Abstract
In cardiac myocytes, initiation of excitation–contraction coupling is highly localized near the T-tubule network. Myocytes with a dense T-tubule network exhibit rapid and homogeneous sarcoplasmic reticulum (SR) Ca2+ release throughout the cell. We examined whether progressive changes in T-tubule organization and Ca2+ release synchrony occur in a murine model of congestive heart failure (CHF). Myocardial infarction (MI) was induced by ligation of the left coronary artery, and CHF was diagnosed by echocardiography (left atrial diameter >2.0 mm). CHF mice were killed at 1 or 3 weeks following MI (1-week CHF, 3-week CHF) and cardiomyocytes were isolated from viable regions of the septum, excluding the MI border zone. Septal myocytes from SHAM-operated mice served as controls. T-tubules were visualized by confocal microscopy in cells stained with di-8-ANEPPS. SHAM cells exhibited a regular striated T-tubule pattern. However, 1-week CHF cells showed slightly disorganized T-tubule structure, and more profound disorganization occurred in 3-week CHF with irregular gaps between adjacent T-tubules. Line-scan images of Ca2+ transients (fluo-4 AM, 1 Hz) showed that regions of delayed Ca2+ release occurred at these gaps. Three-week CHF cells exhibited an increased number of delayed release regions, and increased overall dyssynchrony of Ca2+ release. A common pattern of Ca2+ release in 3-week CHF was maintained between consecutive transients, and was not altered by forskolin application. Thus, progressive T-tubule disorganization during CHF promotes dyssynchrony of SR Ca2+ release which may contribute to the slowing of SR Ca2+ release in this condition.
In adult mammalian ventricular myocytes, contraction is initiated by a series of events beginning with the opening of Ca2+ channels during the action potential. The resulting Ca2+ influx triggers release of a larger amount of Ca2+ from the sarcoplasmic reticulum (SR) by a process known as Ca2+-induced Ca2+ release (CICR, for review see Bers, 2002). In ventricular myocytes, this process occurs at junctional complexes between T-tubules and the SR where L-type Ca2+ channels and SR Ca2+-release channels (ryanodine receptors) are in close proximity (Flucher & Franzini-Armstrong, 1996). Thus, initiation of excitation–contraction coupling is highly localized near the T-tubule network (Shacklock et al. 1995).
In myocytes with a high density of T-tubules, such as in rats and mice, SR Ca2+ release occurs almost simultaneously throughout the cell (Berlin, 1995; Shacklock et al. 1995; Heinzel et al. 2002). However, myocytes with less-dense T-tubule networks exhibit less synchronous Ca2+ transients, with regions of delayed Ca2+ release occurring where T-tubules are not present (Heinzel et al. 2002). Cells which are devoid of T-tubules, such as atrial myocytes and Purkinje cells, exhibit very dyssynchronous Ca2+ transients. In these cells, SR Ca2+ release is triggered rapidly by Ca2+ influx near the sarcolemma, but more slowly in the cell interior following propagation of Ca2+ (Berlin, 1995; Cordeiro et al. 2001). Experimentally promoting loss of T-tubules by cell culture or de-tubulation techniques has also been shown to reduce the synchrony of Ca2+ transients, which results in slower spatially averaged Ca2+ release (Lipp et al. 1996; Yang et al. 2002; Louch et al. 2004). Thus, there is considerable evidence to suggest that a dense and intact T-tubular network is required for rapid and homogeneous SR Ca2+ release.
Several reports have suggested that the T-tubular network may be altered in heart failure. A marked loss of T-tubules has been observed in failing canine ventricular myocytes (He et al. 2001; Balijepalli et al. 2003), although it is unclear whether such changes occur in human heart failure (Kaprielian et al. 2000; Wong et al. 2001; Ohler et al. 2001). However, the structural organization of T-tubules may be altered in failing human cardiomyocytes (Kostin et al. 1998; Kaprielian et al. 2000; Wong et al. 2001; Louch et al. 2004). It is not known how such disorganization may influence excitation–contraction coupling.
We postulate that disorganization of T-tubules in heart failure contributes to the slowing of the rising phase of the Ca2+ transient in this condition by promoting dyssynchrony of Ca2+ release. However, such effects may be difficult to ascertain in models of chronic heart failure in which reduction of SR Ca2+ stores (for review see Houser et al. 2000) may also contribute to slowing of Ca2+ release (Sitsapesan & Williams, 1994; Lukyanenko et al. 1996). We have recently developed a mouse model of the early stages of congestive heart failure (CHF) following myocardial infarction. At this stage of CHF, overall cardiac function is dramatically reduced but reduced shortening of the non-infarcted myocardium has not yet occurred (Finsen et al. 2005). Cardiomyocyte SR Ca2+ content is also not decreased at this time point although there is marked slowing of Ca2+ release. In the present study, we demonstrate that progressive T-tubule disorganization promotes dyssynchronous SR Ca2+ release in this model, and quantify the extent to which these alterations contribute to slowing of the Ca2+ transient.
Methods
Induction of myocardial infarction (MI) and criteria for CHF
Animals were cared for according to the Norwegian Animal Welfare Act, which conforms to NIH guidelines (NIH publication no. 85-23, revised 1996). Two animals were kept in each cage and housed in a temperature regulated room with a 12 h day/12 h night cycling.
MI was induced in 9-week-old female C57BL/6 mice, as previously described (Woldbaek et al. 2002). Briefly, mice were anaesthetized with intravenous tail injections of 0.02 ml propofol (10 mg ml−1), tracheotomized, and exposed to a mixture of 1.8–2.2% isoflurane and 98% oxygen using a rodent ventilator (model 874092, B. Braun, Melsungen, Germany). A thoracotomy was performed and the left coronary artery was ligated to produce a large MI. Sham-operated (SHAM) mice underwent the same procedure without coronary artery ligation.
At 1 week following surgery, post-MI mice which had developed CHF were selected based on our previously reported criteria (Finsen et al. 2005). Specifically, using echocardiography, animals with CHF were distinguished from non-failing animals by increased left atrial diameter (>2.0 mm, versus 1.4 ± 0.0 mm in 1-week SHAM) and infarct size >40% of the total left ventricle circumference. Animals selected by these criteria exhibit CHF characterized by congestion and markedly reduced cardiac function (Finsen et al. 2005). Following evaluation at 1-week, CHF animals were either killed (1-week CHF group) or saved for an additional 2 weeks (3-week CHF group). Equivalent time points were used with SHAM animals (1-week SHAM, 3-week SHAM).
