Abstract
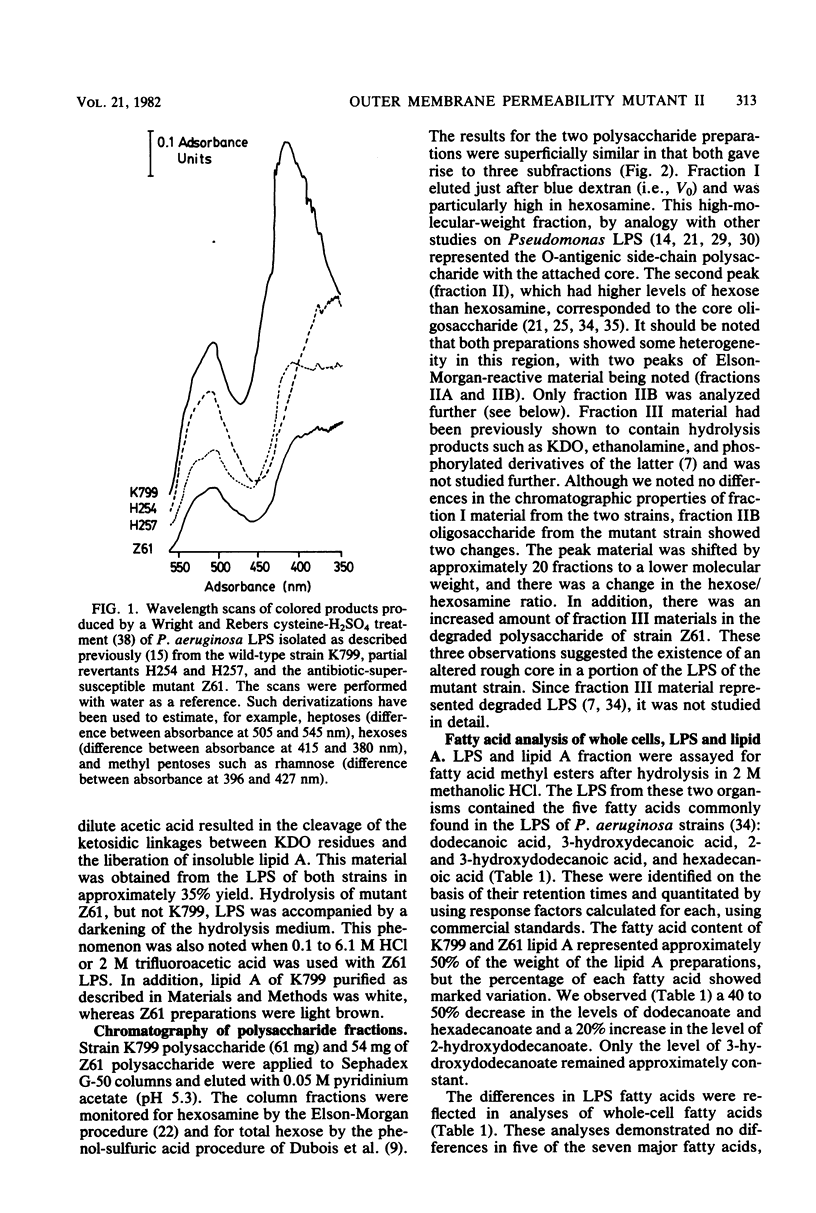
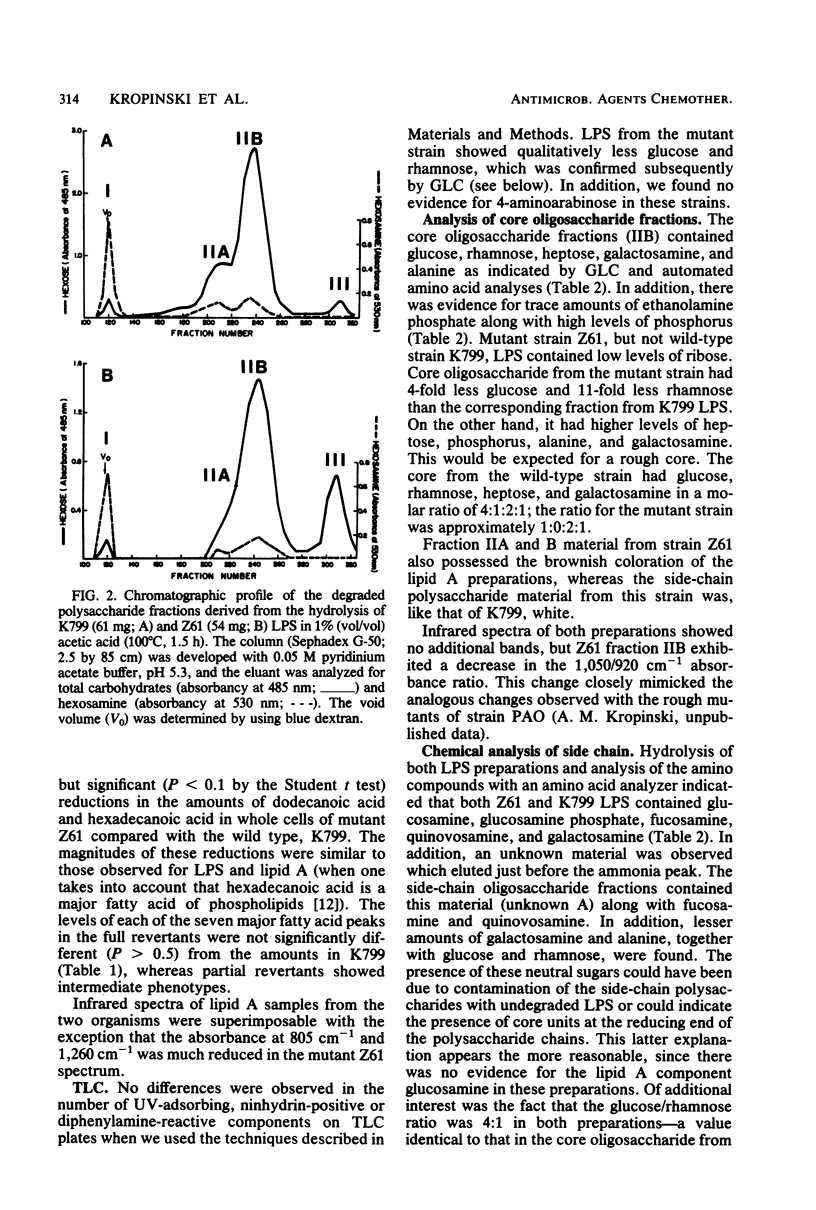
Lipopolysaccharides extracted from Pseudomonas aeruginosa strain K799 and its antibiotic-supersusceptible derivative Z61 were analyzed chemically and chromatographically. The side-chain polysaccharides purified by gel exclusion chromatography were compositionally identical, being composed of fucosamine (2-amino-2,6-dideoxygalactose), quinovosamine (2-amino-2,6-dideoxyglucose), and an unidentified amino sugar. In addition, low amounts of the core-specific components (glucose, rhamnose, alanine, and galactosamine) were found associated with the side chains from both strains. An average molecular weight of 38,000 to 50,000 was calculated for this fraction based on the glucose and rhamnose levels. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated that the lipopolysaccharides from these two strains were microheterogeneous. Qualitative analysis of the lipopolysaccharide neutral sugars, using a series of single-step revertants of mutant Z61, demonstrated that full revertants showed patterns indistinguishable from those of the wild-type strain K799, whereas partial revertants had intermediate levels and mutant Z61 low levels of neutral sugars. Quantitative analysis revealed that the core oligosaccharide fraction from the wild-type strain had a glucose/rhamnose/galactosamine ratio of 4:1:1, whereas the core from Z61 exhibited major deficiencies in both glucose and rhamnose. The lipid A from both strains contained five fatty acids, namely, 3-hydroxydecanoate, dodecanoate, 2- and 3-hydroxydodecanoate, and hexadecanoate. Whereas the overall fatty acid content was equal, the mutant strain showed markedly lower levels of dodecanoate and hexadecanoate and increased levels of 2-hydroxydodecanoate. Results of whole-cell fatty acid analyses were consistent with this observation. Evidence for an additional alteration of the lipid A of strain Z61 was obtained from acid hydrolysis studies and infrared spectra of isolated lipid A, although the actual chemical basis could not be determined by a variety of techniques. It is suggested that the state of the lipopolysaccharide is able to influence the number of open functional protein F pores in the outer membrane of P. aeruginosa.