Abstract
The α7 subtype of the nicotinic acetylcholine receptor (α7 nAChR) is prominently expressed in the hippocampus where it is thought to play a role in the regulation of cognitive function. In this study, we have investigated the effects of 5-hydroxyindole (5-HI), a positive modulator of the α7 nAChR, on GABAergic activity in hippocampal CA1 stratum radiatum interneurons in acute rat brain slices. Superfusion of 5-HI (100 μm) increased the mean frequency and amplitude of spontaneous IPSCs (sIPSCs). The potentiation was occluded by pretreatment of slices with: (1) a high concentration of the broad-spectrum agonist nicotine to desensitize the α7 receptor, (2) an α7 nAChR antagonist, and (3) tetrodotoxin to block action potential firing. These results indicate that facilitation by 5-HI was mediated by the α7 nAChR and required neuronal excitation. In contrast, 5-HI had no effect on sIPSCs recorded in hippocampal slices from younger animals, even though the expression of functional α7 nAChRs was confirmed by agonist application experiments. In these slices, 5-HI only enhanced sIPSCs after pretreatment with the acetylcholinesterase inhibitor Bw284c51. Taken together, our results suggest that 5-HI facilitates GABAergic transmission via excitation of the α7 nAChR, and that this effect requires the presence of the endogenous agonist ACh in the extracellular environment of the receptor.
Nicotinic acetylcholine receptors containing the α7 subunit (α7 nAChRs) are expressed at high levels in the rodent hippocampus and are characterized by blockade by α-bungarotoxin and methyllycaconitine (MLA), selective activation by choline, high permeability to Ca2+, and rapid desensitization (Couturier et al. 1990; Séguéla et al. 1993; Alkondon et al. 1997b). Disruption of α7 nAChR activity has been implicated in the pathophysiology of psychiatric and neurological conditions such as schizophrenia and Alzheimer's disease (Freedman et al. 1997; Court et al. 1999; Guan et al. 2000). For example, analysis of post-mortem tissue from schizophrenia patients has revealed a reduction in α7 nAChR protein levels in various cortical regions (Freedman et al. 1995; Guan et al. 1999), while the β-amyloid protein associated with the pathophysiology of Alzheimer's disease modulates α7 nAChR function (Wang et al. 2000; Pettit et al. 2001). Although the broad-spectrum nAChR agonist nicotine has long been reported to enhance cognitive processes in animal models and in humans (reviewed by Levin, 2002; Newhouse et al. 2004), the involvement of the α7 nAChR subtype in cognition was speculative until the recent development of selective pharmacological agents that promote or inhibit α7 nAChR activity and the generation of α7 nAChR receptor knockout mice. Selective activation of the α7 nAChR was found to improve sensory processing and cognition in animal models (Stevens et al. 1998; Levin et al. 1999; Cilia et al. 2005; Hajós et al. 2005), whereas impairments were elicited by application of antagonists (Felix & Levin, 1997; Bettany & Levin, 2001) or deletion of the gene encoding α7 nAChR (Young et al. 2004; Keller et al. 2005). In light of these findings, the α7 nAChR shows promise as a therapeutic target in the treatment of various cognitive, neurological and psychiatric disorders (Martin et al. 2004).
Its rapid activation/deactivation kinetics makes the α7 nAChR suitable for mediating fast synaptic transmission and indeed, α7 nAChR-mediated synaptic currents have been demonstrated in the rat hippocampus (Frazier et al. 1998a; Hefft et al. 1999). In addition, its presence at extrasynaptic and presynaptic locations indicates the involvement of α7 nAChRs in modulatory or ‘volume’ transmission in the CNS (Descarries et al. 1997; Fabian-Fine et al. 2001; Coggan et al. 2005). The α7 nAChR has been shown to modulate the release of various neurotransmitters, including glutamate (McGehee et al. 1995; Gray et al. 1996), GABA (Alkondon et al. 1997a), dopamine (Schilstrom et al. 1998) and noradrenaline (Li et al. 1998). Furthermore, α7 nAChR activity may also regulate neuronal excitability and plasticity (Radcliffe & Dani, 1998; Frazier et al. 2003; Maggi et al. 2004).
Interneurons in the rat hippocampus express high levels of α7 nAChR, and functional α7 nAChR responses in this system have been well characterized (Alkondon & Albuquerque, 1993; Jones & Yakel, 1997; Frazier et al. 1998b; McQuiston & Madison, 1999; Fabian-Fine et al. 2001). Although brief local applications of nicotinic agonists can temporarily enhance neuronal activity (Alkondon et al. 1997a, 1999; Ji & Dani, 2000), receptor desensitization may limit α7 nAChR signalling in the prolonged presence of an agonist (Briggs & McKenna, 1998; Frazier et al. 1998b). An alternative approach to receptor activation with an exogenous agonist is the use of a positive allosteric modulator which would enhance receptor function elicited by endogenous agonists without directly activating or desensitizing the receptor. A number of positive modulators of the α7 nAChR have been reported, including ivermectin (Krause et al. 1998), galantamine (Santos et al. 2002), 5-hydroxyindole (5-HI; (Zwart et al. 2002) and PNU-120596 (Hurst et al. 2005). In this study, we tested the ability of 5-HI to enhance α7 nAChR function and modulate GABAergic transmission in rat hippocampal CA1 interneurons. We examined the relative efficacy of 5-HI at two stages of postnatal development and investigated the dependence of 5-HI efficacy on the presence of endogenous α7 nAChR agonists.
