Abstract
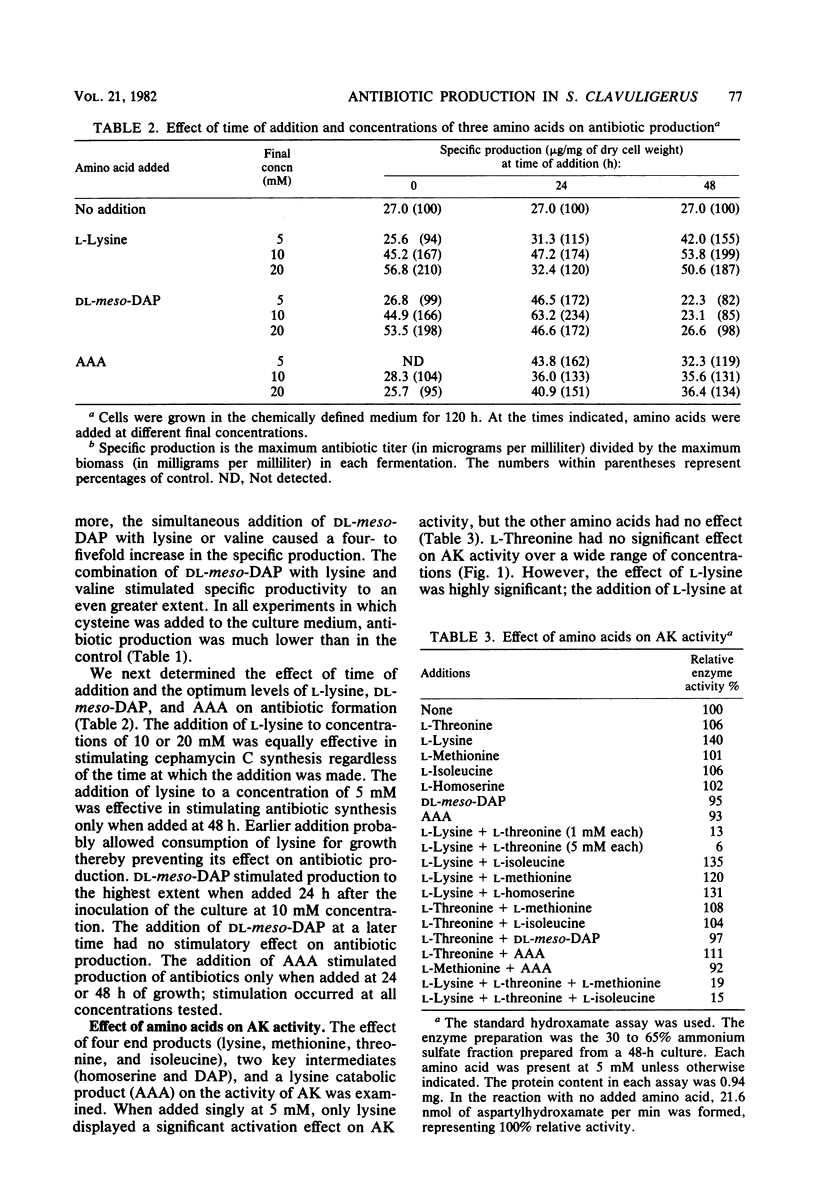
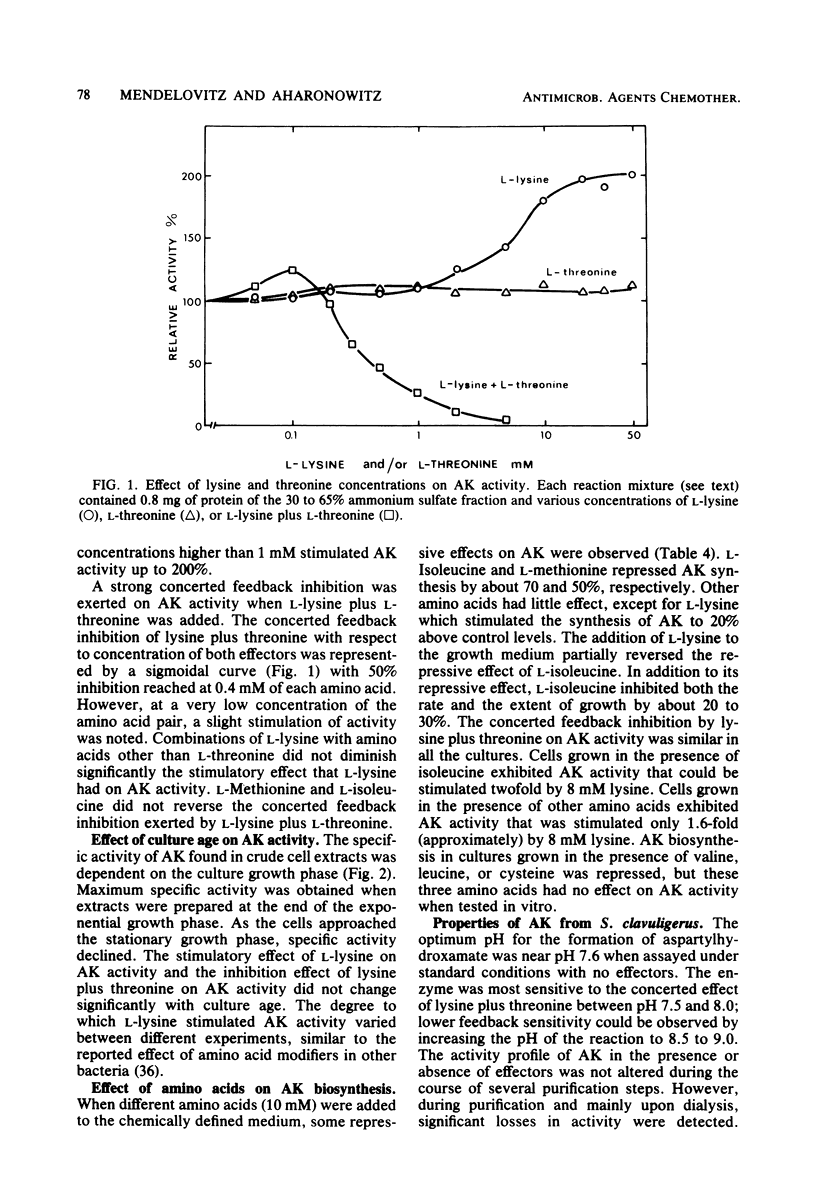
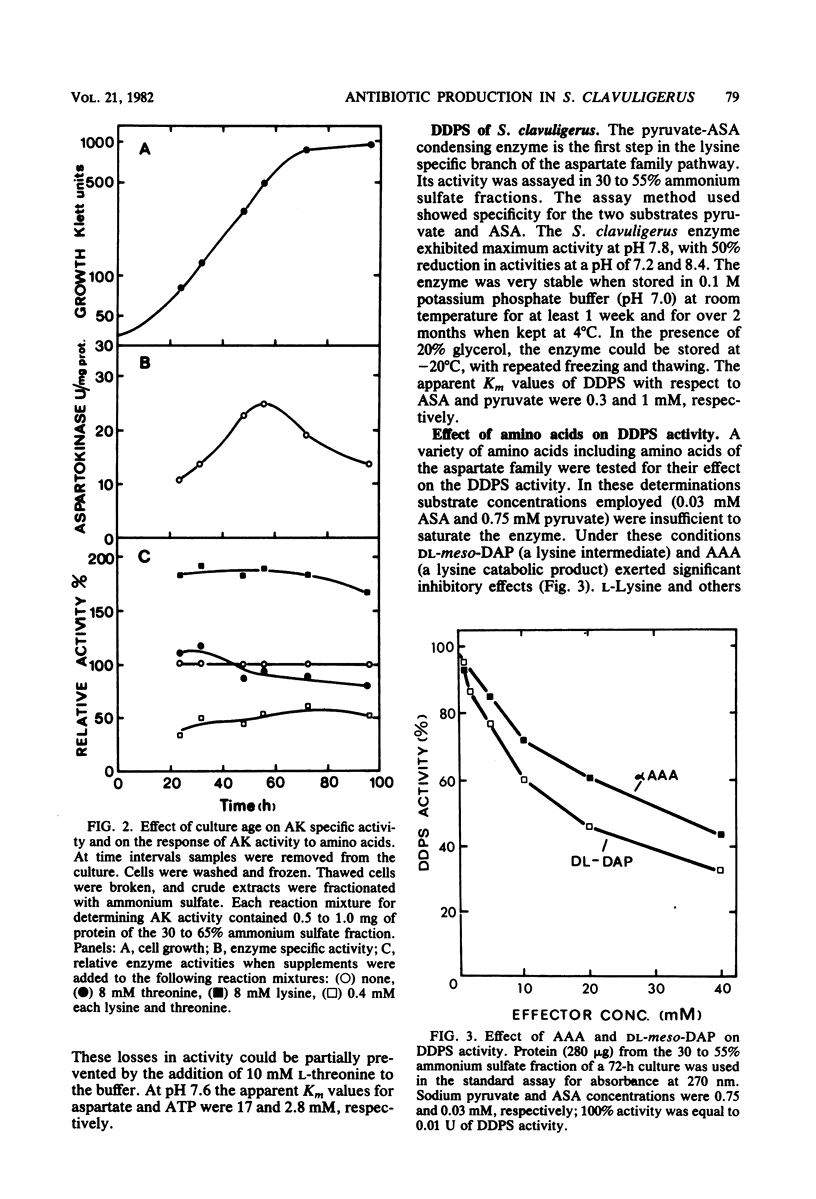
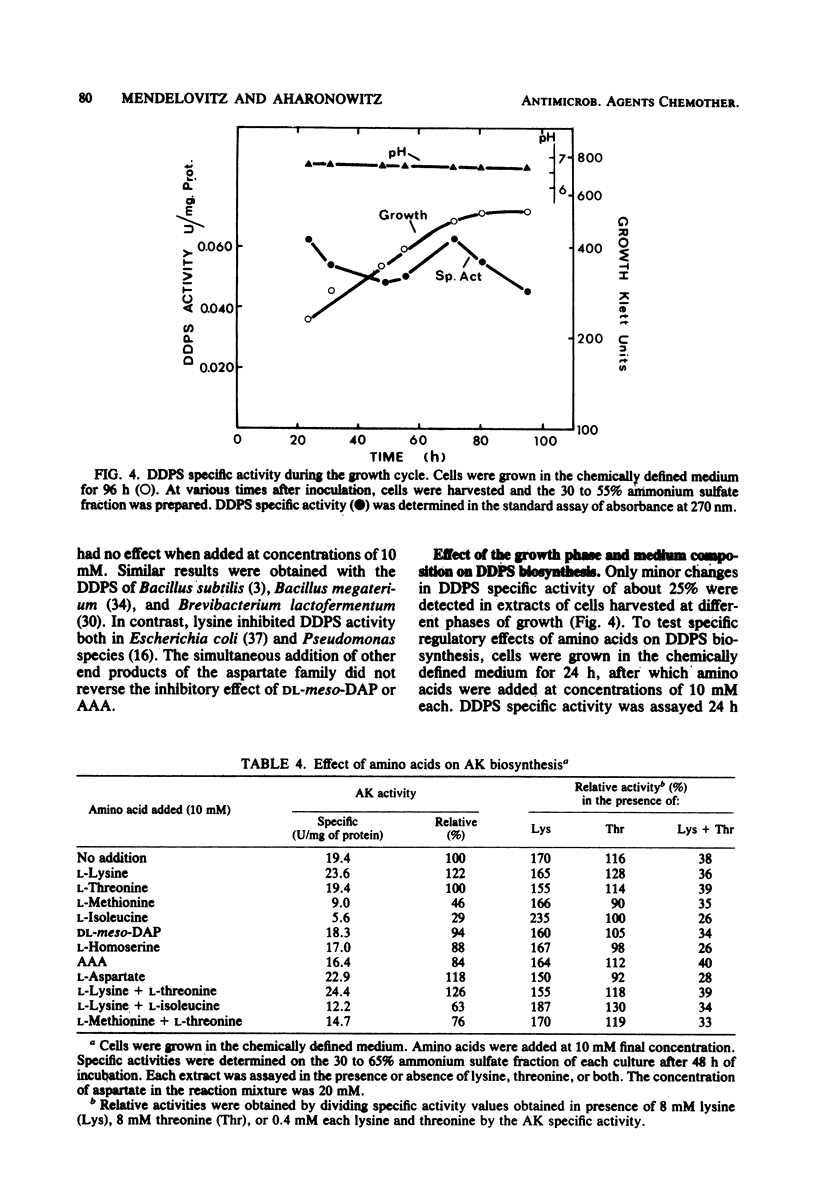
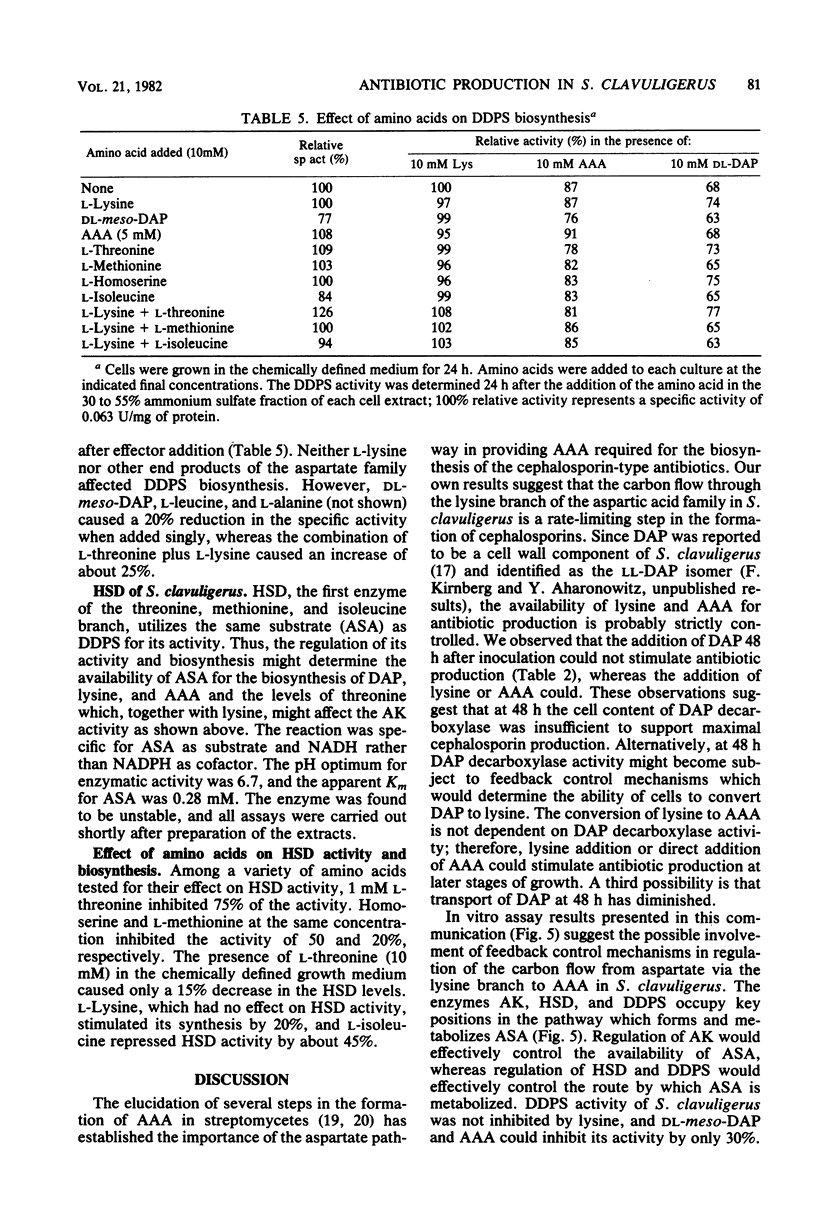
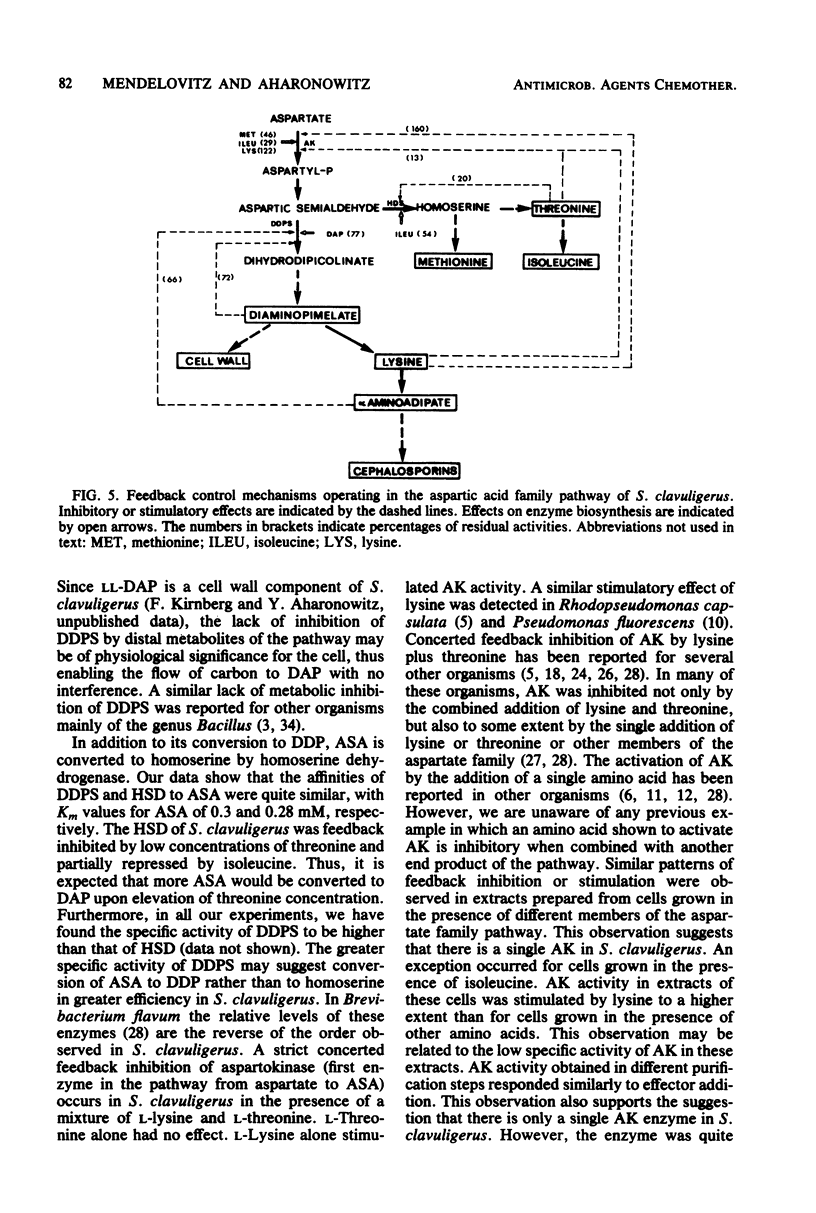
The effect of the cephalosporin precursors and amino acids of the aspartic acid family on antibiotic production by Streptomyces clavuligerus was investigated DL-meso-Diaminopimelate and L-lysine each stimulated specific antibiotic production by 75%. A fourfold increase in specific production was obtained by simultaneous addition of the two compounds. The stimulation could be further increased by adding valine to the two effectors. In the streptomycetes the alpha-aminoadipyl side chain of the cephalosporin antibiotics is derived from lysine. Streptomycetes, like other bacteria, are expected to produce lysine from aspartic acid; therefore, the feedback control mechanisms operating in the aspartic acid family pathway of S. clavuligerus, which may affect the flow of carbon to alpha-aminoadipic acid, were investigated. Threonine inhibited antibiotic production by 41% when added to minimal medium at a concentration of 10 mM. Simultaneous addition of 10 mM lysine completely reversed this inhibition. The