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Passage of Pseudomonas aeruginosa in compromised mice. Infect Immun. 1979 Oct;26(1):118–124. doi: 10.1128/iai.26.1.118-124.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Deneke C. F., MacLeod R. A. Heterogeneity and distribution of lipopolysaccharide in the cell wall of a gram-negative marine bacterium. J Bacteriol. 1978 Oct;136(1):148–157. doi: 10.1128/jb.136.1.148-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev B. A., Knirel Y. A., Kocharova N. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of the polysaccharide chain of Ps. aeruginosa O-serogroup 7 (Lanyi) lipopolysacharide. Eur J Biochem. 1980 May;106(2):643–651. doi: 10.1111/j.1432-1033.1980.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Gray G. W., Wilkinson S. G. Low-molecular-weight solutes released during mild acid hydrolysis of the lipopolysaccharide of Pseudomonas aeruginosa. Biochem J. 1972 Nov;130(1):289–295. doi: 10.1042/bj1300289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Symes K. C., Gray G. W., Wilkinson S. G. Studies of polysaccharide fractions from the lipopolysaccharide of Pseudomonas aeruginosa N.C.T.C. 1999. Biochem J. 1975 Jul;149(1):93–106. doi: 10.1042/bj1490093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensom A. H., Meadow P. M. Evidence for two regions in the polysaccharide moiety of the lipopolysaccharide of Pseudomonas aeruginosa 8602. FEBS Lett. 1970 Jul 29;9(2):81–84. doi: 10.1016/0014-5793(70)80318-0. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Meadow P. M. The extractable lipids of Pseudomonas aeruginosa. Biochim Biophys Acta. 1969 Oct 28;187(3):366–379. doi: 10.1016/0005-2760(69)90010-1. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Lipopolysaccharide-deficient, bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):98–108. doi: 10.1128/jb.127.1.98-108.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton D., Rodemeyer G., Rodemeyer R. Characterization of 2-amino-2,6-dideoxy-D-glucose as a constituent of the lipopolysaccharide antigen of Pseudomonas aeruginosa immunotype 4. Carbohydr Res. 1977 Jun;56(1):129–138. doi: 10.1016/s0008-6215(00)84244-5. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K., Kropinski A. M. The chemical composition of the lipopolysaccharide from Pseudomonas aeruginosa strain PAO and a spontaneously derived rough mutant. Microbios. 1977;19(76):103–116. [PubMed] [Google Scholar]