Methods
Hippocampal neurons in primary culture
Neuronal cultures were prepared from embryonic rat brains harvested following kill by CO2 inhalation in accordance with GlaxoSmithKline animal welfare guidelines and the UK Animals (Scientific Procedures) Act 1986. The dissected hippocampi were placed into an ice-cold medium: Hank's balanced salt solution (HBSS; Ca2+- and Mg2+-free); pyruvate, 1 mm; penicillin, 100 mg ml−1; streptomycin, 100 mg ml−1; Hepes, 10 mm; NaHCO3, 0.035%. Trypsin/EDTA was diluted in HBSS with sodium pyruvate (Ca2+- and Mg2+-free) and the tissue was trypsinized for 30 min at 37°C. Tissue pieces were physically dissociated and neurons were plated onto poly-d-lysine-coated coverslips in the following plating medium: neurobasal medium + 1 mm sodium pyruvate; penicillin, 100 mg ml−1; streptomycin, 100 mg ml−1; B27 supplement 1×; l-glutamine, 1 mm. Half of the volume of medium was replaced twice weekly, and the cells were used for recordings from 7 to 16 days in vitro.
Hippocampal slice preparation
Sprague-Dawley rats (postnatal day 12–35) were deeply anaesthetized with isoflurane by inhalation, and the brains were removed following decapitation in accordance with GlaxoSmithKline animal welfare guidelines and the UK Animals (Scientific Procedures) Act 1986. Using a vibratome (Campden Instruments), horizontal hippocampal slices (300 or 350 μm thick) were cut in ice-cold artificial cerebrospinal fluid (aCSF) solution of the following composition (mm): NaCl, 125; KCl, 2.5; NaHCO3, 26; NaH2PO4.H2O, 1.25; glucose, 25; CaCl2, 1, MgCl2, 2, bubbled with 95% CO2/5% O2. Slices were incubated at room temperature in aCSF containing 50 μmd-aminophosphonovalerate (d-AP5) for an hour before experimentation and used for up to 8 h later.
Electrophysiology recordings
Cultured hippocampal neurons were perfused with an external solution containing (mm): NaCl, 145; KCl, 2.5; Hepes, 10; glucose, 10; CaCl2, 1.5; MgCl2, 1; pH 7.4 with NaOH. Tetrodotoxin (0.5 μm) was perfused throughout the recordings to block action potential firing. Patch pipettes were filled with (mm): potassium gluconate, 130; Hepes, 10; EGTA, 5; 300 mOsmol kg−1. Membrane currents were recorded by whole-cell voltage clamp using an Axopatch 200B amplifier and pClamp9 software (Molecular Devices). Solutions containing test compounds were applied via a dual-barrel fast perfusion system (RSC-160; Biologic, Grenoble, France).
Hippocampal slices were superfused (2–3 ml min−1) at room temperature with aCSF (mm): NaCl, 125; KCl, 2.5; NaHCO3, 26; NaH2PO4.H2O, 1.25; glucose, 25; CaCl2, 2; MgCl2, 1; bubbled with 95% CO2/5% O2. Neurons in the CA1 stratum radiatum of acutely isolated brain slices were visualized under IR-DIC optics (Nikon). Membrane currents were recorded by whole-cell voltage clamp using a Multipatch 700B amplifier and pClamp9 software (Molecular Devices). For sIPSC recordings, patch pipettes were filled with an internal solution containing a high concentration of chloride (mm): CsCl, 140; NaCl, 2; CsHEPES, 10; CsEGTA, 10; 300 mOsmol kg−1. Patch pipettes had tip resistances of 4–8 MΩ when filled with internal solution. NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulphonamide disodium) (5 μm) and d-AP5 (50 μm) were perfused throughout the experiment to isolate sIPSCs pharmacologically. In pressure ejection experiments, the following internal solution was used (mm): KMeSO4, 130; NaCl, 4; Hepes, 10; EGTA, 0.5; MgATP, 4; Na2GTP, 0.2; sodium phosphocreatine, 300 mOsmol kg−1. A glass pipette of tip diameter 50–150 μm was positioned adjacent to the target cell body and was used to apply ACh by pressure microejection (50–100 ms duration, 5–15 p.s.i. (34.5–103.5 kPa); Picospritzer; Warner Instruments). All chemicals were purchased from Sigma-Aldrich or Tocris Cookson, except tetrodotoxin (TTX; Affiniti Research Products). Data analysis of agonist-evoked currents was performed using pClamp9 (Molecular Devices) and Origin5 (Original Lab Corp., Northampton, MA, USA) software. Concentration–response curves were fitted with the Hill equation:
where y is the membrane current, EC50 is the concentration of half-maximal efficacy, x is the agonist concentration, and nH is the Hill coefficient. Synaptic currents were detected and analysed using MiniAnalysis (Synaptosoft, Inc.). All data are reported as means ±s.e.m. Statistical significance was determined using a two-tailed Student's t test (Excel, Microsoft).
Results
5-HI is a positive modulator at the rat α7 nAChR
Under whole-cell voltage clamp (Vh−70 mV), agonist-evoked α7 nAChR responses were elicited in rat cultured hippocampal neurons using a dual-barrel fast perfusion system. As shown in Fig. 1A, application of ACh (300 ms duration at 2 min intervals) elicited fast inward currents in 9 of 22 cells. The ACh-evoked currents were abolished by the selective α7 nAChR antagonist MLA (10 nm) in all cells tested. Application of 5-HI (100 μm) increased the peak amplitude and charge transfer of the ACh-evoked responses. The ACh concentration–response curve was shifted to the left by 5-HI, indicating an increase in the potency of ACh at the rat native α7 nAChR. The EC50 for ACh was 194 μm and 65 μm (n = 4 cells per data point) in the absence and in the presence of 5-HI (100 μm), respectively. We also observed an increase in the Hill slope of the ACh concentration–response curve from 1.2 to 2.1. In the six cells tested, 5-HI alone (100 μm) did not induce any current responses (data not shown), confirming that 5-HI does not act as an agonist at the α7 nAChR under these experimental conditions.
