Abstract
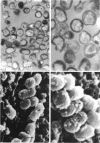
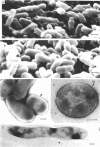
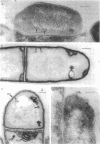
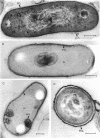
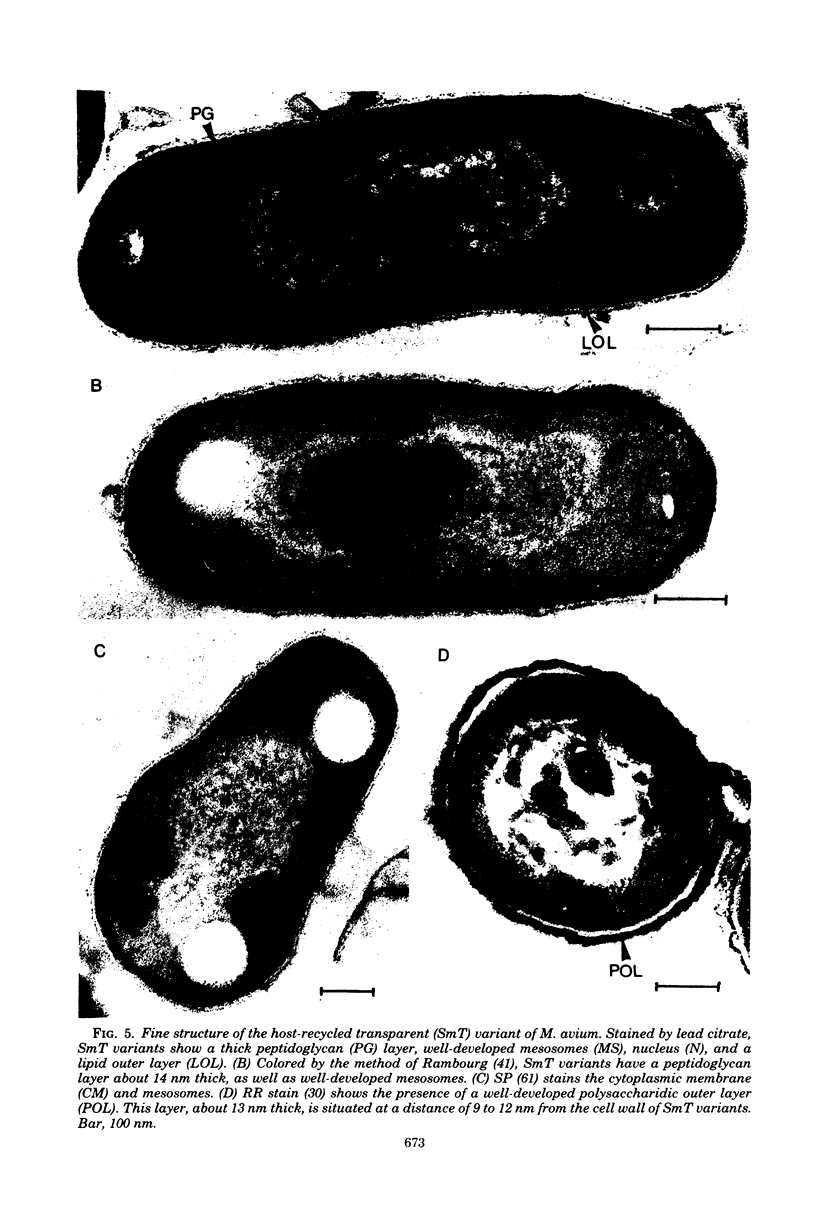
Whole cells of Mycobacterium avium, characterized by their negative response in the nine biochemical tests used for mycobacterial identification in our laboratory, turned positive for nitrate reductase, Tween-80 hydrolysis, beta-glucosidase, acid phosphatase, alkaline phosphatase, penicillinase, and trehalase after their wall portion was removed to yield spheroplasts. This suggested that the negative results in most of the biochemical procedures were caused by the exclusion mechanism at the wall level. Preliminary transmission and scanning electron microscopic studies showed differences at wall level between laboratory-maintained opaque, dome-shaped (SmD) and host-recycled smooth, transparent (SmT) colony type variants of M. avium and suggested the presence of an outer regularly structured layer in SmT variants. Comparative ultrastructural studies utilizing different polysaccharide coloration methods confirmed the presence of an outer polysaccharide layer in SmT variants which was probably related to their enhanced pathogenicity for experimental animals and drug resistance as compared to that of SmD variants. These findings are discussed with respect to multiple drug resistance, virulence, and gene expression of M. avium.
