harvested at 96-hours. The total protein profiles (HUVEC plus Bartonella) were analyzed on immunoblots probed with anti-B. bacilliformis GroEL antiserum. These data show that GroEL cannot be detected in uninfected HUVECs and is markedly greater in the LSS100 infection as compared to co-cultures with the other Bartonella strains (e.g., a four-fold increase over the JB584-infected co-cultures by densitometry) (Fig. 4). Taken as a whole, the data suggest that the decrease in HUVECs observed in the LSS100-infected co-cultures is due to an elevated quantity of GroEL, since infection with LSS200 (containing a deletion mutation in the cloned groEL and otherwise isogenic) abrogates the inhibitory effect on growth and restores cell numbers to levels that are similar to JB584 or LSS001 co-cultures. In fact, differences between HUVEC co-cultures infected with JB584 and LSS001, or LSS200 (Fig. 3) are not significantly different from one another (p> 0.05). The data also suggest that GroES is not responsible for the inhibitory effect seen in a LSS100 infection, as both the groES gene and the groESL operon promoter are intact in the pGRO1Δ plasmid harbored by LSS200 (Fig. 3). Nevertheless, these data do not rule out the possibility that a GroES-GroEL complex could be involved in the activity.
FIG. 4.
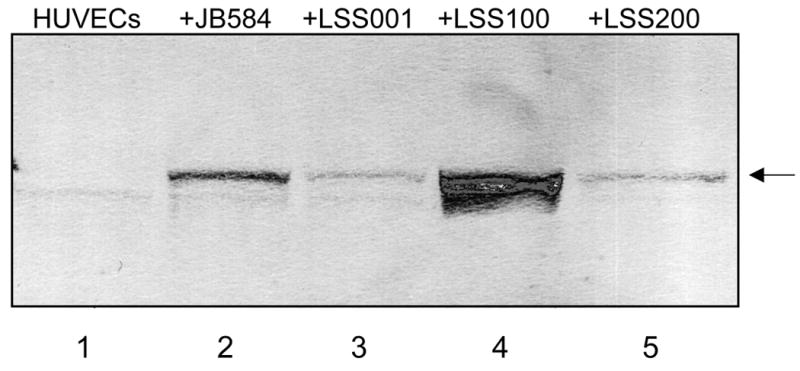
Immunoblot showing GroEL levels in infected HUVECs cultivated in M199-FCS, following a 96-h co-culture with the indicated B. bacilliformis strains. Lane 1, uninfected HUVECs (no detectable GroEL); Lanes 2–5, HUVECs infected with JB584, LSS001, LSS100, and LSS200, respectively. The GroEL protein is arrowed.
The morphology of the 96-h infected HUVEC co-cultures was subsequently examined by microscopy following fixing and staining with Hema-Quik (Fisher Scientific). HUVECs infected with JB584, LSS001 or LSS200 were indistinguishable from one another by visual inspection of their gross cellular morphology (Fig. 5), although infected cells were for the most part more vacuolated and granulated than uninfected HUVECs. Similar changes in morphology have been previously reported for HUVECs infected with B. henselae and B. quintana (5) or B. bacilliformis (8, 23). Unlike the other co-cultures, HUVECs infected with LSS100 consistently showed cell shrinkage, chromatin condensation and membrane blebbing, all characteristics of cells undergoing apoptosis (Fig. 5), and DAPI and TUNEL staining qualitatively supported this observation (not shown).
FIG. 5.