Figure 1. 5-Hydroxyindole potentiates currents through the rat native α7 nAChR.
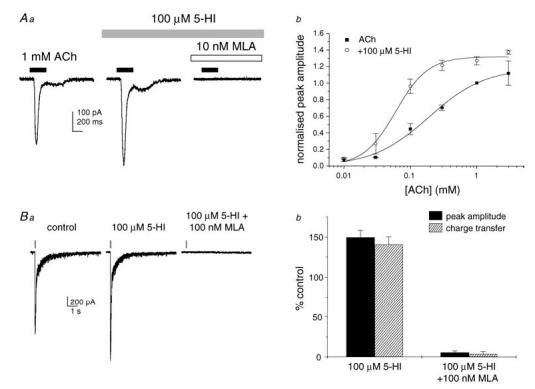
Aa, representative ACh-evoked inward currents in rat hippocampal neurons in primary culture. Currents were enhanced in the presence of 5-hydroxyindole (5-HI; 100 μm) and abolished by methyllycaconitine (MLA; 10 nm). Ab, concentration–response curves for ACh in the absence (▪) and in the presence (○) of 5-HI (100 μm; n = 4 cells per data point). In the presence of 5-HI, the ACh EC50 shifted from 194 to 65 μm (P < 0.05) and the Hill slope increased from 1.2 to 2.1 (P < 0.05). The maximal efficacy was not significantly affected. Ba, representative fast inward currents elicited by pressure microejection of ACh (1 mm) onto CA1 stratum radiatum interneurons in a rat hippocampal slice. The responses were observed in the presence of picrotoxin (100 μm), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulphonamide disodium (NBQX; 5 μm), d-aminophosphonovalerate (d-AP5; 50 μm), MDL72222 (0.5 μm) and dihydro-β-erythroidine (DHβE; 0.1 μm). The time course of agonist application is indicated by the bars above the traces. Bb, mean agonist-evoked currents were enhanced in the presence of 5-HI (100 μμ) and inhibited in the presence of MLA (100 nm; n = 5–8 cells).
We next evaluated the effect of 5-HI on α7 nAChRs in isolated hippocampal slices from 3- to 5-week-old rats. CA1 stratum radiatum interneurons were visually identified and held under whole-cell voltage clamp (Vh−70 mV). Brief pulses (50–100 ms at 2 min intervals) of ACh (1 mm) were applied locally to the cell body by pressure microejection in the presence of the glutamate receptor antagonists NBQX (5 μm) and d-AP5 (50 μm), the GABAA receptor antagonist picrotoxin (100 μm), the 5-HT3 receptor antagonist tropanyl 3,5-dichlorobenzoate (MDL72222; 0.5 μm) and the α4β2 nAChR antagonist dihydro-β-erythroidine (DHβE; 0.1 μm). In 57 of 70 cells, ACh elicited fast-rising inward currents with kinetics similar to those previously described (Alkondon et al. 1993; Frazier et al. 1998b). Superfusion of 5-HI (100 μm) potentiated ACh-evoked currents, increasing both their peak amplitude and charge transfer by 50% (n = 7 cells; Fig. 1B) but with no effect on the holding current (data not shown). Perfusion of MLA (100 nm) at the end of the experiment abolished ACh-induced responses in all cells tested. Furthermore, pressure application of 5-HI (100 μm) alone did not evoke any current responses (n = 23 cells; data not shown). These results confirm that 5-HI is a positive modulator at the rat α7 nAChR in both cultured hippocampal neurons and CA1 stratum radiatum interneurons in isolated slices.
5-HI increases GABAergic transmission in CA1 stratum radiatum via the α7 nAChR
Using a chloride-based internal solution, and in the presence of the glutamate receptor antagonists NBQX (5 μm) and d-AP5 (50 μm), sIPSCs were recorded as inward currents at a holding potential of −70 mV in CA1 stratum radiatum interneurons in hippocampal slices from 3- to 5-week-old rats. Bath application of 5-HI (100 μm) increased the sIPSC frequency and average amplitude (19 of 20 cells tested; Fig. 2), effects which were sustained over a 15 min application period and were reversible on wash-out. To test if the 5-HI-induced potentiation required action potential firing, TTX (0.5 μm) was applied 10 min before 5-HI perfusion. In all cells tested, 5-HI had no effect on sIPSC properties following TTX preincubation (n = 6 cells; Fig. 3). This indicates that 5-HI-induced facilitation was dependent on the firing of connected interneurons in the slice. Interestingly, the frequency and mean amplitude of miniature IPSCs (mIPSCs) and control sIPSCs were the same (Table 1). This indicates that, under control conditions, spontaneous GABAergic activity recorded from CA1 stratum radiatum interneurons is action-potential independent.
Figure 2. 5-HI facilitates sIPSCs in hippocampal interneurons.