aspartokinase of S. clavuligerus was found to be subject to concerted feedback inhibition by threonine and lysine. Threonine may act to limit the supply of lysine available for cephamycin C biosynthesis via this concerted mechanism. Single or simultaneous addition of any other amino acid of the aspartate family in the in vitro assay did not inhibit aspartokinase activity. Activity was stimulated by lysine. Aspartokinase biosynthesis was partially repressed by methionine or isoleucine at concentrations higher than 10 mM. Methionine, but not isoleucine, inhibited cephamycin C synthesis by 27% when added to minimal medium at a concentration of 10 mM. Dihydrodipicolinate synthetase, the first specific enzyme of the lysine branch, was not inhibited by lysine but was partially inhibited by high concentrations of 2,6-diaminopimelate and alpha-aminoadipate; it was slightly repressed by diaminopimelic acid. Homoserine dehydrogenase activity was inhibited by threonine and partially repressed by isoleucine. It appears that S. clavuligerus aspartokinase is a key step in the control of carbon flow toward alpha-aminoadipic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Demain A. L. Influence of inorganic phosphate and organic buffers on cephalosporin production by Streptomyces clavuligerus. Arch Microbiol. 1977 Nov 18;115(2):169–173. doi: 10.1007/BF00406371. [DOI] [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Chasin L. A., Szulmajster J. Biosynthesis of dipicolinic acid in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Dec 15;29(5):648–654. doi: 10.1016/0006-291x(67)90265-3. [DOI] [PubMed] [Google Scholar]
- D'Amato R. F., Pisano M. A. A chemically defined medium for cephalosporin C production by Paecilomyces persicinus. Antonie Van Leeuwenhoek. 1976;42(3):299–308. doi: 10.1007/BF00394127. [DOI] [PubMed] [Google Scholar]
- DATTA P., GEST H. ALTERNATIVE PATTERNS OF END-PRODUCT CONTROL IN BIOSYNTHESIS OF AMINO-ACIDS OF THE ASPARTIC FAMILY. Nature. 1964 Sep 19;203:1259–1261. doi: 10.1038/2031259a0. [DOI] [PubMed] [Google Scholar]
- DATTA P., GEST H. CONTROL OF ENZYME ACTIVITY BY CONCERTED FEEDBACK INHIBITION. Proc Natl Acad Sci U S A. 1964 Oct;52:1004–1009. doi: 10.1073/pnas.52.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. Biochemistry of penicillin and cephalosporin fermentations. Lloydia. 1974 Jun;37(2):147–167. [PubMed] [Google Scholar]