Cardiomyocyte isolation
Anaesthetized (1.8–2.2% isoflurane, 98% oxygen) mice were killed by cervical dislocation. Hearts were then quickly excised and placed in cold buffer-1 containing (mm): 130 NaCl, 25 Hepes, 22 d-glucose, 5.4 KCl, 0.5 MgCl2, 0.4 NaH2PO4, 0.01 μg ml−1 insulin (pH 7.4). Hearts were then cannulated, mounted on a Langendorff setup, and perfused retrogradely through the aorta with buffer-1 containing 200 U ml−1 collagenase Type II (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 0.1 mm Ca2+. Following 15–25 min of perfusion, hearts were cut down. In CHF mice, the MI and surrounding border zone were then carefully excised. Since the MI covered nearly the entire left ventricular free wall, CHF myocytes were only isolated from the noninfarcted part of the septum. Septal myocytes from SHAM hearts served as controls. The septum was minced and gently shaken at 37°C for 3–4 min in the same solution used in the perfusion with the addition of 1% BSA and 0.02 U ml−1 deoxyribonuclease I (Worthington). Following filtration (200 μm nylon mesh) and sedimentation, the cell pellet was washed three times in buffer-1 plus 1% BSA with progressively increasing [Ca2+] (0.1, 0.2 and 0.5 mm). Isolated cardiomyocytes were stored at room temperature until use.
Experimental protocols
In all experiments, myocytes were plated on laminin-coated coverslips placed in an open-perfusion chamber mounted on the stage of an inverted microscope. When myocytes had adhered, they were superfused at 22°C with Hepes Tyrode solution containing (mm): 140 NaCl, 1.0 CaCl2, 0.5 MgCl2, 5.0 Hepes, 5.5 glucose, 0.4 NaH2PO4, 5.4 KCl, pH 7.4. All experiments were performed on randomly selected myocytes which were free of membrane blebs.
Ca2+ transients were examined in myocytes loaded with 20 μm fluo-4 AM (Molecular Probes, Eugene, OR, USA) for 30 min. Ca2+ transients were elicited by field stimulating myocytes through a pair of platinum electrodes, with a 3 ms biphasic pulse 50% above threshold voltage. The T-tubular network was visualized by incubating cells with di-8-ANEPPS (10 μm; Molecular Probes) for 10 min, followed by 5 min of washout. In some experiments, cells were treated with 1 μm forskolin (Sigma, St Louis, MO, USA) in the superfusing solution. SR Ca2+ content was assessed by rapidly applying 10 mm caffeine (Sigma) and measuring the resulting Ca2+ transient.
Confocal fluorescence imaging
An LSM 510 scanning system (Zeiss GmbH, Jena, Germany) with a ×40 water-immersion objective was used for confocal fluorescence imaging. Fluo-4 and di-8-ANEPPS were excited at 488 nm, and emission intensity was measured at 510 nm. Pinhole width was set at 100 μm (1.41 Airy unit) to provide an optical slice thickness of 1 μm. For recording of Ca2+ transients, myocytes were scanned with a 512 pixel line; pixel width was between 0.19 and 0.37 μm. The scan line was positioned randomly along the longitudinal axis of the cell, although care was taken to avoid crossing nuclei. Myocytes were only examined if it was possible to focus on the entire length of the cell in a single plane. Cells were scanned every 1.5 ms, and sequential scans were stacked to create two-dimensional images with time in the x-axis. A light-emitting diode was used to record the start of the stimulus on a separate channel from the fluo-4 signal. In the line scan images, the stimulus appears as a vertical line 3 ms (2 pixels) in duration.
Images of the T-tubular network were obtained with 1024 × 1024 pixel XY images. Some of these images framed the cell (pixel width 0.12–0.25 μm) to show the complete cross-section of the T-tubular structure, while others showed smaller sections in greater detail (pixel width 0.04–0.07 μm).
Image analysis
Image analysis was performed using Image J (NIH), MATLAB (The MathWorks, Inc., Natick, MA, USA), and SigmaPlot software (Systat Software, Inc., Point Richmond, CA, USA). Collected images of T-tubules for all cell groups were randomized and then examined blindly. For each cell, T-tubular structure was categorized by eye as ‘organized’, ‘somewhat disorganized’ or ‘markedly disorganized’. ‘Organized’ T-tubule structure was defined as a predominantly striated staining pattern with the majority of tubules appearing in the transverse direction. The extent of disorganization was estimated by the degree of departure from this regular striated pattern.
All line-scan images of Ca2+ transients were smoothed along both the temporal and spatial axes by a function which replaced each pixel with the average of its 3 × 3 pixel neighbourhood. Although line-scan images are presented with the stimulus line indicated, analysis of Ca2+ transients was performed on the basis of the fluo-4 signal. The half-maximal fluorescence of the entire spatially averaged line-scan (F50) was used as a threshold level in the analysis of Ca2+-release synchrony. In all cells examined, F50 was reached along the entire length of the line scan. For analysis of local Ca2+ transients, line-scan images were additionally smoothed with a 5 point running average in the time direction to reduce noise. Since local transients often exhibited noisy, plateau-like peaks, peak fluorescence and time-to-peak values were measured at the centre of these plateau regions.
Statistics
Statistical analyses were performed using SigmaPlot and R software (R Development Core Team, 2005). Mean data are presented (±s.e.m.) Most data comparisons were performed with two-tailed paired or unpaired t tests. Differences in proportions were determined using the Fishers Exact test. Beat-to-beat consistency of F50 profiles was calculated using correlations between five replicate measurements at each point along the scan line (see online Supplemental Material).
Results
Animal and cardiomyocyte characteristics
Mean animal characteristics are shown in Table 1. Both 1-week and 3-week CHF mice exhibited congestion, as evidenced by larger lung weight and lung weight/body weight values than in time-matched SHAMs. Mean heart weight and heart weight/body weight ratios were also larger in CHF mice at both time points. However, myocyte hypertrophy was only detected in the 3-week CHF group, as measurements of cell length and cross-sectional area were increased from values in 3-week SHAM (P < 0.05).
Table 1.