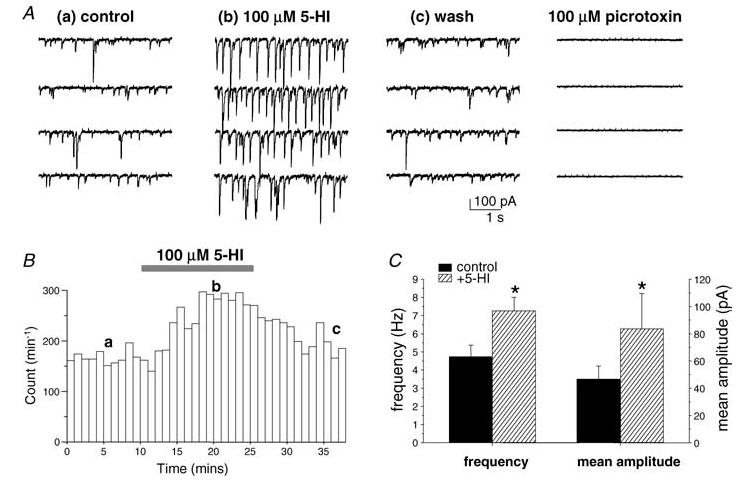
A, sample traces of a representative recording from a CA1 stratum radiatum interneuron (from postnatal day (P)26 rat). sIPSC frequency and mean amplitude were reversibly potentiated in the presence of 5-HI (100 μm). Aa–c correspond to time points represented in B. sIPSC activity was abolished in the presence of picrotoxin (100 μm). B, frequency–time plot from the recording shown in A, illustrating sIPSC frequency in control conditions (a), in the presence of 5-HI (100 μm; b) and after wash-out of 5-HI (c). C, mean sIPSC frequency and amplitude data from 20 cells (from 3- to 5-week-old rats). *P < 0.05 versus control, paired t test.
Figure 3. 5-HI-mediated facilitation of sIPSCs is occluded by inhibition of α7 nAChR activity.
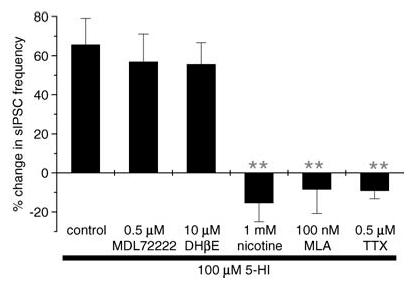
Pretreatment (10–15 min, unless otherwise stated) with, and coapplication of, nicotine (1 mm; n = 5 cells), MLA (100 nm, 30 min preincubation; n = 4 cells) or TTX (0.5 μm; n = 6 cells) blocked the facilitatory effect of 5-HI (100 μm) on sIPSC frequency. No significant changes in sIPSCs were observed on application of 5-HI in the presence of nicotine, MLA or TTX. In contrast, MDL72222 (0.5 μm; n = 6 cells) and DHβE (10 μm; n = 4 cells) had no effect on 5-HI-induced potentiation of sIPSC frequency. **P < 0.005 versus control, unpaired t test.
Table 1.
Comparison of sIPSC, mIPSC and ACh-evoked current properties recorded from hippocampal slices taken from 2- to 3-, and 3- to 5-week-old rats
| 2–3 weeks old | 3–5 weeks old | |
|---|---|---|
| sIPSCs | ||
| Frequency (Hz) | 3.5 ± 0.5 (n = 14) | 4.7 ± 0.5 (n = 20) |
| Amplitude (pA) | −43.4 ± 4.0 | −52.5 ± 8.4 |
| Charge transfer (fC) | −773.1 ± 94.6 | −973.7 ± 193.3 |
| 10–90% rise (ms) | 4.3 ± 0.4 | 3.9 ± 0.2 |
| Half-width (ms) | 12.5 ± 0.9 | 12.6 ± 1.3 |
| mIPSCs | ||
| Frequency (Hz) | ND | 4.3 ± 2.0 (n = 6) |
| Amplitude (pA) | ND | −45.8 ± 17.3 |
| ACh-evoked currents | ||
| Amplitude (pA) | −233.4 ± 55.0 (n = 26) | −247.5 ± 75.9 (n = 19) |
| Charge transfer (pC) | −158.5 ± 64.3 | −206.1 ± 96.2 |
No significant differences were observed in sIPSC or ACh (1 mm)-evoked current properties between the two ages. There were also no significant differences between sIPSC and mIPSC properties recorded from 3- to 5-week-old animals. ND, not determined.
To determine whether 5-HI facilitates GABAergic transmission via modulation of nAChRs, a high concentration of nicotine (1 mm) was preapplied for 5–10 min to desensitize α7 nAChRs (Frazier et al. 1998b; McQuiston & Madison, 1999). As summarized in Fig. 3, 5-HI had no significant effect on sIPSCs following preincubation with nicotine (n = 5 cells). To further investigate the involvement of the α7 nAChR subtype, we evaluated the effect of blocking α7 nAChRs with MLA. MLA (100 nm, 30 min preincubation; n = 4 cells) occluded the facilitatory actions of 5-HI. It should be noted that a sufficient period of preincubation was necessary for MLA to completely block the 5-HI-induced potentiation. Pretreating slices for 10 min only inhibited the 5-HI effect by 50% (data not shown; n = 6 cells). In contrast to the occlusion by MLA, the 5-HI-induced potentiation was unaffected by the 5-HT3 receptor antagonist MDL72222 (0.5 μm) or the α4β2 nAChR antagonist DHβE (10 μm) in all cells tested (Fig. 3). Taken together, these results strongly suggest that 5-HI facilitated GABAergic transmission via the modulation of α7 nAChR function.