Full text
PDF


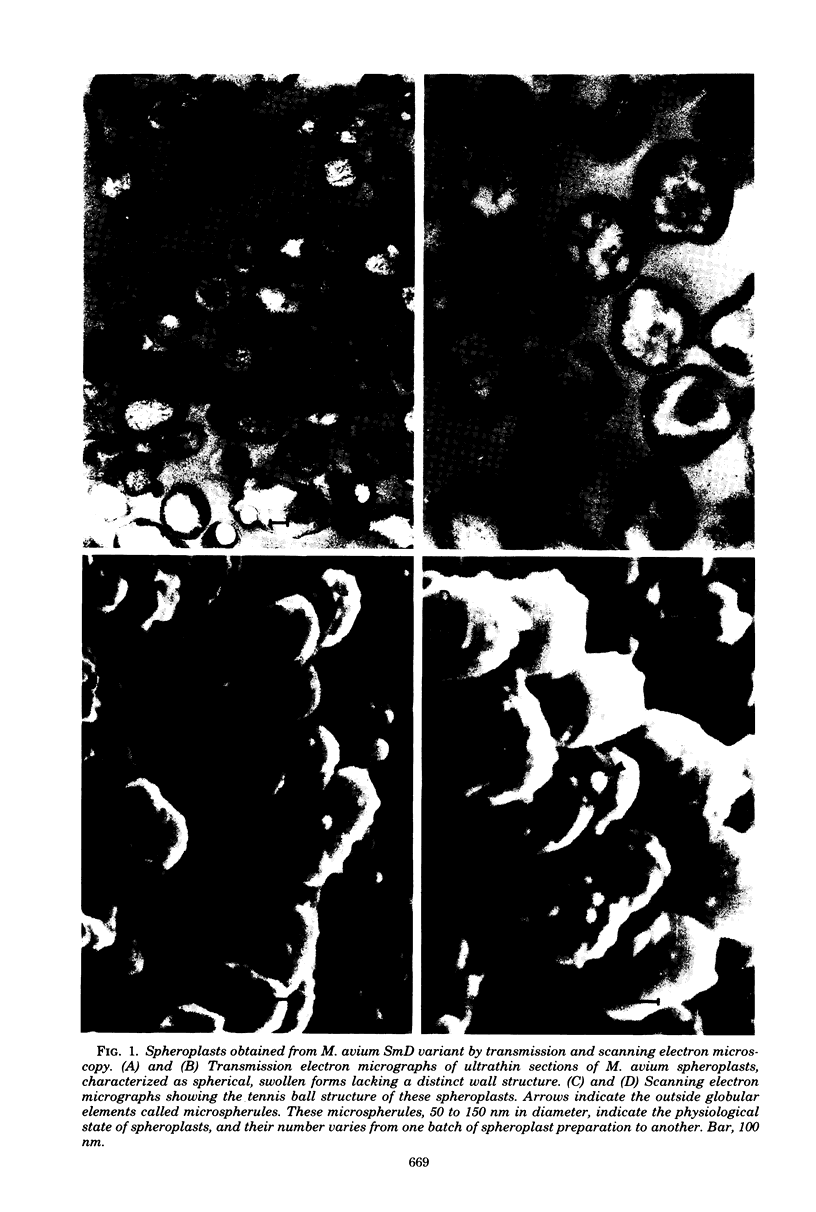
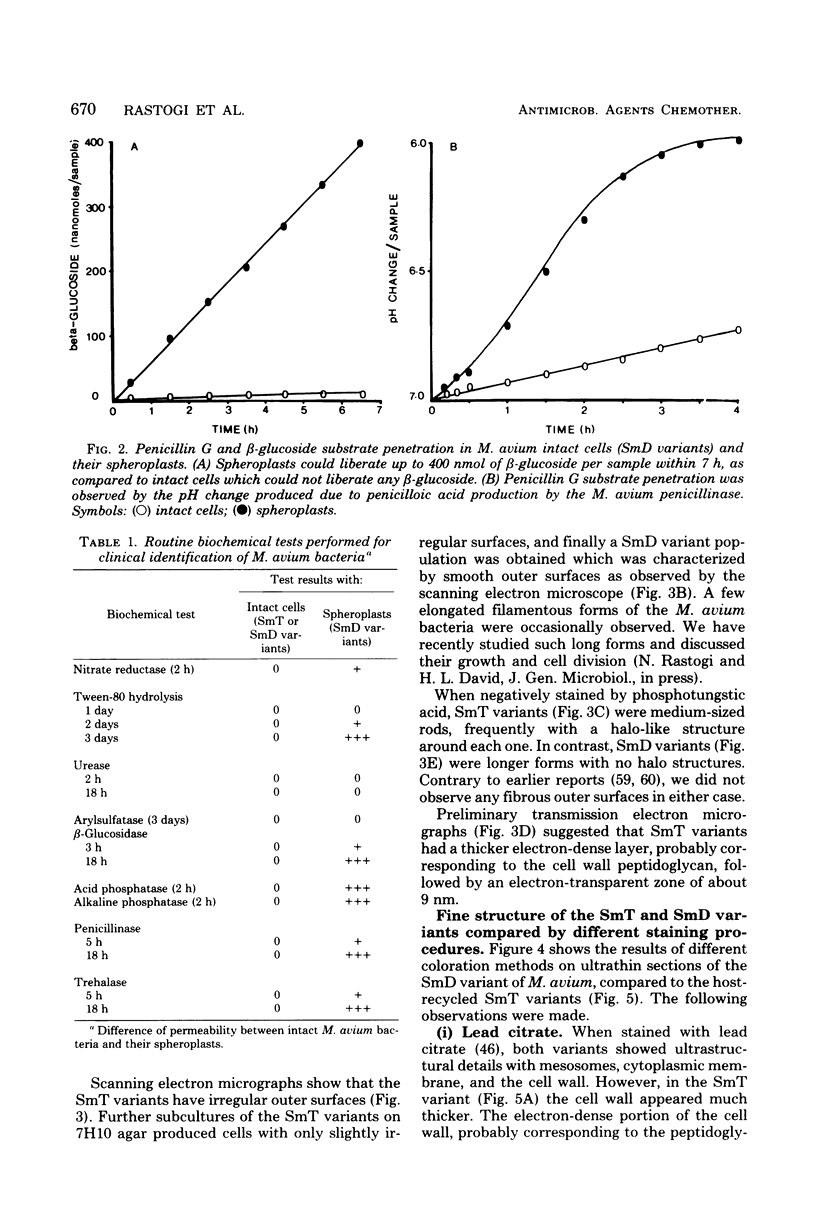
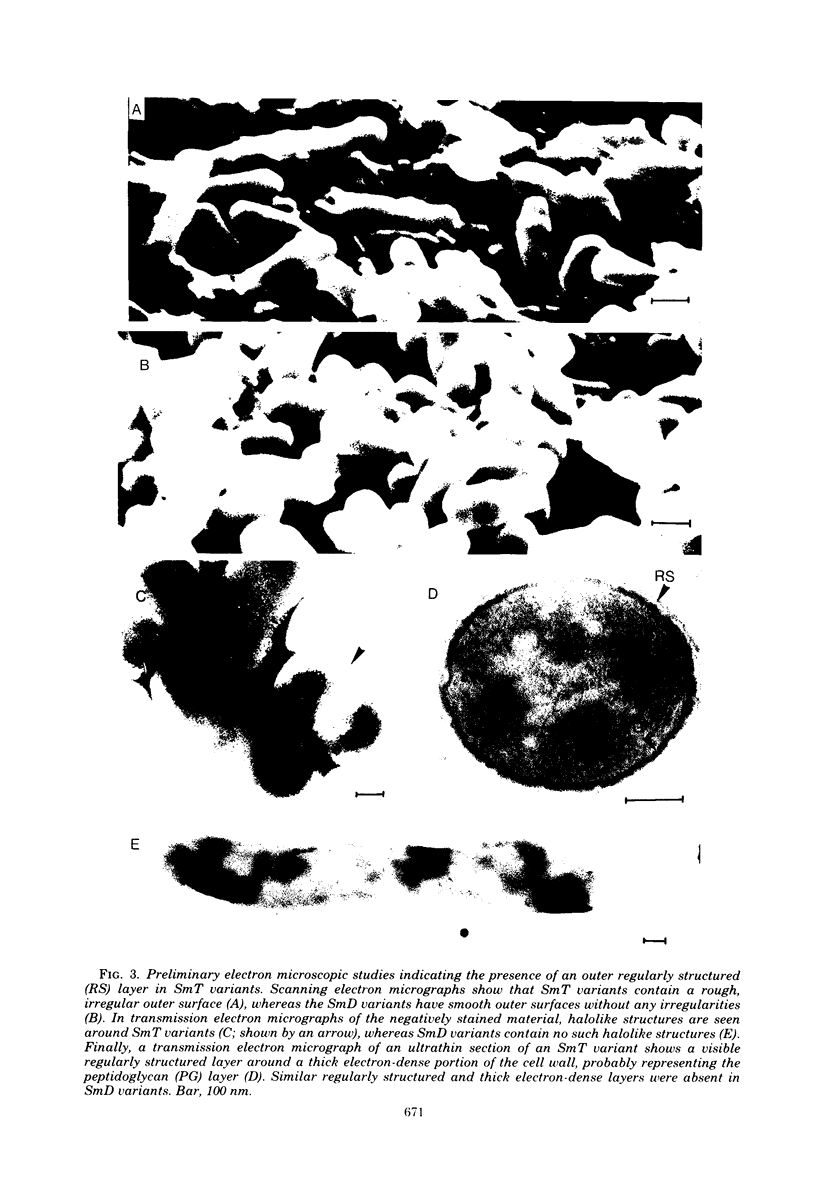
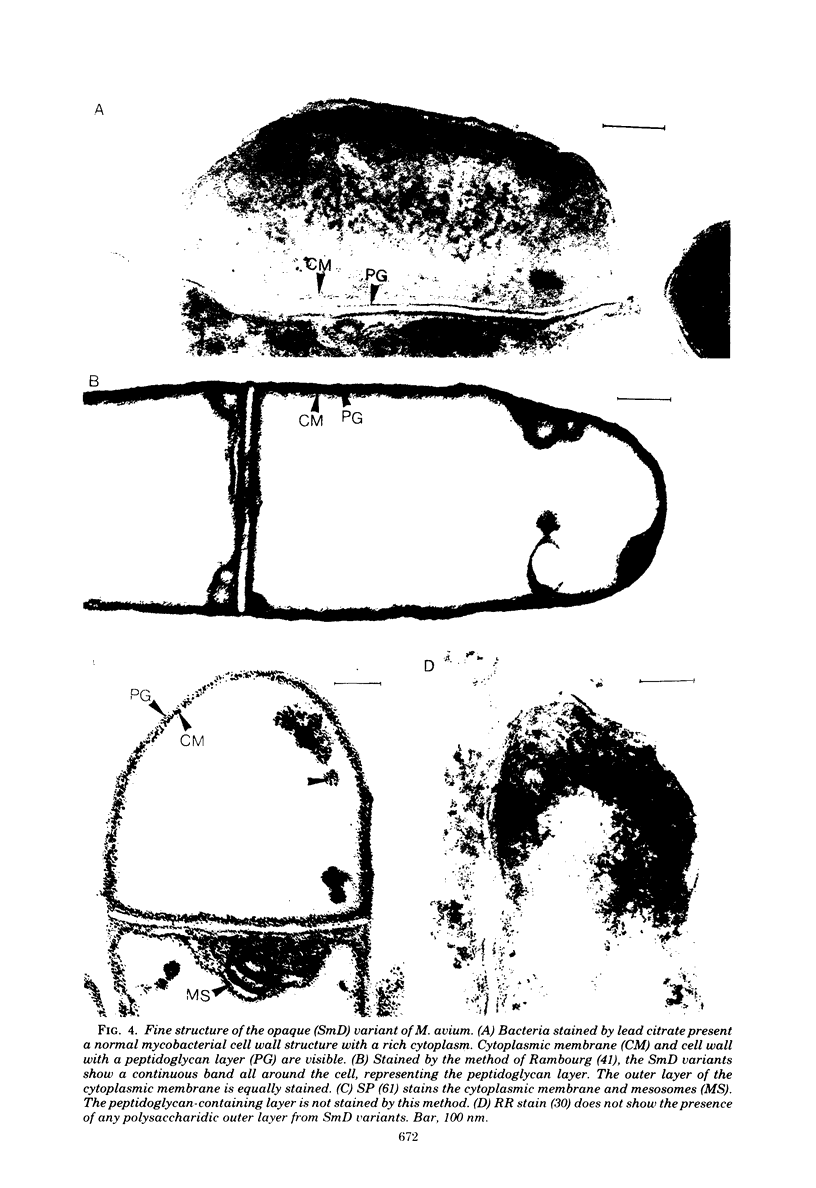




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adámek L., Mison P., Mohelská H., Trnka L. Ultrastructural organization of spheroplasts induced in Mycobacterium sp. smegmatis by lysozyme or glycine. Arch Mikrobiol. 1969;69(3):227–236. doi: 10.1007/BF00408975. [DOI] [PubMed] [Google Scholar]
- Asano A., Cohen N. S., Baker R. F., Brodie A. F. Orientation of the cell membrane in ghosts and electron transport particles of Mycobacterium phlei. J Biol Chem. 1973 May 25;248(10):3386–3397. [PubMed] [Google Scholar]
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. The response of cell walls of Bacillus subtilis to metals and to electron-microscopic stains. Can J Microbiol. 1978 Feb;24(2):89–104. doi: 10.1139/m78-018. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Isolation of plasmids from mycobacteria. Infect Immun. 1979 Jun;24(3):979–981. doi: 10.1128/iai.24.3.979-981.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Drug-resistance in M. tuberculosis and other mycobacteria. Clin Chest Med. 1980 May;1(2):227–230. [PubMed] [Google Scholar]
- David H. L., Jahan M. T. beta-Glucosidase activity in mycobacteria. J Clin Microbiol. 1977 Mar;5(3):383–384. doi: 10.1128/jcm.5.3.383-384.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L., Jones W. D., Jr, Newman C. M. Ultraviolet light inactivation and photoreactivation in the mycobacteria. Infect Immun. 1971 Sep;4(3):318–319. doi: 10.1128/iai.4.3.318-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Regulatory mechanisms in the mycobacteria. Ann Microbiol (Paris) 1978 Jan;129(1):71–73. [PubMed] [Google Scholar]
