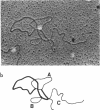
Abstract
Clinical isolates of Haemophilus ducreyi from patients with chancroid were shown to have one or more 4.9- to 7.0-megadalton non-self-transferable plasmids and to have in vitro resistance to sulfonamides. Transformation of Escherichia coli to sulfonamide resistance was associated with the acquisition of a 4.9-megadalton plasmid, which did not confer linked resistance to streptomycin. The guanine-plus-cytosine content of this plasmid was found to be 57%. Filter-blot hybridization and restriction endonuclease digestion studies suggested a relationship of this plasmid to RSF1010. Electron microscope heteroduplex analysis confirmed this relationship. The identification in H. ducreyi of a plasmid closely related to plasmids found in enteric species, rather than transposition of a resistance determinant to an indigenous plasmid, suggests that further dissemination of the enteric plasmid pool to this genus is possible since plasmid transfer between certain Haemophilus species is readily demonstrated.
Full text
PDF
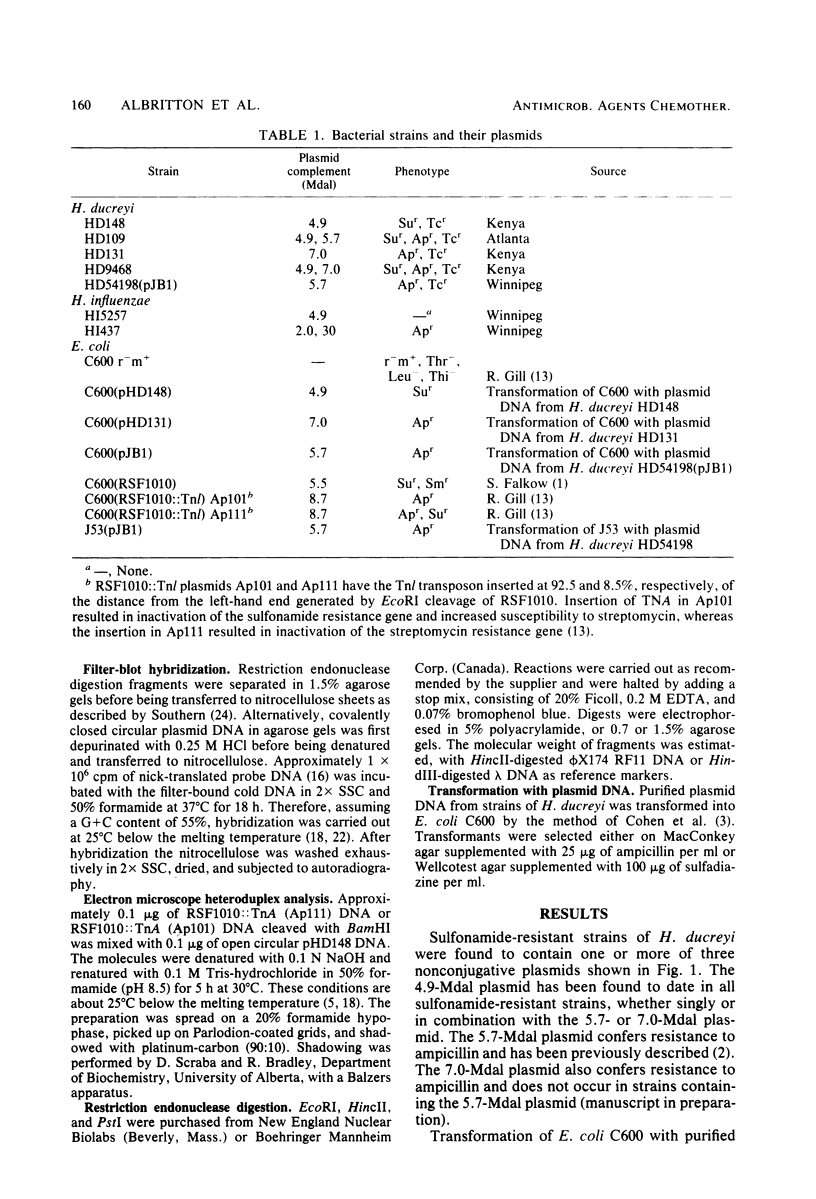
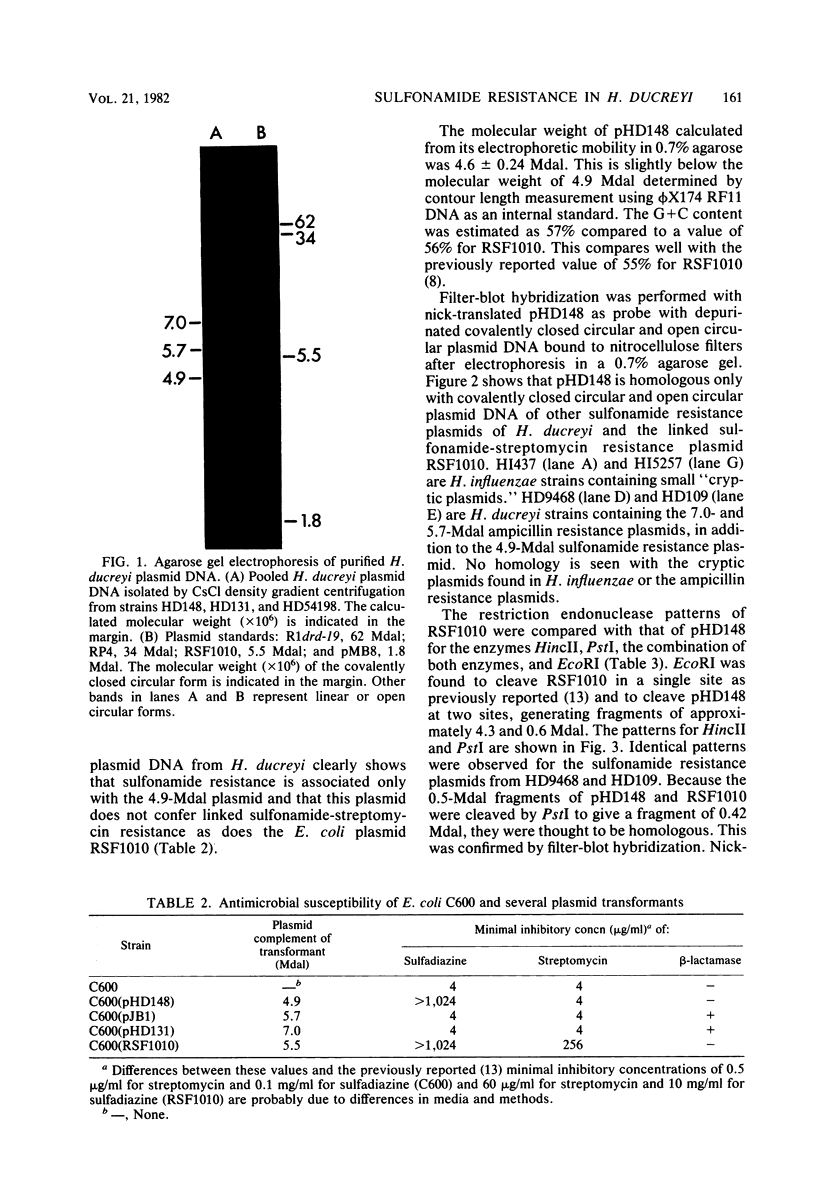
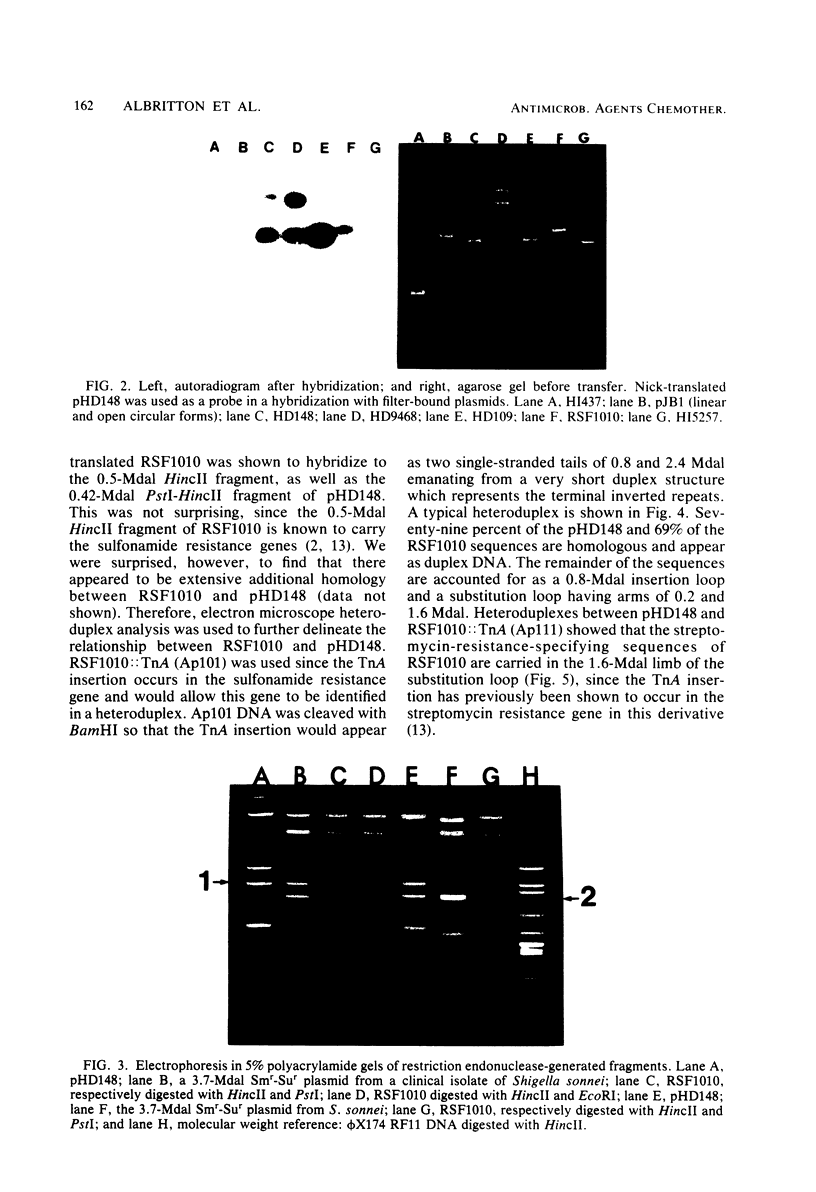


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J. L., Maclean I., Ronald A. R., Albritton W. L. Plasmid-mediated ampicillin resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1979 Feb;15(2):294–299. doi: 10.1128/aac.15.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. B., Pittard J. Genetic and biophysical study of R plasmids conferring sulfonamide resistance in Shigella strains isolated in 1952 and 1956. J Bacteriol. 1974 Dec;120(3):1186–1195. doi: 10.1128/jb.120.3.1186-1195.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Slutchuk M., Lian C. J., Wilt J. C., Ronald A. R. The treatment of chancroid: comparison of one week of sulfisoxazole with single dose doxycycline. J Antimicrob Chemother. 1979 May;5(3):261–265. doi: 10.1093/jac/5.3.261. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber R. E., Rowe C. E., Gilbert K. R. Treatment of chancroid. A comparison of tetracycline and sulfisoxazole. Arch Dermatol. 1969 Nov;100(5):604–607. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar J. L. The management of resistant chancroid in Vietnam. J Urol. 1972 May;107(5):807–808. doi: 10.1016/s0022-5347(17)61144-3. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Inoue K., Inoue M. Nonconjugative plasmids encoding sulfanilamide resistance. Antimicrob Agents Chemother. 1977 Sep;12(3):418–422. doi: 10.1128/aac.12.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Elwell L. P., Falkow S., Sykes R. B., Richmond M. H. beta-lactamases and R-plasmids of Haemophilus influenzae. Scand J Infect Dis Suppl. 1978;(13):16–22. [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Anderson E. S. Genetic and molecular characterisation of some non-transferring plasmids. Mol Gen Genet. 1974 Mar 27;129(3):229–242. doi: 10.1007/BF00267915. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]