5-HI had no effect on sIPSCs in slices from ‘juvenile’ rats
In experiments carried out in slices from younger rats aged 2–3 weeks, we observed that 5-HI had no effect on GABAergic sIPSCs in 14 interneurons from these ‘juvenile’ animals (%control: sIPSC frequency, 106.2 ± 4.2%; mean amplitude, 105.6 ± 3.7%; Fig. 4). As summarized in Table 1, comparison of control sIPSC properties revealed no significant differences in frequency, mean amplitude, charge transfer, rise time or event half-width between recordings from ‘juvenile’ (2–3 weeks old) and ‘adolescent’ rats (3–5 weeks old).
Figure 4. 5-HI had no effect on sIPSCs recorded from juvenile rats (2–3 weeks old).
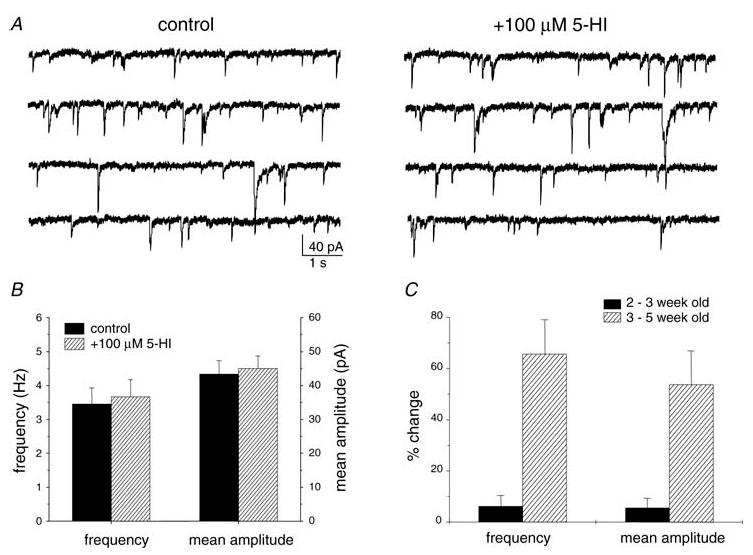
A, sample traces from a representative recording of a CA1 stratum radiatum interneuron (from P14 rat). Perfusion of 5-HI (100 μm) for 30 min had no effect on sIPSC frequency or mean amplitude. B, mean sIPSC frequency and amplitude in the presence and absence of 5-HI (100 μm; n = 14 cells) in recordings from juvenile rats. C, comparison of 5-HI effect in slices from 2- to 3-, and 3- to 5-week-old rats (n = 14 and 20 cells, respectively).
To investigate whether the absence of 5-HI-induced facilitation in recordings from juvenile rats might reflect a lack of α7 nAChR expression, we applied ACh onto interneurons in slices from juvenile rats to test for functional α7 nAChRs. Pressure application of ACh (1 mm) onto the soma of CA1 stratum radiatum interneurons elicited fast inward currents in most interneurons tested in slices from juvenile rats (50 of 58 cells; adolescent rats, 57 of 70 cells) which did not differ in peak amplitude or charge transfer to those evoked in slices from adolescent rats (Table 1). The pharmacology of the agonist-evoked responses from juvenile rat neurons was also similar to those recorded from the adolescent neurons. The ACh-evoked currents were potentiated by 5-HI (100 μm; Fig. 5) and abolished by MLA (100 nm; data not shown) in all cells tested. Interestingly, 5-HI affected the charge transfer of the ACh-evoked response to a greater degree than the peak amplitude. This effect was manifest as a significant slowing of the decay kinetics, suggesting that 5-HI may differentially affect receptor desensitization and/or deactivation in juvenile relative to adolescent slices. Taken together, the data confirm the expression of functional α7 nAChRs in stratum radiatum interneurons from juvenile rat brain slices and that 5-HI is effective as a positive modulator at these receptors.
Figure 5. CA1 stratum radiatum interneurons from juvenile rats express functional α7 nAChRs.
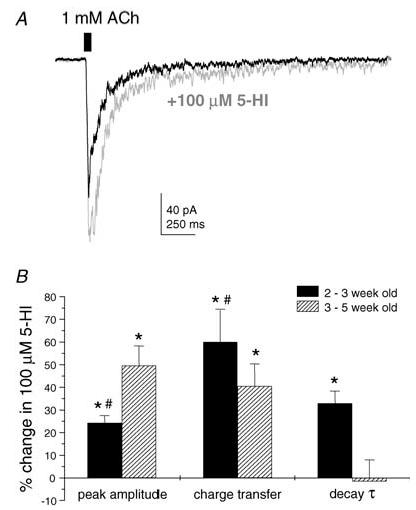
A, pressure ejection of ACh (1 mm) locally onto the soma of a representative CA1 stratum radiatum interneuron (from P19 rat) evoked a fast-rising inward current. Bath application of 5-HI (100 μm) increased the peak amplitude, charge transfer and decay time constant of the ACh-evoked response. B, comparison of mean 5-HI-induced potentiation of ACh-evoked responses between slices from 2- to 3-, and 3- to 5-week-old rats (n = 26 and 19 cells, respectively). In recordings from 2- to 3-week-old rats, 5-HI had a greater effect on charge transfer and a significant effect on the decay time constant τ. *P < 0.05 versus control, paired t test; #P < 0.05 peak amplitude versus charge transfer, unpaired t test.
