Abstract
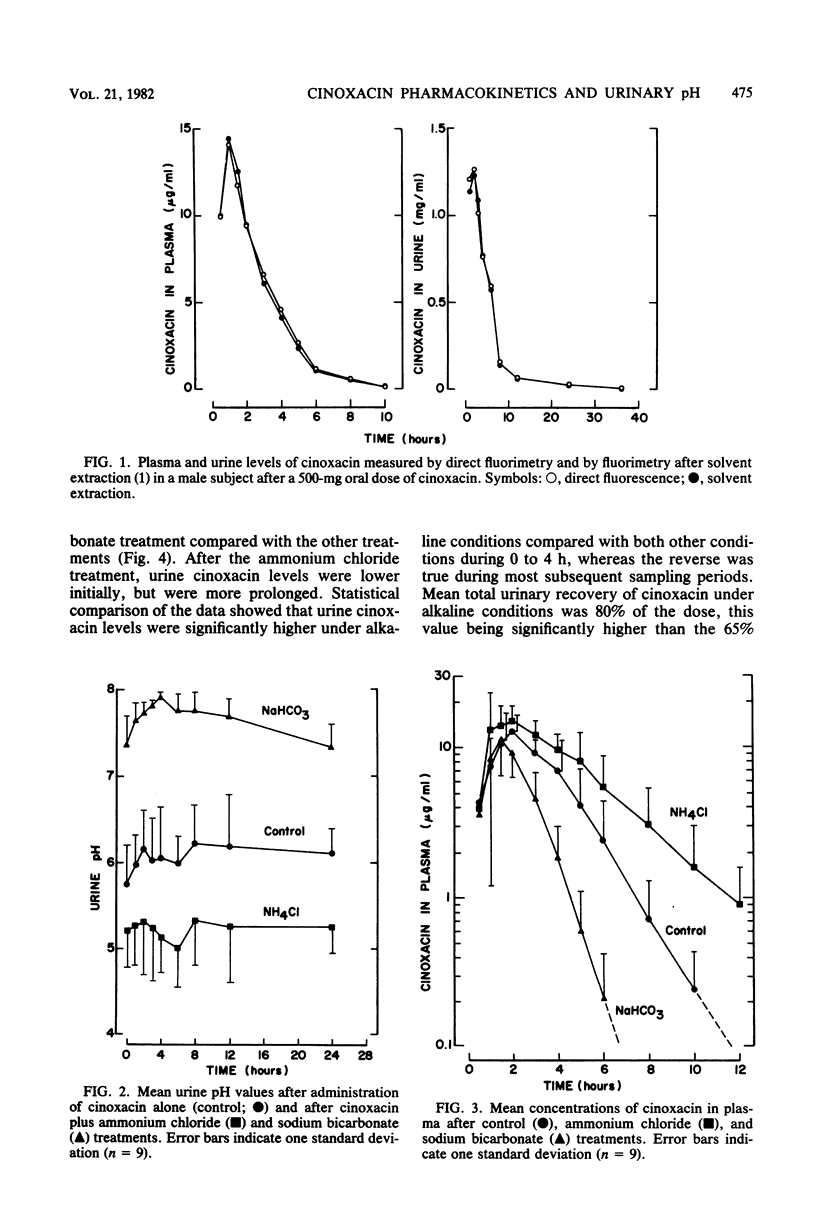
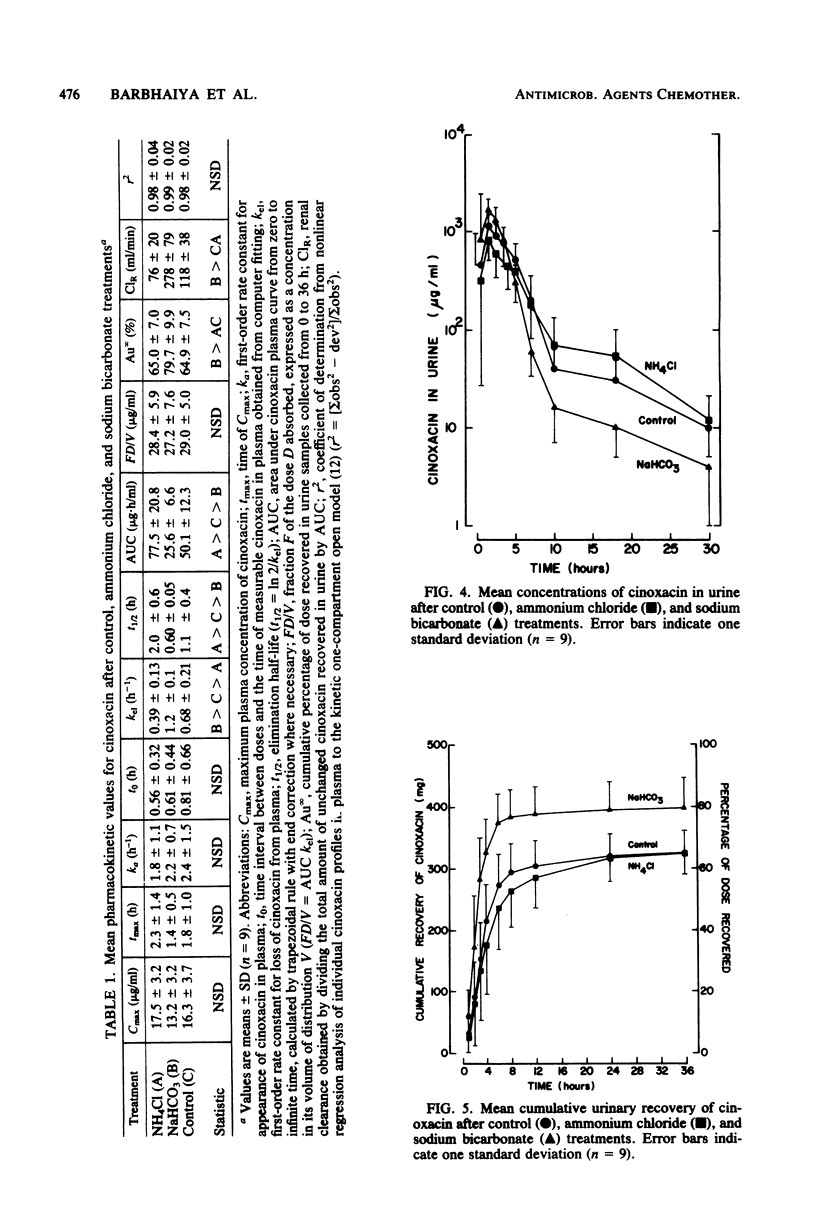
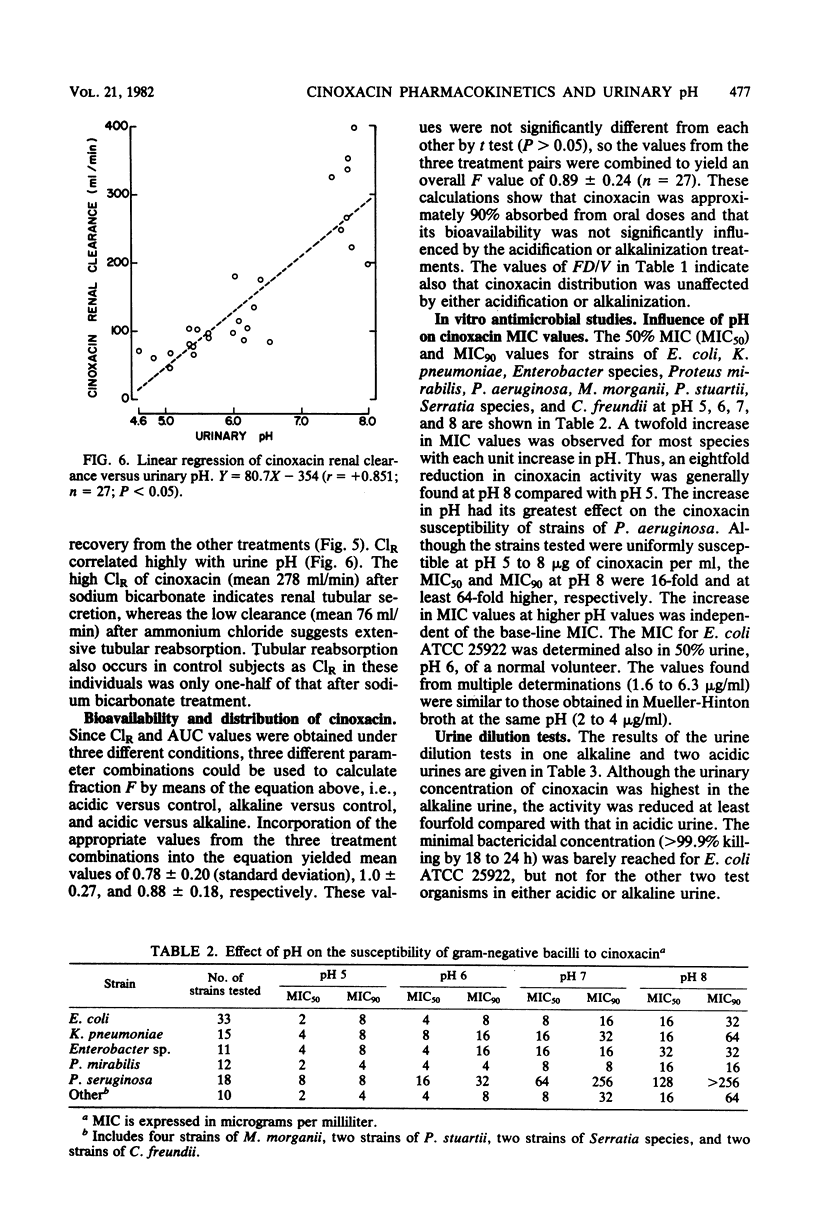
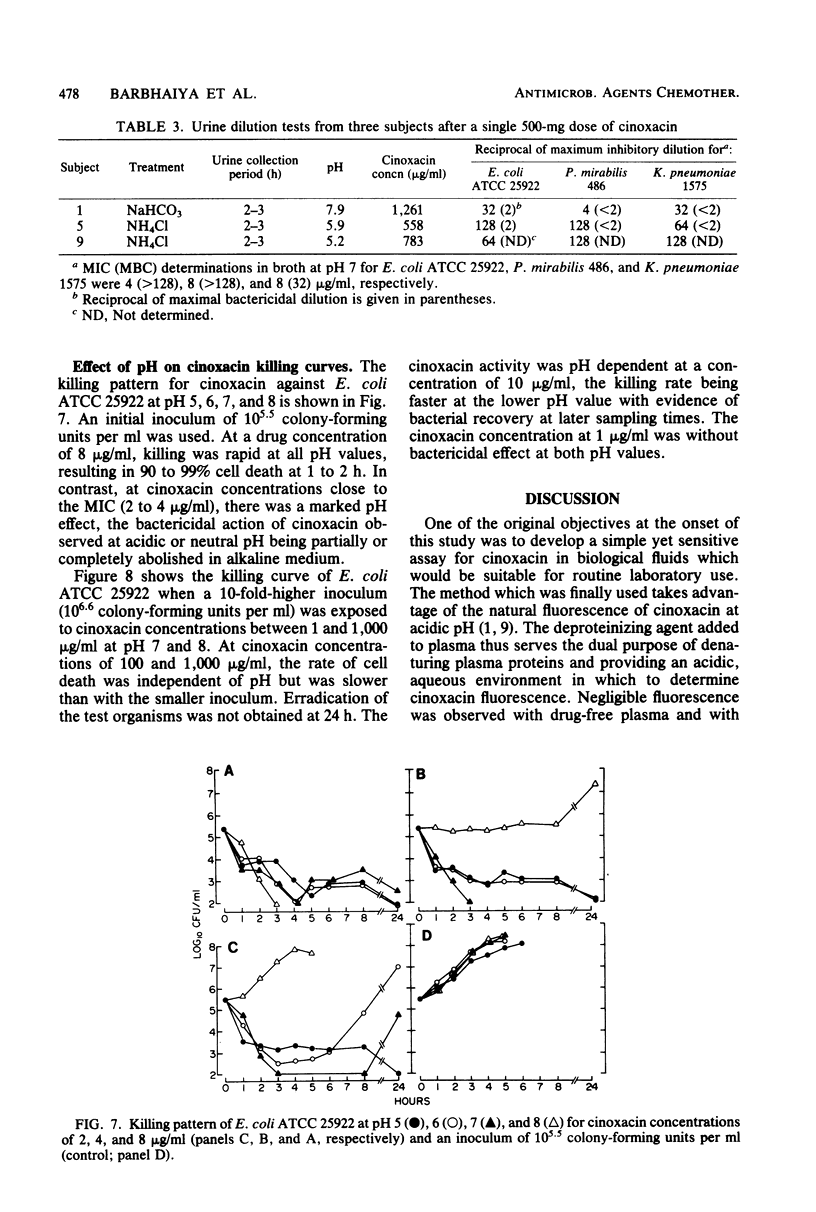
The impact of acidification and alkalinization of the urine on the pharmacokinetics of cinoxacin was examined after single 500-mg oral doses were administered to nine healthy male volunteers. Acidic and alkaline conditions were achieved by repeated oral doses of ammonium chloride or sodium bicarbonate, respectively. Plasma cinoxacin levels in all subjects were adequately described in terms of one-compartment-model kinetics with first-order absorption and elimination. Acidification and alkalinization treatment had no effect on cinoxacin absorption or distribution. The mean elimination half-life of cinoxacin in plasma was 1.1, 2.0, and 0.6 h in control subjects and with acidification