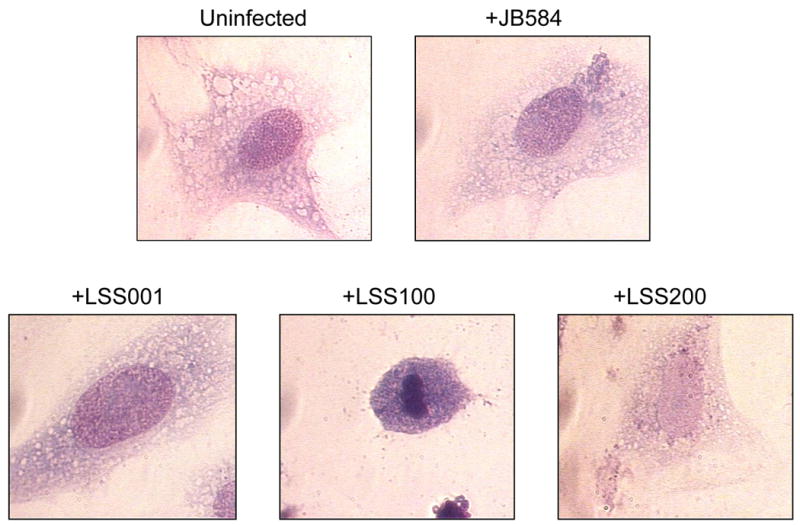
Micrographs showing representative cell morphologies of infected HUVECs following a 96-h co-culture with four B. bacilliformis strains including JB584, LSS001, LSS100 and LSS200. An uninfected HUVEC is provided for comparison. Cells were visualized using Hema-Quik Stain (Fisher Scientific) and are shown at 1000x magnification. Note the marked apoptosis in +LSS100 cells.
To investigate whether apoptosis was occurring in response to the elevated intracellular GroEL levels, HUVEC co-cultures infected with LSS001 or LSS100 were incubated for 96 h in the presence of the pan-caspase inhibitor, Z-VAD-FMK, or an equal volume of its respective solvent (100% ethanol) as a negative control (Fig. 6). Results show that infection with LSS001 significantly increases HUVEC numbers relative to the respective uninfected control cultures incubated with ethanol (14.9% increase) or Z-VAD-FMK (30.4% increase) (p <0.0001 for both). However, infection with LSS100 in the presence of ethanol results in a marked and significant decrease (84.9%; p < 0.0001) relative to the uninfected control. Overall, these results are similar to those observed in the absence of ethanol (Fig. 3). When Z-VAD-FMK is included in the LSS001 co-cultures, HUVEC numbers increase slightly but significantly (8.1%; p<0.008) relative to the respective negative control, likely reflecting inhibition of spontaneous apoptosis. Perhaps most notable was the marked and significant increase (549%; p <0.0001) in HUVEC numbers in the LSS100 co-cultures treated with Z-VAD-FMK relative to the ethanol control. In fact, the inhibitor restored HUVEC numbers in these particular co-cultures to levels similar to what is observed in uninfected host cells. Taken together, data in Fig. 6 suggest that the LSS100 strain, with its overproduction of GroEL during infection of the cultured host cells, can trigger HUVEC apoptosis by 96 h post-infection, and that the process can be inhibited by treatment with Z-VAD-FMK.
FIG. 6.
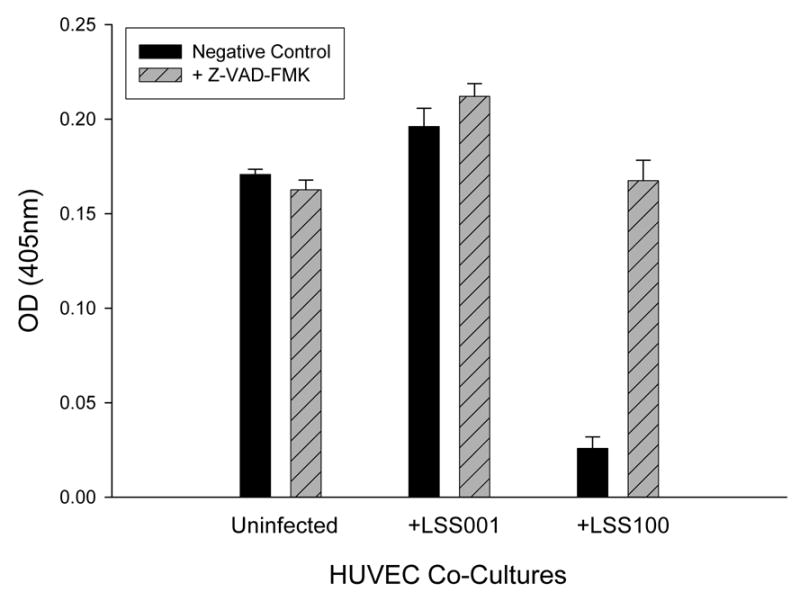
Z-VAD-FMK inhibits the reduction of HUVEC numbers observed with a B. bacilliformis LSS100 co-culture. HUVEC numbers at 96 h of co-culture in M199-FCS medium plus the indicated B. bacilliformis strain together with Z-VAD-FMK (50 μM) or an equal volume of solvent (100% ethanol) as a negative control are shown. Data represent the average of six experiments ± SD.