5-HI efficacy depends on activation of α7 nAChRs by endogenous agonist
As 5-HI is a positive modulator and has no apparent agonist activity at the α7 nAChR, its facilitatory effect on sIPSCs in slices from adolescent rats implies the presence of an endogenous α7 nAChR agonist in the extracellular environment. Reduced levels of endogenous agonists in juvenile rat slices may accordingly underlie the lack of 5-HI activity in this preparation. To test this hypothesis, we pretreated slices from juvenile rats with the acetylcholinesterase inhibitor 1,5-bis(4-allyldimethyl-ammoniumphenyl)-pentan-3-one dibromide (Bw284c51) to reduce ACh degradation and elevate the levels of endogenous ACh in the extracellular space. Superfusion of Bw284c51 (1 μm) had no effect on sIPSC frequency or amplitude (data not shown; n = 6 cells). After 10–15 min preincubation with and in the presence of Bw284c51 (1 μm), an increase in sIPSC frequency was observed on bath application of 5-HI (100 μm; percentage increase 48.3 ± 24.6%, n = 6 cells; Fig. 6). In contrast, 5-HI had no significant effect on sIPSCs in the presence of Bw284c51 and MLA (100 nm; n = 4 cells), supporting mediation by the α7 nAChR.
Figure 6. 5-HI facilitated sIPSCs in recordings from juvenile rats following pretreatment with Bw284c51.
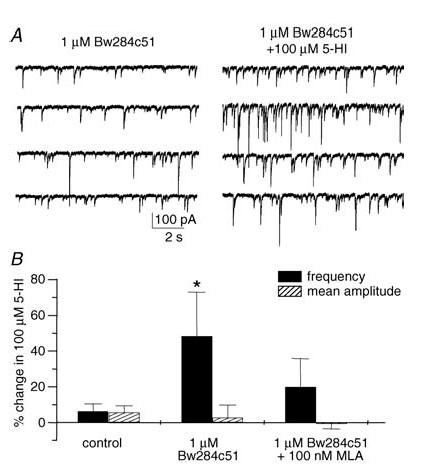
A, representative traces showing the effect of 5-HI (100 μm) on sIPSCs after preapplication of the acetylcholinesterase inhibitor Bw284c51 (1 μm, 30 min preapplication time). B, following pretreatment with Bw284c51, 5-HI significantly facilitated sIPSC frequency, but had no effect on the mean sIPSC amplitude (n = 6 cells). In contrast, 5-HI had no significant effect on sIPSCs in the presence of Bw284c51 and MLA (100 nm; n = 4 cells). *P < 0.05, paired t test.
Although the presence of an agonist is necessary for 5-HI activity, high levels of endogenous agonist in the extracellular space may actually limit the effect of 5-HI due to receptor desensitization. To test this, we investigated the effect of Bw284c51 (1 μm) on 5-HI-mediated potentiation of sIPSCs in slices from adolescent rats aged 3–5 weeks. In Bw284c51-treated slices, 5-HI enhanced sIPSC frequency and mean amplitude by 30% (n = 5 cells; p < 0.05 for both frequency and amplitude, paired t test; Fig. 7), effects not significantly different to those observed under control conditions. It should also be noted that Bw284c51 alone had no effect on sIPSC properties in all cells tested (Fig. 7B).
Figure 7. Effect of Bw284c51 on 5-HI-induced facilitation in interneurons from adolescent rats.
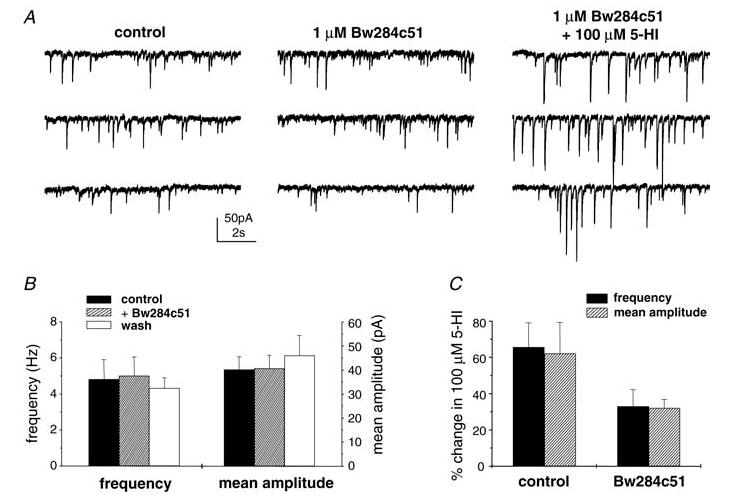
A, representative traces showing the effect of Bw284c51 (1 μm, 15 min incubation; middle panel) and coapplication of Bw284c51 and 5-HI (100 μm; right panel) on sIPSCs from a P25 rat. B, perfusion of Bw284c51 (1 μm) had no effect on sIPSC frequency or mean amplitude in 7 cells tested. C, 5-HI significantly increased sIPSC frequency and mean amplitude in slices pretreated with Bw284c51 (1 μm; n = 5 cells; P < 0.05, paired t test). The magnitude of potentiation was not significantly different to that observed under control conditions (P > 0.1, unpaired t test).
Discussion
In the present study, we have shown that 5-HI is a positive modulator at the rat α7 nAChR in cultured hippocampal neurons and in CA1 stratum radiatum interneurons in acutely isolated slices. 5-HI increased the apparent potency of ACh at the rat α7 nAChR in cultured neurons. This was previously reported for recombinant human α7 nAChRs expressed in Xenopus oocytes (Zwart et al. 2002). However, we also observed a significant increase in the Hill slope of the ACh concentration–response curve in the presence of 5-HI, and this indicates an increase in agonist co-operativity under our experimental conditions.