- Demain A. L., Masurekar P. S. Lysine inhibition of in vivo homocitrate synthesis in Penicillium chrysogenum. J Gen Microbiol. 1974 May;82(1):143–151. doi: 10.1099/00221287-82-1-143. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Effect of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977;31:343–356. doi: 10.1146/annurev.mi.31.100177.002015. [DOI] [PubMed] [Google Scholar]
- Dungan S. M., Katta P. Concerted feedback inhibition. Purification and some properties of aspartokinase from Pseudomonas fluorescens. J Biol Chem. 1973 Dec 25;248(24):8534–8540. [PubMed] [Google Scholar]
- Filer D., Kindler S. H., Rosenberg E. Aspartokinase of Micrococcus luteus. 'Feedback-stimulation" by methionine. FEBS Lett. 1977 Mar 15;75(1):177–179. doi: 10.1016/0014-5793(77)80080-x. [DOI] [PubMed] [Google Scholar]
- Filer D., Rosenberg E., Kindler S. H. Aspartokinase of Myxococcus xanthus: "feedback stimulation" by required amino acids. J Bacteriol. 1973 Jul;115(1):23–28. doi: 10.1128/jb.115.1.23-28.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Demain A. L. Homocitrate synthase as the crucial site of the lysine effect on penicillin biosynthesis. J Antibiot (Tokyo) 1977 Sep;30(9):760–761. doi: 10.7164/antibiotics.30.760. [DOI] [PubMed] [Google Scholar]
- Friedrich C. G., Demain A. L. Uptake and metabolism of alpha-aminoadipic acid by Penicillium chrysogenum Wis 54-1255. Arch Microbiol. 1978 Oct 4;119(1):43–47. doi: 10.1007/BF00407926. [DOI] [PubMed] [Google Scholar]