Animal and cardiomyocyte characteristics
| 1-week SHAM | 1-week CHF | 3-week SHAM | 3-week CHF | |
|---|---|---|---|---|
| BW (g) | 21.5 ± 0.4 | 18.5 ± 0.6* | 22.3 ± 0.8 | 22.9 ± 0.6 |
| HW (g) | 0.225 ± 0.020 | 0.322 ± 0.020* | 0.248 ± 0.018 | 0.383 ± 0.040* |
| LW (g) | 0.152 ± 0.005 | 0.301 ± 0.016* | 0.162 ± 0.004 | 0.278 ± 0.032* |
| HW/BW (×10−3) | 10.4 ± 1.0 | 17.6 ± 1.1* | 11.1 ± 0.7 | 16.6 ± 1.5* |
| LW/BW (×10−3) | 7.1 ± 0.3 | 16.5 ± 1.1* | 7.3 ± 0.4 | 12.1 ± 1.4* |
| Cell length (μm) | 140.1 ± 3.2 | 143.7 ± 4.1 | 139.2 ± 7.0 | 159.0 ± 3.6* |
| Cell width (μm) | 23.8 ± 0.9 | 24.5 ± 0.8 | 24.4 ± 1.7 | 24.1 ± 0.7 |
| Cell area (μm2) | 2981 ± 126 | 3024 ± 145 | 2960 ± 261 | 3400 ± 137* |
CHF, congestive heart failure; BW, body weight; HW, heart weight; LW, lung weight;
P < 0.05 versus time-matched SHAM; nanimals: 1-week SHAM = 13, 1-week CHF = 13, 3-week SHAM = 6, 3-week CHF = 6; ncells: 1-week SHAM = 37, 1-week CHF = 64, 3-week SHAM = 38, 3-week CHF = 51.
Alterations in the T-tubular network during heart failure progression
Alterations in T-tubular structure during heart failure were examined by staining cells with di-8-ANEPPS. Representative myocytes are presented in Fig. 1. Both 1-week (Fig. 1A) and 3-week SHAMs (Fig. 1C) had very organized T-tubular structure. These cells exhibited a striated staining pattern since most tubules appeared in the transverse direction, although some longitudinal tubules were also observed. In 1-week CHF (Fig. 1B), T-tubular structure appeared somewhat disorganized; there was a general loss of the striated staining pattern and there appeared to be an increase in the proportion of longitudinal tubules. T-tubules were further disorganized in 3-week CHF (Fig. 1D). Indeed, in some 3-week CHF cells, no evidence of the striated pattern remained and there was disordered staining in both the transverse and longitudinal directions. Many 3-week CHF cells showed irregular gaps between adjacent T-tubules which were observed infrequently in SHAM.
Figure 1. T-tubular structure is progressively disorganized during congestive heart failure (CHF) progression.
T-tubules were visualized with 10 μm di-8-ANEPPS. Shown are confocal cross-sections of living cells, with greater detail shown in the smaller panels. In SHAM cells (A and C), T-tubules were organized in a regular striated pattern. In 1-week CHF (B), T-tubular structure appeared somewhat disorganized, while more pronounced disorganization was observed in 3-week CHF (D).
Blinded characterization of T-tubule images confirmed our impressions of altered T-tubule structure during CHF. The majority of T-tubule images in 1-week and 3-week SHAM (13 of 15 cells, 36 of 42 cells, respectively) were deemed to have ‘organized’ structure, while very few images were so classified in CHF (2 of 24 1-week CHF cells, 1 of 30 3-week CHF cells). ‘Somewhat disorganized’ T-tubules were observed in 71% of cells in 1-week CHF, compared with only 13% in 1-week SHAM (P < 0.05). A greater proportion of 3-week CHF cells exhibited ‘marked disorganization’ of T-tubules than 1-week CHF cells (53 versus 21%, P < 0.05) and this degree of disorganization was never observed in SHAM.
Is T-tubule disorganization associated with increased dyssynchrony of Ca2+ release?
We quantified dyssynchrony of Ca2+ release using the method described in Fig. 2A. Line-scan images were thresholded to F50 values, and then outlined to more clearly illustrate the timing of Ca2+ release along the cell. For each point along the scan line, the first time at which F50 was reached was recorded to create an F50 profile (Fig. 2A, far right panel). More dyssynchronous Ca2+ transients would be expected to have greater variation in the time to reach F50 at different points along the cell. Therefore, we defined the dyssynchrony index as the standard deviation of the F50 profile.
Figure 2. Three-week CHF myocytes exhibit increased dyssynchrony of Ca2+ transients.
Longitudinal line scans in fluo-4-AM-loaded myocytes are displayed with the stimulus shown as a vertical line. A, Ca2+ release synchrony was quantified by thresholding images to half-maximal fluorescence (F50). The leading edge of the thresholded image was outlined to create a profile of the earliest time at which F50 was reached along the cell (A, far right). The standard deviation of these values was defined as the dyssynchrony index. B, Ca2+ release was quite uniform in most 1-week SHAM, 3-week SHAM, and 1-week CHF cells (see insets for greater detail of regions between arrowheads). More dyssynchronous Ca2+ release was observed in many 3-week CHF cells. C, mean dyssynchrony index values (1-week SHAM, ncells = 27; 1-week CHF, ncells = 24; 3-week SHAM, ncells = 25; 3-week CHF, ncells = 39; *P < 0.05 versus 3-week SHAM). D, distributions of dyssynchrony index measurements (Gaussian curve fits).
Figure 2B shows representative line-scan images from field-stimulated cells. SR Ca2+ release was quite uniform along most 1-week and 3-week SHAM cells (Fig. 2B, left panels), as indicated by relatively low dyssynchrony index values. In 1-week CHF (Fig. 2B, upper right), synchrony of Ca2+ release resembled that observed in SHAM. However, more non-uniform Ca2+ release was observed in many 3-week CHF cells (Fig. 2B, lower right). Line-scan images in these cells typically exhibited several regions where Ca2+ release was markedly delayed. While not all myocytes in 3-week CHF exhibited very dyssynchronous Ca2+ transients, the mean dyssynchrony index was significantly larger in 3-week CHF (4.3 ± 0.3 ms; ncells = 39) from 3-week SHAM (3.0 ± 0.2 ms; ncells = 25, P < 0.05; Fig. 2C) and the distribution of dyssynchrony index values was right-shifted (Fig. 2D).