To unequivocally demonstrate HUVEC apoptosis and to examine the process over time, we measured histone-associated DNA fragments from LSS001- and LSS100- HUVEC co-cultures by assaying with an ELISA at three post-infection time points (Fig. 7). At 18 h, both LSS001 and LSS100 co-cultures significantly suppress spontaneous host cell apoptosis (39% and 32% of the apoptosis observed in uninfected HUVECs; p< 0.0001), and LSS100 causes a slight but significantly greater suppression of apoptosis than LSS001 (p = 0.011). To our knowledge, these are the first data showing that B. bacilliformis can interfere with spontaneous apoptosis of HUVECs, as previously reported for B. henselae–HUVEC co-cultures (12, 20). As the Bartonella infection proceeds, the LSS100 co-cultures show a significant increase in HUVEC apoptosis at 48 h relative to uninfected HUVECs (139% increase; p=0.0032) and over 200% greater apoptosis than co-cultures infected with LSS001 (p = 0.0003) . In marked contrast, LSS001 co-cultures maintained a significant inhibition of spontaneous apoptosis (~67% of what is observed in uninfected cells; p=0.0004). It is interesting to note that although the LSS001 strain continues to inhibit spontaneous apoptosis of HUVECs at 48 h, the level of inhibition is roughly half that observed in the 18 h co-cultures (Fig. 7). This last observation may be due to an accumulation of GroEL in the cell as the bacterium replicates over time. Assays on culture supernatants from both 18- and 48-h infections contained only trace amounts (<0.07 OD405 units) of fragmented DNA (not shown), suggesting that secondary necrosis was not a major factor at these timepoints. At 96 h, both strains enhance apoptosis as compared to uninfected HUVECs, and culture supernatants suggest that secondary necrosis occurs in both the uninfected cells and those co-cultured with LSS100. On the contrary, supernatants from LSS001 co-cultures showed only trace amounts of DNA, suggesting that secondary necrosis is not occurring (not shown). Taken as a whole, the data suggest that: a) both strains inhibit host cell apoptosis within the first 18 h of infection, b) that overproduction of GroEL by LSS100 markedly enhances apoptosis by 48 h and c) by 96 h both strains enhance HUVEC apoptosis.
FIG. 7.
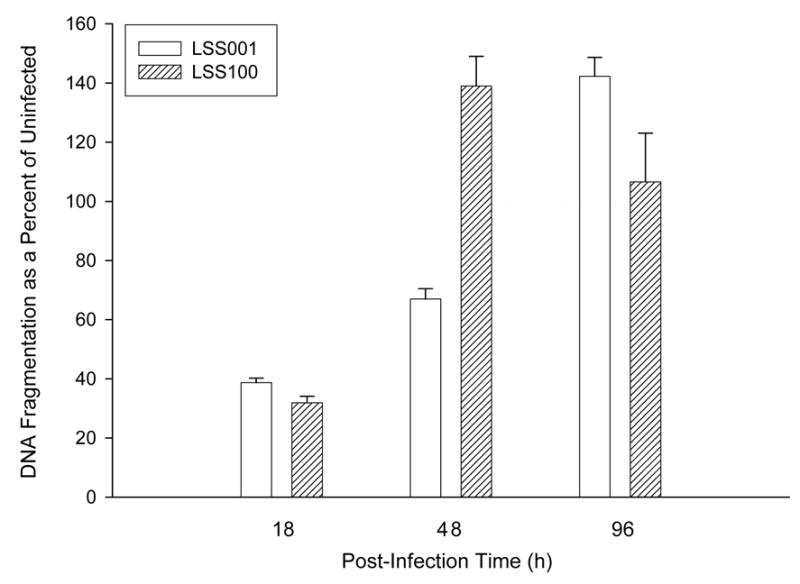
HUVEC DNA fragmentation during a B. bacilliformis LSS001 and LSS100 co-culture as determined by ELISA (Roche). Results from co-cultured HUVECs at 18 h, 48 h and 96 h are shown as a percentage of uninfected HUVECs, with the 100% line outlined. Data represent the average of three experiments ± SD (3 independent determinations were done per experiment).