- Hermann M., Thevenet N. J., Coudert-Maratier M. M., Vandecasteele J. P. Consequences of lysine oversynthesis in Pseudomonas mutants insensitive to feedback inhibition. Lysine excretion or endogenous induction of a lysine-catabolic pathway. Eur J Biochem. 1972 Oct 17;30(1):100–106. doi: 10.1111/j.1432-1033.1972.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock M. H., Hodgson B. Lysine- and lysine-plus-threonine-inhibitable aspartokinases in Bacillus brevis. Biochim Biophys Acta. 1976 Sep 14;445(2):350–363. doi: 10.1016/0005-2744(76)90089-9. [DOI] [PubMed] [Google Scholar]
- Kern B. A., Hendlin D., Inamine E. L-lysine epsilon-aminotransferase involved in cephamycin C synthesis in Streptomyces lactamdurans. Antimicrob Agents Chemother. 1980 Apr;17(4):679–685. doi: 10.1128/aac.17.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick J. R., Doolin L. E., Godfrey O. W. Lysine biosynthesis in Streptomyces lipmanii: implications in antibiotic biosynthesis. Antimicrob Agents Chemother. 1973 Nov;4(5):542–550. doi: 10.1128/aac.4.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., López M. J., Villanueva J. R., Martín J. F. Inhibition and repression of homocitrate synthase by lysine in Penicillium chrysogenum. J Bacteriol. 