To compare Ca2+ release at different locations along the line scans, we defined regions of early and delayed Ca2+ release using the F50 profiles (Fig. 3A). This was accomplished by first marking the earliest time point at which F50 was reached along the scan line. Interestingly, this point occurred earlier in 3-week SHAM (6.0 ± 0.5 ms, ncells = 22) than 3-week CHF (8.6 ± 0.6, ncells = 25, P < 0.05). Regions of the cell which reached F50 within 10 ms of the earliest F50 point were considered to have early Ca2+ release. Ca2+ release that reached F50 at later times was defined as delayed release. Delayed Ca2+ release comprised a larger proportion of the line scan (i.e. a larger number of data points outside the 10 ms window) in 3-week CHF cells than in 3-week SHAM (26 ± 5% versus 9 ± 2%, P < 0.05). Regions of delayed Ca2+ release were defined by a minimum width of 2 μm (arrows in Fig. 3A), and corresponded closely with delayed areas detectable by eye in line-scan images (Fig. 2B). Delayed release regions occurred approximately 2.5-fold more frequently in 3-week CHF than in 3-week SHAM (Fig. 3B). Measurements of the widths of delayed release regions were similarly distributed in the two groups (Fig. 3C), with the exception of a few very large (>10 μm) regions which were observed in 20% of 3-week CHF cells (5 of 25) and never in 3-week SHAM (0 of 22 cells, P = 0.051). The mean delayed release region width was 4.1 ± 0.4 μm in 3-week SHAM, and 7.7 ± 2.2 μm in 3-week CHF.
Figure 3. Three-week CHF cells show a greater number of delayed release regions.
A, representative F50 profiles from a 3-week SHAM and 3-week CHF cell. Early Ca2+ release was defined for regions which reached F50 within 10 ms of the earliest F50 point (between dashed lines). Delayed release was defined outside this window, and delayed regions with a minimum width of 2 μm were considered (arrows). B, number of delayed release regions per cell (3-week SHAM, ncells = 22; 3-week CHF, ncells = 25; *P < 0.05). C, distributions of width measurements for delayed release regions (3-week SHAM, nregions = 26 in 22 cells; 3-week CHF, nregions = 73 in 25 cells).
Does increased dyssynchrony in CHF result from T-tubule disorganization?
We directly examined the relationship between changes in T-tubule organization and Ca2+ release synchrony by staining fluo-4-loaded myocytes with di-8-ANEPPS. Figure 4 shows paired cross-sectional and line-scan images from these experiments in a 3-week SHAM and 3-week CHF cell (Fig 4A and B, respectively). Due to background Ca2+ fluorescence, the resolution of T-tubules in these images is somewhat reduced from that possible in cells loaded with only di-8-ANEPPS. Locations where the scan line (shown in white) intersects T-tubules appear as bright horizontal lines in the line-scan images. Since T-tubule organization was quite uniform in 3-week SHAM cells, the scan line rarely crossed regions without T-tubules and a regular T-tubule pattern was observed in line-scan images. In 3-week CHF cells, T-tubule disorganization caused the longitudinal scan line to intersect T-tubules less frequently, leading to a more sparse appearance of T-tubules in line-scan images. In these cells we observed that in regions within close proximity to T-tubules, Ca2+ release occurred soon after the stimulus. This was visualized as an increase in fluorescence along the horizontal lines indicating T-tubules. Occasionally, we observed early Ca2+ release without the presence of a T-tubule in line-scan images, but examination of cross-sectional images showed that T-tubules were always very nearby the scan line at these locations. Importantly, regions where Ca2+ release was delayed always appeared in parts of the cell devoid of T-tubules. This observation was also true of 3-week SHAM cells, although delayed release regions and irregular gaps between T-tubules occurred less frequently in these cells. These findings strongly suggest that gaps between adjacent T-tubules can lead to the formation of delayed Ca2+ release regions and that the marked disorganization of T-tubules in 3-week CHF contributes to the increased dyssynchrony of Ca2+ transients in these cells.
Figure 4. Regions of delayed Ca2+ release occur at irregular gaps between T-tubules.
T-tubules and Ca2+ transients were visualized simultaneously in fluo-4-AM-loaded myocytes stained with di-8-ANEPPS. Paired T-tubule and line-scan images are shown, with the position of the scan line indicated by a dotted vertical white line. In both 3-week SHAM (A) and 3-week CHF (B), Ca2+ release occurred early in regions near T-tubules, while delayed Ca2+ release regions always occurred at gaps between T-tubules. More gaps and delayed release regions were apparent in 3-week CHF than 3-week SHAM.
Changes in the pattern of Ca2+ release resulting from structural changes such as T-tubule disorganization would be expected to be consistent between beats (Heinzel et al. 2002). We compared the pattern of Ca2+ release over consecutive beats using F50 profiles, as shown in Fig. 5A and B, for a 3-week SHAM and a 3-week CHF cell, respectively. In every cell examined, F50 profiles of any two beats were highly correlated, and there was no indication of neighbouring beats being more or less correlated than replicates further apart. The overall average correlation across all cells and beats was 0.534 ± 0.143 (n = 16) in 3-week SHAM, and 0.486 ± 0.135 (n = 16) in 3-week CHF. Therefore, in both cell types, approximately 50% of the variance in each F50 profile results from a common underlying signal. This signal was extracted using smoothing of four profiles to predict the fifth profile (described in the online Supplemental Material; see examples in Fig. 5A and B). We calculated that the variance of the common signal was greater in 3-week CHF than 3-week SHAM (9.52 versus 6.37 ms2, P < 0.05), supporting the notion there is an increase in dyssynchrony in 3-week CHF resulting from structural alterations. Interestingly, subtraction of the common signal from the data revealed that the random beat-to-beat component of the F50 profile was also more variable in 3-week CHF than 3-week SHAM (Fig. 5C; mean beat-to-beat variance 9.55 ms2 in 3-week CHF, versus 5.66 ms2 in 3-week SHAM, P < 0.05). Thus, increased beat-to-beat variability is likely to contribute to the greater Ca2+ release dyssynchrony observed in 3-week CHF. Importantly, however, release that was variable between beats was never markedly delayed. Thus, delayed release regions as defined by the method in Fig. 3A resulted from irregular gaps between T-tubules but not from beat-to-beat variability in Ca2+ release. Figure 5D shows that error in predicting the common signal decreased as a greater number of beats were included in the averaging procedure. This decline closely mirrored values predicted by a common signal across beats, confirming our method for signal extraction.