Previous work has shown that the type IV secretion system (VirB) of B. henselae is essential to rearrangement of the host cell cytoskeleton during infection, activation of a pro-inflammatory response via NF-κB, inhibition of HUVEC apoptosis and mediation of a cytotoxic/cytostatic effect when HUVECs are co-cultured with bacteria at high titer (20). Interestingly, VirB is not involved in Bartonella mitogenicity for HUVECs. Based upon our results, it is tempting to speculate that accumulation of secreted GroEL during the high titer B. henselae infection may have contributed to the observed HUVEC cytotoxicity in that study. Whether Bartonella GroEL is actually secreted by VirB is unknown, but recent data showing that GroEL is a substrate for the type IV secretion system in Brucella, a closely-related pathogen, suggests that it is at least a possibility (24).
Hsp60, a eukaryotic analog of GroEL released by the mitochondria, has been shown to accelerate pro-caspase 3 activation by enhancing its vulnerability to upstream activator caspases. As such, Hsp60 constitutes an important regulator of apoptotic cell death (18, 25). Hsp60 activation of caspase 3 is apparently dependent upon an excess (16-fold) of the chaperonin, whereas equimolar amounts of Hsp60 are inefficient at stimulating caspase 3 maturation. Based upon these observations and results of this study, we hypothesize that intracellular infection of HUVECs by Bartonella is accompanied by release of GroEL and that the protein eventually accumulates in the cytosol to a threshold level that stimulates caspase 3 maturation and apoptosis. Normally, this process would require considerable time and increase in bacterial load as infection progresses. In contrast, infection by a GroEL-overproducing strain, like LSS100, dramatically increases the kinetics of this process and the resulting apoptosis.
To reconcile the different activities we have ascribed to GroEL, it is important to note that the previous study which implicates the protein as a participant in HUVEC mitogenicity was done using cell cultures exposed to soluble fractions of B. bacilliformis (16), whereas the current study examines the effects of GroEL synthesis by the pathogen during active infection. Undoubtedly, HUVEC surface receptors for GroEL are different from those located within the confines of the cell and may contribute to the different effects observed. Previous studies with Chlamydia pneumoniae have shown that GroEL binds the TLR4 of human vascular smooth muscle cell and is a potent mitogen (19). In eukaryotes, extracellular Hsp60 binds to TLR-2, TLR-4, CD91 and CD36 (21) and it is conceivable that extracellular binding of Bartonella GroEL to these or other receptors might enhance endothelial cell proliferation. Alternatively or in addition, GroEL could serve as a chaperonin which protects or maintains proper folding of some uncharacterized bacterial mitogen(s) in the cell lysate. We do not believe the mitogenic /extracellular (16) and the apoptotic/intracellular effects (this study) are mutually exclusive. It is even conceivable that accumulation of Bartonella GroEL during an intracellular infection and subsequent induction of apoptosis could provide a host cell escape route for the bacterium. Regardless, our data suggest that GroEL released by Bartonella during intracellular infection may represent another strategy for regulating host cell growth.
Acknowledgments
This work was supported by Public Health Service Grant AI52101 to MFM, who was also supported by Public Health Service grant AI053111. Automated nucleotide sequencing was done at The Murdock Sequencing Facility at The University of Montana-Missoula. We are grateful to Dr. Sonja Best for helpful discussions and suggestions.