- Johnson A. R. Improved method of hexosamine determination. Anal Biochem. 1971 Dec;44(2):628–635. doi: 10.1016/0003-2697(71)90252-1. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Key B. A., Gray G. W., Wilkinson S. G. The purification and chemical composition of the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1970 Dec;120(3):559–566. doi: 10.1042/bj1200559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L. C., Milazzo F. H. The extraction and analysis of lipopolysaccharides from Pseudomonas aeruginosa strain PAO, and three rough mutants. Can J Microbiol. 1979 Mar;25(3):390–398. doi: 10.1139/m79-060. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tang J., Barzilay I., Khorana H. G. Structure of the lipopolysaccharide from an Escherichia coli heptose-less mutant. I. Chemical degradations and identification of products. J Biol Chem. 1979 Jul 10;254(13):5906–5917. [PubMed] [Google Scholar]
- Ryan E. A., Kropinski A. M. Separation of amino sugars and related compounds by two-dimensional thin-layer chromatography. J Chromatogr. 1980 Jul 4;195(1):127–132. doi: 10.1016/s0021-9673(00)81550-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Amino components of the lipopolysaccharide from Pseudomonas aeruginosa N.C.T.C. 8505. Presence of 2,4-diamino-2,4,6-trideoxy-D-glucose. Biochem J. 1977 Jan 1;161(1):103–109. doi: 10.1042/bj1610103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Taylor D. P. Occurrence of 2,3-diamino-2,3-dideoxy-d-glucose in lipid A from lipopolysaccharide of pseudomonas diminuta. J Gen Microbiol. 1978 Dec;109(2):367–370. doi: 10.1099/00221287-109-2-367. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Welbourn A. P. 2-Amino-2-deoxygalacturonic acid in lipopolysaccharides from Pseudomonas aerugimosa. Biochem J. 1975 Sep;149(3):783–784. doi: 10.1042/bj1490783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Zilić Z., Blau N., Knob M. Simple rapid method for the separation and quantitative analysis of carbohydrates in biological fluids. J Chromatogr. 1979 Sep 11;164(1):91–94. doi: 10.1016/s0378-4347(00)81575-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]