- David H. L. Response of Mycobacteria to ultraviolet light radiation. Am Rev Respir Dis. 1973 Nov;108(5):1175–1185. doi: 10.1164/arrd.1973.108.5.1175. [DOI] [PubMed] [Google Scholar]
- David H. L., Traore I., Feuillet A. Differential identification of Mycobacterium fortuitum and Mycobacterium chelonei. J Clin Microbiol. 1981 Jan;13(1):6–9. doi: 10.1128/jcm.13.1.6-9.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diderichsen B. flu, a metastable gene controlling surface properties of Escherichia coli. J Bacteriol. 1980 Feb;141(2):858–867. doi: 10.1128/jb.141.2.858-867.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Edwards R. P. Electron-microscope illustrations of division in Mycobacterium leprae. J Med Microbiol. 1970 Aug;3(3):493–499. doi: 10.1099/00222615-3-3-493. [DOI] [PubMed] [Google Scholar]
- Engbaek H. C., Vergmann B., Baess I. Non-photochromogenic mycobacteria serotype Davis. The inhomogeneity within the serological group and the relationship to Mycobacterium avium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):619–631. [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Kanetsuna F., Galindo B. Ultrastructure of cell walls of genus Mycobacterium. J Ultrastruct Res. 1968 Oct;25(1):46–63. doi: 10.1016/s0022-5320(68)80059-0. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn J. O., O'Donnell K. F., Silcox V. A., David H. L. Preparation of a stable mycobacterial tween hydrolysis test substrate. Appl Microbiol. 1973 Nov;26(5):826–826. doi: 10.1128/am.26.5.826-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- McCarthy C. M., Schaefer J. O. Response of Mycobacterium avium to ultraviolet irradiation. Appl Microbiol. 