In hippocampal slices from weaned ‘adolescent’ (3–5 weeks old) rats, sustained application of 5-HI led to an increase in the frequency and magnitude of GABAergic sIPSCs. Desensitization of α7 nAChRs by pretreatment with a high concentration of the non-selective nAChR agonist nicotine (Frazier et al. 1998b; McQuiston & Madison, 1999) completely occluded the 5-HI-mediated facilitation. Blockade of the α7 nAChR by the antagonist MLA also inhibited the effect of 5-HI on sIPSCs. Previously, Mannaioni et al. (2003) showed that 5-HI potentiated sIPSCs recorded from CA1 pyramidal neurons but this effect was not sensitive to (10 min) preincubation with MLA. The authors speculated the involvement of nAChRs other than the α7 subtype. As CA1 stratum radiatum interneurons also express α4β2 nAChRs (Alkondon et al. 1999; McQuiston & Madison, 1999) and 5-HI also facilitates 5-HT3 receptor activity (Kooyman et al. 1993), we investigated the possible involvement of these receptors. Preincubation with the antagonists DHβE or MDL72222 had no effect on the 5-HI-induced potentiation of sIPSCs, indicating that 5-HI was not acting via α4β2 nAChRs or 5-HT3 receptors, respectively. Although we cannot rule out the possibility that 5-HI may also act on targets other than α7 nAChRs, the pharmacological data presented here provide strong evidence that 5-HI facilitates hippocampal sIPSCs via modulation of α7 nAChRs. Furthermore, these α7 nAChRs do not appear to be located at the presynaptic GABAergic axon terminals, as TTX occluded the effect of 5-HI even though action potential-independent mIPSCs persisted. Thus, our data suggest that positive modulation of α7 nAChRs leads to excitation of GABAergic interneurons, which is sufficient to cause cell firing and neurotransmitter release.
A number of published reports have shown that brief (milliseconds or seconds) and local (pressure microejection or U-tube) applications of α7 nAChR agonists elicited GABAergic IPSCs in CA1 interneurons in an MLA- and TTX-sensitive manner (Alkondon et al. 1997a, 1999; Ji & Dani, 2000). Indeed, brief α7 nAChR agonist applications can directly generate depolarization and cell firing in hippocampal interneurons (Alkondon et al. 1999; McQuiston & Madison, 1999; Ji & Dani, 2000). In our experiments, we globally applied an α7 nAChR positive modulator for over 15 min and observed a robust and sustained potentiation of sIPSCs. Although the time course and strategy of receptor activation were different (sustained versus phasic; positive modulator versus agonist), both approaches elicited GABAergic IPSCs via α7 nAChR activation and action potential firing.
As 5-HI did not exhibit any agonist activity at the α7 nAChR, its facilitatory effect on sIPSCs suggests the presence of endogenous agonists (ACh and choline) in the extracellular space. We did not observe any changes in the holding current on perfusion of MLA, suggesting that α7 nAChRs are not tonically active. We also observed no changes in sIPSC properties on application of MLA (data not shown) or the acetylcholinesterase inhibitor Bw284c51, in contrast to Alkondon et al. (1999) who reported a transient increase in sIPSC frequency and amplitude on perfusion of MLA. As 5-HI (100 μm) lowered the EC50 for ACh in cultured neurons, it is likely that 5-HI enhances the affinity of α7 nAChRs for endogenous agonists in the slice, promoting receptor activation, neuronal depolarization and firing. We attempted to demonstrate directly the presence of endogenous agonists in the slice by pressure application of 5-HI onto the cell body of interneurons in slices from adolescent animals. Although no 5-HI-induced currents were observed, this does not necessarily rule out the presence of endogenous ACh in the slice. Pressure ejection of 5-HI might displace any endogenous tonal ACh in the vicinity. Moreover, the concentration of endogenous agonist is likely to be very low (as α7 nAChRs were not tonically desensitized in our experiments) and the cell bodies of the neurons recorded from in these experiments were near the surface of the slice where ACh may easily diffuse away. Interestingly, Fayuk & Yakel (2004) observed desensitization of α7 nAChRs after superfusion of acetylcholinesterase inhibitors only when ACh was applied exogenously in pressure application experiments.
Application of 5-HI did not facilitate sIPSCs recorded from younger ‘juvenile’ rats (2–3 weeks old). This was not due to the absence of α7 nAChRs, as expression of functional receptors was confirmed by the observation of MLA-sensitive ACh-evoked responses. This is in agreement with α-bungarotoxin-binding studies which demonstrated the expression of α7 nAChR protein in the neonatal rat hippocampus, with protein levels actually decreasing over the first three postnatal weeks (Adams et al. 2002; Tribollet et al. 2004). In addition to validating receptor expression, we have also confirmed that 5-HI is effective as a positive modulator of ACh-evoked currents at α7 nAChRs in slices from juvenile animals. Our observation of a 5-HI-induced increase in decay kinetics of agonist-evoked currents in juvenile slices is intriguing. As there is very little information on the developmental regulation of the functional properties of α7 nAChRs in the literature, we can only speculate on the possible explanations for this, e.g. the receptors are differentially regulated by intracellular binding proteins or receptor phosphorylation.