References
- 1.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. John Wiley & Sons, Inc; New York, N.Y: 1995. [Google Scholar]
- 2.Battisti JM, Minnick MF. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Eviron Microbiol. 1999;65:3441–3448. doi: 10.1128/aem.65.8.3441-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DJ, O'Connor SP, Hollis DG, Weaver RE, Steigerwalt AG. Molecular characterization and proposal of a neotype strain for Bartonella bacilliformis. J Clin Microbiol. 1991;29:1299–1302. doi: 10.1128/jcm.29.7.1299-1302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll JA, Coleman SA, Smitherman LS, Minnick MF. Hemin-binding surface protein from Bartonella quintana. Infect Immun. 2000;68:6750–6757. doi: 10.1128/iai.68.12.6750-6757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conley T, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 6.Connolly DT, Knight MB, Harakas NK, Wittwer AJ, Feder J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal Biochem. 1986;152:136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrman O, Arvand M, Göhler A, Schmid M, Krüll M, Hippenstiel S, Seybold J, Dehio C, Suttorp N. Bartonella henselae induces NF-kB-dependent upreguation of adhesion molecules in cultured human endothelial cells: possible role of outer membrane proteins as pathogenic factors. Infect Immun. 2001;69:5088–5097. doi: 10.1128/IAI.69.8.5088-5097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia FU, Wojta J, Hoover RL. Interactions between live Bartonella bacilliformis and endothelial cells. J Infect Dis. 1992;165:1138–1141. doi: 10.1093/infdis/165.6.1138. [DOI] [PubMed] [Google Scholar]
- 9.Garcia FU, Wojta J, Broadley KN, Davidson JM, Hoover RL. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol. 1990;136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 10.Goulhen F, Hafezi A, Uitto VJ, Hinode D, Nakamura R, Grenier D, Mayrand D. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect Immun. 1998;66:5307–5313. doi: 10.1128/iai.66.11.5307-5313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf VAJ, Volkmann B, Schaller M, Sander CA, Alitalo K, Rieβ T, Autenrieth IB. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 2001;3:623–632. doi: 10.1046/j.1462-5822.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 12.Kirby JE, Nekorchuk DM. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc Natl Acad Sci USA. 2002;99:4656–4661. doi: 10.1073/pnas.072292699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler JE. Bartonella infections. Adv Pediatric Infect Dis. 1996;11:1–27. [PubMed] [Google Scholar]
- 14.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. Four new derivatives of the broad-host-range vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 15.Maeno N, Oda H, Yoshiie K, Wahid MR, Fujimura T, Matayoshi S. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 16.Minnick MF, Smitherman LS, Samuels DS. Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun. 2003;71:6933–6942. doi: 10.1128/IAI.71.12.6933-6942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resto-Ruiz SI, Schmiederer M, Sweger D, Newton C, Klein TW, Friedman H, Anderson BE. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect Immun. 2002;70:4564–4570. doi: 10.1128/IAI.70.8.4564-4570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244–250. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 20.Schmid MC, Schulein R, Dehio M, Denecker G, Carena L, Dehio C. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol Microbiol. 2004;52:81–92. doi: 10.1111/j.1365-2958.2003.03964.x. [DOI] [PubMed] [Google Scholar]
- 21.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy a comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Davis GE, Ihler GM. Formation of stress fibres in human endothelial cells infected with Bartonella bacilliformis is associated with altered morphology, impaired migration and defects in cell morphogenesis. Cell Microbiol. 2001;3:169–180. doi: 10.1046/j.1462-5822.2001.00104.x. [DOI] [PubMed] [Google Scholar]
- 24.Watarai M, Kim S, Erdenebaatar J, Makino S, Horiuchi M, Shirahata T, Sakaguchi S, Katamine S. Cellular prion protein promotes Brucella infection into macrophages. J Exp Med. 2003;198:5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049–2056. doi: 10.1093/emboj/18.8.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Pelech SL, Mayrand D, Grenier D, Heino J, Uitto VJ. Bacterial heat shock protein-60 increases epithelial cell proliferation through the ERK1/2 MAP kinases. Exp Cell Res. 2001;266:11–20. doi: 10.1006/excr.2001.5199. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Pelech S, Uitto VJ. Long-term effect of heat shock protein 60 from Actinobacillus actinomycetemcomitans on epithelial cell viability and mitogen-activated protein kinases. Infect Immun. 2004;72:38–45. doi: 10.1128/IAI.72.1.38-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