1974 Jul;28(1):151–153. doi: 10.1128/am.28.1.151-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough W. G., Merkal R. S. Iron-chelating compound from Mycobacterium avium. J Bacteriol. 1976 Oct;128(1):15–20. doi: 10.1128/jb.128.1.15-20.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Wittler R. G., Gooder H., Charache P. Wall-defective microbial variants: terminology and experimental design. J Infect Dis. 1971 Apr;123(4):433–438. doi: 10.1093/infdis/123.4.433. [DOI] [PubMed] [Google Scholar]
- Meyer L., David H. L. Evaluation de l'activité uréase et de l'activité beta-glucosidase pour l'identification pratique des mycobactéries. Ann Microbiol (Paris) 1979 Oct;130B(3):323–332. [PubMed] [Google Scholar]
- Miegeville M., Morin O. Nouvelle contribution de la microscopie électronique à balayage à l'étude des protoplastes de levures. C R Acad Sci Hebd Seances Acad Sci D. 1977 May 16;284(19):1935–1938. [PubMed] [Google Scholar]
- Nicholson A., Lepow I. H. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979 Jul 20;205(4403):298–299. doi: 10.1126/science.451601. [DOI] [PubMed] [Google Scholar]
- Olitzki A. L., Davis C. L., Schaefer W. B., Cohn M. L. Colony variants of avian-Battey group Mycobacteria intracerebrally injected into mice. Pathol Microbiol (Basel) 1969;34(5):316–323. doi: 10.1159/000162176. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rastogi N., David H. L. Ultrastructural and chemical studies on wall-deficient forms, spheroplasts and membrane vesicles from Mycobacterium aurum. J Gen Microbiol. 1981 May;124(1):71–79. doi: 10.1099/00221287-124-1-71. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Venkitasubramanian T. A. Preparation of protoplasts and whole cell ghosts from Mycobacterium smegmatis. J Gen Microbiol. 1979 Dec;115(2):517–521. doi: 10.1099/00221287-115-2-517. [DOI] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Sato H., Diena B. B., Greenberg L. Spheroplast induction and lysis of BCG strains by glycine and lysozyme. Can J Microbiol. 1966 Apr;12(2):255–261. doi: 10.1139/m66-035. [DOI] [PubMed] [Google Scholar]
- Sato H., Diena B. B., Greenberg L. The production of spheroplasts by rapid-growing non-virulent mycobacteria. Can J Microbiol. 1965 Oct;11(5):807–810. doi: 10.1139/m65-109. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Scudamore R. A., Beveridge T. J., Goldner M. Outer-membrane penetration barriers as components of intrinsic resistance to beta-lactam and other antibiotics in Escherichia coli K-12. Antimicrob Agents Chemother. 1979 Feb;15(2):182–189. doi: 10.1128/aac.15.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore R. A., Beveridge T. J., Goldner M. Penetrability of the outer membrane of Neisseria gonorrhoeae in relation to acquired resistance to penicillin and other antibiotics. Antimicrob Agents Chemother. 1979 Jun;15(6):820–827. doi: 10.1128/aac.15.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980 Apr;19(4):845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Silverman M., Zieg J., Hilmen M., Simon M. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci U S A. 1979 Jan;76(1):391–395. doi: 10.1073/pnas.76.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Zieg J., Simon M. Flagellar-phase variation: isolation of the rh1 gene. J Bacteriol. 