The cholinergic innervation of the hippocampus continues to develop postnatally, and staining studies have shown that the septohippocampal projection reaches adult patterns during the second postnatal week in the rat (Milner et al. 1983; Linke & Frotscher, 1993). More importantly in terms of ACh production and release, Aznavour et al. (2005) used choline acetyltransferase immunocytochemistry to show that the density of ACh-containing varicosities in the rat CA1 doubled between postnatal day (P)8 and P16, with a further increase of 50% to adult levels between P16 and P32. In light of this, it is possible that the level of ACh in the extracellular environment is lower in slices from juvenile rats due to the reduced cholinergic innervation. Our experimental results are in line with this hypothesis such that the facilitation of sIPSCs by 5-HI was ‘rescued’ after boosting levels of endogenous ACh with perfusion of Bw284c51, an acetylcholinesterase inhibitor with no direct actions on rat α7 nAChRs (Fayuk & Yakel, 2004). Similarly, the novel and selective α7 nAChR positive modulator PNU-120596 and the less selective galantamine were found only to facilitate sIPSCs when coapplied with ACh in hippocampal slices from P16–25 rats (Santos et al. 2002; Hurst et al. 2005). Our results also offer an explanation for the discrepancy in the Hurst et al. (2005) paper between the effectiveness of PNU-120596 alone in vivo and the need for the coapplication of exogenous ACh in electrophysiological recordings in vitro. Later in postnatal development, the extracellular levels of endogenous ACh in the rat hippocampal slice are sufficient for a positive modulator to have an effect without addition of an exogenous agonist. However, the relationship between 5-HI efficacy and the concentration of agonist present is likely to be complex as α7 nAChRs are rapidly desensitized not only after agonist-mediated activation but also in the presence of agonists at subactivation threshold (Briggs & McKenna, 1998). We observed a trend towards reduction of 5-HI effect in the presence of Bw284c51 in slices from adolescent rats. This is consistent with the idea that higher levels of agonist will desensitize α7 nAChRs and thereby limit the ability of a positive modulator to enhance receptor function. That Bw284c51 affected the efficacy of 5-HI in our experiments indicates that ACh is actively degraded, limiting its exposure at the receptor, and hence suggests that the release of ACh continues in the deafferented hippocampal slice (Benardo & Prince, 1980). In addition to the cholinergic septohippocampal projection, a possible source of ACh release is the cholinergic interneurons which have been identified in the CA1 subfield of the hippocampus (Freund & Buzsáki, 1996).
In summary, 5-HI is effective as a positive modulator at the rat α7 nAChR. Potentiation of α7 nAChR function by the sustained presence of 5-HI facilitates GABAergic transmission onto hippocampal interneurons. The lack of 5-HI efficacy in slices from younger animals, together with rescue of the 5-HI-induced potentiation by an acetylcholinesterase inhibitor, indicate that the extracellular levels of ACh in the microenvironment of α7 nAChRs are lower in these slices. Enhanced α7 nAChR signalling may offer therapeutic potential in psychiatric and neurological disorders. In view of our findings, one may postulate that α7 nAChR positive modulators might be more efficacious in the treatment of conditions with reduced receptor expression (as in schizophrenia) than in disorders such as Alzheimer's disease where the extracellular level of endogenous cholinergic agonists may be compromised.
Acknowledgments
We thank Olga Krylova for the neuronal cultures, and Ceri H. Davies for helpful comments on the manuscript.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997a;283:1396–1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997b;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: Quantitative light and electron microscopic immunocytochemical study. J Comp Neurol. 2005;486:61–75. doi: 10.1002/cne.20501. [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. Cholinergic pharmacology of mammalian hippocampal pyramidal cells. Neuroscience. 1980;7:1703–1712. doi: 10.1016/0306-4522(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Bettany JH, Levin ED. Ventral hippocampal alpha 7 nicotinic receptor blockade and chronic nicotine effects on memory performance in the radial-arm maze. Pharmacol Biochem Behav. 2001;70:467–474. doi: 10.1016/s0091-3057(01)00643-8. [DOI] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology. 1998;37:1095–1102. doi: 10.1016/s0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Cilia J, Cluderay JE, Robbins MJ, Reavill C, Southam E, Kew JN, Jones DN. Reversal of isolation-rearing-induced PPI deficits by an alpha7 nicotinic receptor agonist. Psychopharmacology (Berl) 2005;182:214–219. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, et al. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, et al. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998a;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998b;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in post-mortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. J Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 (N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride) enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol. 1999;515:769–776. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurons in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of alpha7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behav Brain Res. 2005;162:143–152. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kooyman AR, van Hooft JA, Vijverberg HP. 5-Hydroxyindole slows desensitization of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells. Br J Pharmacol. 1993;108:287–289. doi: 10.1111/j.1476-5381.1993.tb12795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J. AR- R17779, an alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R, Frotscher M. Development of the rat septohippocampal projection: tracing with DiI and electron microscopy of identified growth cones. J Comp Neurol. 1993;332:69–88. doi: 10.1002/cne.903320106. [DOI] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol. 2004;559:863–874. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni G, Carpenedo R, Moroni F. 5-Hydroxyindole causes convulsions and increases transmitter release in the CA1 region of the rat hippocampus. Br J Pharmacol. 2003;138:245–253. doi: 10.1038/sj.bjp.0705007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Loy R, Amaral DG. An anatomical study of the development of the septo-hippocampal projection in the rat. Brain Res. 1983;284:343–371. doi: 10.1016/0165-3806(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. Beta-Amyloid (1–42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002;61:1222–1234. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, AnD'drea MR, Peterson PA, Shank RP, Reitz AB. Beta-Amyloid (1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, Sher E. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology. 2002;43:374–384. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]