Figure 5. The general shape of F50 profiles is maintained between beats.
F50 profiles are shown for five consecutive beats in a 3-week SHAM cell (A) and 3-week CHF cell (B). The extracted common signal for each set of profiles is indicated. C, the random beat-to-beat component of F50 profiles was more variable in 3-week CHF than 3-week SHAM, as indicated for beat 3 of the profiles in A and B. D, mean prediction error variance decreased in both 3-week CHF (ncells = 16) and 3-week SHAM (ncells = 16) as more replicates were included in the calculation. This decline closely followed theoretical values.
Increased dyssynchrony of Ca2+ release can result from decreased phosphorylation status of Ca2+ cycling proteins (Litwin et al. 2000) However, Fig. 6 shows that Ca2+ transients were not synchronized in either 3-week SHAM or 3-week CHF by treatment with forskolin. In both cell types, regions of delayed Ca2+ release observed in control conditions remained clearly delayed in the presence of forskolin. In 3-week SHAM, the mean dyssynchrony index was 3.0 ± 0.4 in control and 3.5 ± 0.6 during forskolin treatment (n = 6, NS). The mean dyssynchrony index was also unaltered in 3-week CHF (control, 4.1 ± 0.8; forskolin, 5.1 ± 0.9; n = 8, NS). However, the signalling pathway downstream of adenylate cyclase appears to remain intact in 3-week CHF. Indeed, forskolin treatment induced a somewhat greater increase in the Ca2+ transient magnitude in 3-week CHF (142 ± 38%) than in 3-week SHAM (58 ± 17%, P = NS). Taken together, our data present considerable evidence that T-tubule disorganization increases the dyssynchrony of Ca2+ release in 3-week CHF cells.
Figure 6. Ca2+ release is not synchronized by forskolin.
In both 3-week SHAM and 3-week CHF cells, the general pattern of Ca2+ release was not altered by forskolin treatment.
How much does dyssynchrony of Ca2+ release contribute to slowing of the Ca2+ transient?
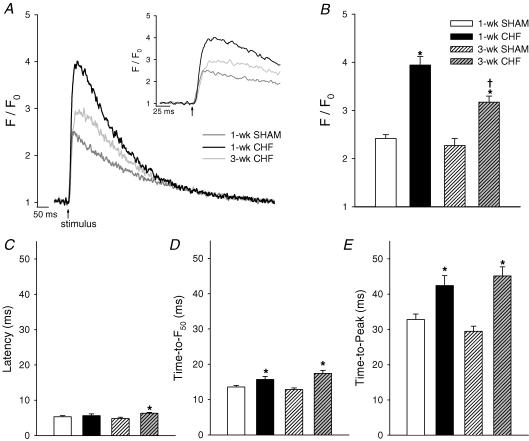
Increased dyssynchrony of Ca2+ release can result in slowing of the rising phase of the Ca2+ transient (Heinzel et al. 2002; Louch et al. 2004; Harris et al. 2005). We therefore investigated changes in the temporal shape of Ca2+ transients during heart failure progression. Figure 7A shows spatially averaged Ca2+ transients from 1-week SHAM, 1-week CHF, and 3-week CHF. Transients were very similar in 1-week and 3-week SHAM. Surprisingly, the magnitude of Ca2+ transients was markedly higher in 1-week CHF than in 1-week SHAM, a phenomenon that was partially reversed in 3-week CHF (Fig. 7B). Rapid application of 10 mm caffeine induced a larger Ca2+ transient in 1-week CHF (F/F0 = 4.9 ± 0.4, ncells = 15) than in 1-week SHAM (F/F0 = 3.6 ± 0.4, ncells = 10, P < 0.05), suggesting that SR Ca2+ content was increased. Thus, increased Ca2+ transients in this model of CHF may result from elevated SR Ca2+ content. Preliminary data collected at a more chronic time point (10-week CHF) suggest that SR Ca2+ content is not reduced from values in 10-week SHAM (not shown). Thus, heart failure progression in mice following MI may differ from that observed in other species in which decreased SR Ca2+ content is reported (for review see Houser et al. 2000).
Figure 7. Overall Ca2+ release becomes progressively slower during CHF.
A, representative spatially averaged Ca2+ transients, with the early phase expanded in the inset. B, mean transient magnitudes were increased in CHF. Mean latency, time-to-F50, and time-to-peak values are shown in C, D, and E, respectively. Slowing of overall Ca2+ release in CHF occurred predominantly between F50 and peak fluorescence (1-week SHAM, ncells = 27; 1-week CHF, ncells = 24; 3-week SHAM, ncells = 25; 3-week CHF, ncells = 39; *P < 0.05 versus respective SHAM, †P < 0.05 versus 1-week CHF).
Prolongation of Ca2+ release was observed in CHF cells throughout the rising phase of the transient. Latency, the delay between the stimulus pulse and the beginning of the Ca2+ transient, was significantly longer in 3-week CHF (6.3 ± 0.2 ms) than in 3-week SHAM (4.8 ± 0.4 ms, Fig. 7C), although it should be noted that this increase was equal to our temporal resolution of 1.5 ms. Time-to-F50 values were significantly prolonged in both 1-week and 3-week CHF, from 1-week and 3-week SHAM, respectively (Fig. 7D). However, the greatest slowing of Ca2+ release in CHF cells occurred between F50 and the peak of the transient. In 1-week and 3-week CHF, time-to-peak values were 29 and 53% larger than values in respective SHAMs (Fig. 7E). Thus, it appears that slowing of Ca2+ release is already apparent in 1-week CHF in this model and becomes more pronounced with progression of CHF. Importantly, prolongation of the Ca2+ transient is not simply the result of larger Ca2+ transients in CHF. In SHAM cells, transient magnitude and time-to-peak values were not positively correlated. As well, increased SR content as occurs in our CHF cells would actually be predicted to cause more rapid Ca2+ release due to increased ryanodine receptor Ca2+ conductance and open probability (Sitsapesan & Williams, 1994; Lukyanenko et al. 1996) resulting in decreased time-to-peak values (Okazaki et al. 1990). Therefore, measuring alterations in time-to-peak values in CHF relative to SHAM might, if anything, underestimate the prolongation of Ca2+ release in CHF cells.