1979 Jan;137(1):517–523. doi: 10.1128/jb.137.1.517-523.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. Humoral bactericidal systems: nonspecific and specific mechanisms. Infect Immun. 1978 Feb;19(2):515–522. doi: 10.1128/iai.19.2.515-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., KOIKE M., TODA T. Paired fibrous structure in mycobacteria. Biochim Biophys Acta. 1958 Oct;30(1):197–198. doi: 10.1016/0006-3002(58)90265-8. [DOI] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., TOKUNAGA T., KOIKE M., HISATSUNE K. Further studies on the paired fibrous structure of mycobacterial cell wall. J Biophys Biochem Cytol. 1961 Feb;9:496–501. doi: 10.1083/jcb.9.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal A. L., Kubica G. P. Differential colonial characteristics of mycobacteria on Middlebrook and Cohn 7H10 agar-base medium. Am Rev Respir Dis. 1966 Aug;94(2):247–252. doi: 10.1164/arrd.1966.94.2.247. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Woodley C. L., David H. L. Effect of temperature on the rate of the transparent to opaque colony type transition in Mycobacterium avium. Antimicrob Agents Chemother. 1976 Jan;9(1):113–119. doi: 10.1128/aac.9.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M. O., Asbury A. K. Intra-axonal bacilli in lepromatous leprosy. A light and electron microscopic study. Acta Neuropathol. 1974 Feb 7;27(1):1–10. doi: 10.1007/BF00687235. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Cramer S., Giphart-Gassler M. Invertible DNA determines host specificity of bacteriophage mu. Nature. 1980 Jul 17;286(5770):218–222. doi: 10.1038/286218a0. [DOI] [PubMed] [Google Scholar]