1980 Dec;144(3):869–876. doi: 10.1128/jb.144.3.869-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurekar P. S., Demain A. L. Lysine control of penicillin biosynthesis. Can J Microbiol. 1972 Jul;18(7):1045–1048. doi: 10.1139/m72-162. [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Chang Y. F. Aspartokinase of Streptococcus mutans: purification, properties, and regulation. J Bacteriol. 1978 May;134(2):483–491. doi: 10.1128/jb.134.2.483-491.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Kinetic studies. J Biol Chem. 1967 Nov 10;242(21):4980–4986. [PubMed] [Google Scholar]
- Rosner A., Paulus H. Regulation of aspartokinase in Bacillus subtilis. The separation and properties of two isofunctional enzymes. J Biol Chem. 1971 May 10;246(9):2965–2971. [PubMed] [Google Scholar]
- Shiio I., Miyajima R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem. 1969 Jun;65(6):849–859. doi: 10.1093/oxfordjournals.jbchem.a129089. [DOI] [PubMed] [Google Scholar]
- TROWN P. W., SMITH B., ABRAHAM E. P. Biosynthesis of cephalosporin C from amino acids. Biochem J. 1963 Feb;86:284–291. doi: 10.1042/bj0860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Warren S. C., Newton G. G., Abraham E. P. Use of alpha-aminoadipic acid for the biosynthesis of penicillin N and cephalosporin C by a Cephalosporium sp. Biochem J. 1967 Jun;103(3):891–901. doi: 10.1042/bj1030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster F. H., Lechowich R. V. Partial purification and characterization of dihydrodipicolinic acid synthetase from sporulating Bacillus megaterium. J Bacteriol. 1970 Jan;101(1):118–126. doi: 10.1128/jb.101.1.118-126.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. G., Brannon D. R., Mabe J. A., Wicker K. J. Incorporation of labeled precursors into A16886B, a novel -lactam antibiotic produced by Streptomyces clavuligerus. Antimicrob Agents Chemother. 1972 Mar;1(3):247–251. doi: 10.1128/aac.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUGARI Y., GILVARG C. Coordinate end-product inhibition in lysine synthesis in Escherichia coli. Biochim Biophys Acta. 1962 Aug 27;62:612–614. doi: 10.1016/0006-3002(62)90256-1. [DOI] [PubMed] [Google Scholar]
- Yen H., Gest H. Regulation of biosynthesis of aspartate family amino acids in the photosynthetic bacterium Rhodopseudomonas palustris. Arch Microbiol. 1974;101(3):187–210. doi: 10.1007/BF00455938. [DOI] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]