To estimate the extent to which T-tubule disorganization contributed to slowing of the Ca2+ transient, we compared Ca2+ release in early and delayed release regions. Figure 8A shows representative local transients spatially averaged from 2 μm sections located within early and delayed regions. The magnitude of Ca2+ release in delayed regions was not significantly altered from that observed in early regions (3-week SHAM: magnitude early = 2.1 ± 0.1, nregions = 27; late = 1.8 ± 0.1, nlate regions = 11, P = NS; 3-week CHF: magnitude early = 3.5 ± 0.2, nregions = 20; late = 3.1 ± 0.2, nregions = 20, P = NS). By definition, local Ca2+ transients were slower in delayed release regions than in early regions (Fig. 8B and C). Interestingly, local transients in early release regions were slower to peak in 3-week CHF than 3-week SHAM. Delayed release regions exhibited a characteristic broadening of the peak in both 3-week CHF and 3-week SHAM, with the most marked slowing of Ca2+ release occurring between F50 and peak fluorescence. Thus, the greater proportion of delayed Ca2+ release regions observed in 3-week CHF would be expected to contribute to the broader peak of the overall Ca2+ transient in these cells. Based on our measurements of local transients, and with the calculated increase in the proportion of delayed Ca2+ release in 3-week CHF, we can estimate that delayed Ca2+ release regions increased time-to-peak values by approximately 3 ms in 3-week CHF.
Figure 8. Alterations in local Ca2+ transients during CHF.
A, representative local Ca2+ transients from early and delayed Ca2+ release regions are shown for a 3-week SHAM cell and 3-week CHF cell. B, mean time-to-F50 values; C, time-to-peak values. Transients in delayed regions exhibited broad peaks, with marked slowing between F50 and peak fluorescence (3-week SHAM, nearly regions = 27, nlate regions = 11, from 10 cells; 3-week CHF, nearly regions = 20, nlate regions = 20, from 10 cells; *P < 0.05 versus respective early region, †P < 0.05 versus equivalent region in SHAM).
Discussion
The present study demonstrates that T-tubule structure in murine cardiomyocytes becomes progressively disorganized during early CHF following MI. This structural rearrangement caused irregular gaps to appear between adjacent T-tubules, especially in 3-week CHF cells. Regions of delayed Ca2+ release observed in line-scan images occurred at these gaps between T-tubules. An increased occurrence of delayed Ca2+ release sites in 3-week CHF contributed to a more dyssynchronous pattern of Ca2+ release. This pattern was consistent between beats and not altered by forskolin treatment. Increased dyssynchrony contributed to slowing of overall Ca2+ release in 3-week CHF cells, although a uniform slowing of Ca2+ release was also observed beginning at 1-week CHF.
T-tubule alterations in heart failure
Few studies have examined the structure of the T-tubule network in heart failure. Kamp and collaborators observed a marked loss of T-tubules in canine ventricular myocytes following tachycardia-induced heart failure (He et al. 2001; Balijepalli et al. 2003). However, only a single preliminary study has reported decreased T-tubule density in human failing cardiomyocytes (Wong et al. 2001). An unchanged (Ohler et al. 2001) and even an increased T-tubule density (Kaprielian et al. 2000) have also been reported. Louch et al. (2004) observed that a prominent T-tubule network was in place in failing human ventricular myocytes, although that study was conducted without human controls. Thus, based on the small amount of literature to date it seems unlikely that there is marked T-tubule loss in human heart failure. However, several reports agree that the T-tubule network may be re-structured in this condition. These changes may include dilation of the T-tubules (Kostin et al. 1998; Kaprielian et al. 2000; Louch et al. 2004) or more complex structural disorganization. Kaprielian et al. (2000) observed more twisted T-tubules in failing human cells, with an increased proportion of tubules running in the longitudinal direction. Similar observations were made in a preliminary report of failing human myocytes (Wong et al. 2001) and in cells from spontaneously hypertensive rats which had developed heart failure (Song et al. 2006). In the present study, we also observed T-tubule disorganization that involved an increased number of longitudinal tubules. At present the trigger for T-tubule disorganization in heart failure is unknown, but possible mechanisms include activation of fetal genes and/or loss of structural proteins.
Local alterations in Ca2+ homeostasis: implications for heart failure
This study directly links alterations in T-tubule structure to alterations in Ca2+ handling during heart failure. However, previous work has shown that similar mechanisms can contribute to dyssynchronous Ca2+ release in healthy cells. Heinzel et al. (2002) observed large inhomogeneities during Ca2+ release in pig ventricular myocytes, and showed that these regions of delayed Ca2+ release occurred at gaps between T-tubules; they observed much more synchronous release of Ca2+ in mouse ventricular myocytes and attributed this difference to a higher density of T-tubules in these cells. In the present study, we have also observed quite uniform release of Ca2+ in SHAM mouse cells. The regions of delayed Ca2+ release and the corresponding gaps between adjacent T-tubules that we observed in these cells were indeed smaller and fewer than those reported in healthy pig myocytes. In the present study, and in the study by Heinzel et al. (2002), regions of delayed Ca2+ release occurred in the same part of the cell during consecutive beats and Ca2+ transients were not synchronized by stimulation of the β-adrenergic signalling pathway.
We observed that T-tubules were disorganized in nearly all 3-week CHF cells examined. However, in some cells the degree of disorganization was not sufficient to cause the formation of irregular gaps between T-tubules, and few delayed Ca2+ release regions were observed. Even in those 3-week CHF cells with the most pronounced T-tubule disorganization delayed release regions were usually not large. In both 3-week SHAM and 3-week CHF, most delayed release regions had a width of less than 4 μm, which is likely to correspond to only one or two missing transverse segments of tubule since the intertubule distance is normally ∼2 μm. If T-tubule disorganization continues with progression of CHF beyond 3 weeks, the size of delayed regions might increase as neighbouring gaps fuse. This effect has been observed previously during progressive loss of T-tubules in cell culture (Louch et al. 2004). This had not happened to any great extent in the current study because of the relatively low number of delayed regions per cell, but might explain the occurrence of a few large delayed release regions (>10 μm) in 3-week CHF.
It seems possible that in 3-week SHAM and 3-week CHF, Ca2+ influx is triggered in early release regions where T-tubules and L-type Ca2+ channels are present, and Ca2+ then diffuses more slowly into delayed release regions where T-tubules are absent (Lipp et al. 1996; Yang et al. 2002; Heinzel et al. 2002; Louch et al. 2004; Song et al. 2006). We believe that this diffusing Ca2+ then triggers SR Ca2+ release in delayed release regions. In support of this view, local transients in delayed release regions were not smaller in magnitude than those in early release regions. Although we have not examined the uniformity of ryanodine receptor distribution and function in CHF, previous studies have also suggested that SR function might remain intact despite the local absence of T-tubules and Ca2+ channels (Lipp et al. 1996; Yang et al. 2002; Heinzel et al. 2002; Louch et al. 2004; Song et al. 2006). Similarly, atrial and Purkinje cells which completely lack T-tubules exhibit SR Ca2+ release throughout the cell which is triggered solely by propagation of CICR (Berlin, 1995; Cordeiro et al. 2001). An important point for our study is that the local Ca2+ transients in delayed release regions had a similar temporal profile in 3-week CHF and 3-week SHAM, suggesting that the mechanisms underlying delayed Ca2+ release are analogous in the two cell types.
Our findings are consistent with the proposal that the ability of Ca2+ current to trigger SR Ca2+ release is compromised in heart failure (Gomez et al. 2001; Sjaastad et al. 2002; Sjaastad et al. 2005; Song et al. 2006). A decrease in CICR gain could be expected if T-tubule remodelling leads to ‘orphan’ ryanodine receptors that are cut off from the triggering Ca2+ signal (Gomez et al. 2001; Song et al. 2006). However, in this situation the effect of ‘autoregulation’ should also be considered (Trafford et al. 2002). Local absence of Ca2+ channels in areas devoid of T-tubules could lead to a compensatory increase in SR content in these regions. This would increase ryanodine receptor conductance and open probability (Sitsapesan & Williams, 1994; Lukyanenko et al. 1996) which could partially offset a decrease in gain of CICR. An increased gap between the T-tubules and SR could also occur so that all ryanodine receptors were located further from Ca2+ channels (Gomez et al. 2001). Such changes could explain the increase in latency of Ca2+ release we have observed in 3-week CHF, as there would be a longer delay between Ca2+ influx and CICR throughout the cell. Perhaps T-tubule disorganization leads to both types of structural changes in CHF: the appearance of ‘orphan’ ryanodine receptors and an increased distance between Ca2+ channels and ryanodine receptors.
Our statistical analyses of F50 profiles over consecutive beats showed that a common signal could be extracted to describe the shape of Ca2+ release along cells. Importantly, this common signal was more dyssynchronous in 3-week CHF than 3-week SHAM, supporting a causative role of structural alterations. Beat-to-beat variability in F50 profiles was also larger in 3-week CHF than 3-week SHAM, suggesting that non-structural mechanisms may also promote dyssynchrony of Ca2+ release. One such mechanism could be alteration of the stochastic activity of ryanodine receptors (Cannell et al. 1994). Beat-to-beat variability in Ca2+ release synchrony can also result from alterations in action potential configuration, as shown previously in normal (Sah et al. 2002) and failing (Harris et al. 2005) ventricular myocytes. Finally, decreased phosphorylation of Ca2+ handling proteins can trigger randomly dyssynchronous Ca2+ release, as reported in MI border zone myocytes (Litwin et al. 2000). However, this latter mechanism for dyssynchrony may be restricted to the myocardium neighbouring the infarction and is an unlikely explanation for our findings since forskolin treatment did not alter the synchrony of Ca2+ release in 3-week CHF.
Our findings are in agreement with previous studies on freshly isolated and cultured cells which have observed that cell regions where T-tubules are absent exhibit delayed Ca2+ release that is not synchronized by isoproterenol (Louch et al. 2004; Heinzel et al. 2002). However, isoproterenol treatment has been reported to synchronize Ca2+ release in de-tubulated myocytes (Brette et al. 2004). In this latter study, synchronization of the Ca2+ transient was attributed to enhanced coupling of clusters of ryanodine receptors and more rapid propagation of Ca2+-induced Ca2+ release due to increased SR Ca2+ content and ryanodine receptor phosphorylation. The apparent discrepancy between de-tubulated and intact myocyte studies could be explained by different regulation of ryanodine receptors in the two situations. Althought the de-tubulation procedure seals off T-tubules from the surface sarcolemma, T-tubules remain inside the cell and dyads may remain intact. Observations from the present study and other studies (Heinzel et al. 2002; Louch et al. 2004; Song et al. 2006) suggest that some ryanodine receptors are ‘orphaned’ in intact cells. Altered regulation of these ryanodine receptors not found in dyads may make them less susceptible to the effects of β-adrenergic stimulation.
In failing human heart, Ca2+ transients are prolonged and exhibit a characteristic broadening of the peak (Gwathmey et al. 1987; Beuckelmann & Erdmann, 1992; Piacentino et al. 2003). In our model of CHF, we observed that peak broadening partly resulted from an increased incidence of delayed release regions. We calculated that increased dyssynchrony of Ca2+ release in 3-week CHF increased time-to-peak values by approximately 3 ms. This is not unexpected based on the relatively small size and incidence of delayed release regions we have observed. Experimentally promoting large decreases in T-tubule density has been observed to slow time-to-peak values by only about 7 ms (Louch et al. 2004). However, Ca2+ transients were actually about 16 ms slower to peak in 3-week CHF than in 3-week SHAM (Fig. 7), and marked slowing of Ca2+ release was observed in 1-week CHF without increased dyssynchrony (Figs 2 and 7). Therefore, factors other than dyssynchrony must also contribute to the slowing of Ca2+ release in CHF. In support of this view, Ca2+ transients in 3-week CHF cells exhibited increased latency (Fig. 7C), the first time-point to reach F50 occurred later in 3-week CHF (Fig. 3A), and local transients in early release regions were slower (Fig. 8B and C). Thus, CHF in our model appears to involve a uniform slowing of Ca2+ release throughout the cell which begins early in CHF. With progression of CHF, a non-uniform dyssynchronous slowing of Ca2+ release also occurs as a result of T-tubule disorganization.
The mechanisms underlying the uniform slowing of Ca2+ release we have observed in CHF are unclear. Hyper-phosphorylation and uncoupling of neighbouring ryanodine receptors (Marx et al. 2000, 2001) could theoretically produce such effects in heart failure. As well, less coordinated Ca2+ channel openings might result in an overall slowing of Ca2+ release. We have previously postulated that this mechanism might result in loss of ‘high-gain’ CICR at relatively negative voltages (Sjaastad et al. 2005). Such alterations might also contribute to increased beat-to-beat variability in the timing of Ca2+ release. Finally, changes in SR content should be considered. Reduced SR content in chronically failing myocytes (for review see Houser et al. 2000) could contribute to slowing of Ca2+ release by reducing Ca2+ conductance and open probability of ryanodine receptors (Sitsapesan & Williams, 1994; Lukyanenko et al. 1996). Reduced SR content could also promote dyssynchronous Ca2+ release if non-uniform alterations in Ca2+ stores occur throughout the cell. However, in the present study we have employed a model of early CHF in which SR function is not reduced. Thus, it seems that reduced SR content is not a prerequisite for either slower or dyssynchronous Ca2+ release in CHF.
In conclusion, murine cardiomyocytes exhibit progressive disorganization of T-tubules during CHF following MI. This structural rearrangement causes irregular gaps to form between adjacent T-tubules which promotes more dyssynchronous Ca2+ release. Our results show that this mechanism can contribute to slowing of the Ca2+ transient in CHF.
Acknowledgments
The authors thank the Norwegian Regional Health Authority East, the Norwegian Research Council, Anders Jahre's Fund for the Promotion of Science, and the VIRUUS program at Ullevaal University Hospital for their generous funding.
Supplemental material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.107227 http://jp.physoc.org/cgi/content/full/jphysiol.2006.107227/DC1 and contains supplemental material on expanded statistics section.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165–H1170. doi: 10.1152/ajpheart.1995.269.3.H1165. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Erdmann E. Ca2+-currents and intracellular [Ca2+]i-transients in single ventricular myocytes isolated from terminally failing human myocardium. Basic Res Cardiol. 1992;87(Suppl. 1):235–243. doi: 10.1007/978-3-642-72474-9_19. [DOI] [PubMed] [Google Scholar]
- Brette F, Rodriguez P, Komukai K, Colyer J, Orchard CH. Beta-adrenergic stimulation restores the Ca2+ transient of ventricular myocytes lacking T-tubules. J Mol Cell Cardiol. 2004;36:265–275. doi: 10.1016/j.yjmcc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation–contraction coupling in cardiac myocytes. Biophys J. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JH. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol. 2001;531:301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol. 2005;98:680–689. doi: 10.1152/japplphysiol.00924.2004. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Franzini-Armstrong C. Formation of junctions involved in excitation–contraction coupling in skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation–contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- Heinzel FR, Bito V, Volders PG, Antoons G, Mubagwa K, Sipido KR. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res. 2002;91:1023–1030. doi: 10.1161/01.res.0000045940.67060.dd. [DOI] [PubMed] [Google Scholar]
- Houser SR, Piacentino V, III, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101:2586–2594. doi: 10.1161/01.cir.101.22.2586. [DOI] [PubMed] [Google Scholar]
- Kostin S, Scholz D, Shimada T, Maeno Y, Mollnau H, Hein S, Schaper J. The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell Tissue Res. 1998;294:449–460. doi: 10.1007/s004410051196. [DOI] [PubMed] [Google Scholar]
- Lipp P, Huser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol. 1996;497:589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules – a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Gyorke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Ohler A, Houser SR, Tomaselli GF, O'Rourke B. Transverse tubules are unchanged in myocytes from failing human hearts. Biophys J. 2001;80:590a. [Google Scholar]
- Okazaki O, Suda N, Hongo K, Konishi M, Kurihara S. Modulation of Ca2+ transients and contractile properties by beta-adrenoceptor stimulation in ferret ventricular muscles. J Physiol. 1990;423:221–240. doi: 10.1113/jphysiol.1990.sp018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino V, III, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. Vienna Austria: R Foundation for Statistical Computing; 2005. R: A language and environment for statistical computing. [Google Scholar]
- Sah R, Ramirez RJ, Backx PH. Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation–contraction coupling. Circ Res. 2002;90:165–173. doi: 10.1161/hh0202.103315. [DOI] [PubMed] [Google Scholar]
- Shacklock PS, Wier WG, Balke CW. Local Ca2+ transients (Ca2+ sparks) originate at transverse tubules in rat heart cells. J Physiol. 1995;487:601–608. doi: 10.1113/jphysiol.1995.sp020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+ J Membr Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- Sjaastad I, Birkeland JA, Ferrier G, Howlett S, Skomedal T, Bjornerheim R, Wasserstrom JA, Sejersted OM. Defective excitation-contraction coupling in hearts of rats with congestive heart failure. Acta Physiol Scand. 2005;184:45–58. doi: 10.1111/j.1365-201X.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Sjaastad I, Bokenes J, Swift F, Wasserstrom JA, Sejersted OM. Normal contractions triggered by ICa,L in ventricular myocytes from rats with postinfarction CHF. Am J Physiol Heart Circ Physiol. 2002;283:H1225–H1236. doi: 10.1152/ajpheart.00162.2001. [DOI] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Integrative analysis of calcium signalling in cardiac muscle. Front Biosci. 2002;7:d843–d852. doi: 10.2741/trafford. [DOI] [PubMed] [Google Scholar]
- Woldbaek PR, Hoen IB, Christensen G, Tonnessen T. Gene expression of colony-stimulating factors and stem cell factor after myocardial infarction in the mouse. Acta Physiol Scand. 2002;175:173–181. doi: 10.1046/j.1365-201X.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- Wong C, Soeller C, Burton L, Cannell MB. Changes in transverse-tubular system architecture in myocytes from diseased human ventricles. Biophys J. 2001;80:588a. [Google Scholar]
- Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+–Ca2+ exchange activity is localized in the T-tubules of rat ventricular myocytes. Circ Res. 2002;91:315–322. doi: 10.1161/01.res.0000030180.06028.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.107227 http://jp.physoc.org/cgi/content/full/jphysiol.2006.107227/DC1 and contains supplemental material on expanded statistics section.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com