and alkalinization of urine, respectively. Recovery of intact cinoxacin in samples of urine collected 0 to 36 h after cinoxacin administration represented 65% of the dose in control subjects and urine acidification and 80% of the dose with alkalinization of urine. The mean renal clearance of cinoxacin was 76, 118, and 278 ml/min with acidification, control, and alkalinization, respectively, and renal clearance was highly correlated with urinary pH. Urine concentrations of cinoxacin were significantly higher with alkalinization compared with control values during the first 4 h after drug administration. Urine cinoxacin concentrations were reduced somewhat by acidification, but these tended not to be significantly different from control values. Changes in cinoxacin elimination owing to urine pH are less pronounced in humans than in dogs. The antibacterial activity of cinoxacin against some common urinary tract pathogens was pH dependent. A four- to eightfold reduction in cinoxacin activity was generally observed at pH 8 compared with lower pH values. However, in view of the high levels of cinoxacin which are obtained in both acidic and basic urine, the impact of urine pH on cinoxacin antibacterial efficacy would be of minor clinical importance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black H. R., Israel K. S., Wolen R. L., Brier G. L., Obermeyer B. D., Ziege E. A., Wolny J. D. Pharmacology of cinoxacin in humans. Antimicrob Agents Chemother. 1979 Feb;15(2):165–170. doi: 10.1128/aac.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalka D., Feldman H. Letter: Absolute drug bioavailability: approximation without comparison to parenteral dose for compounds exhibiting perturbable renal clearance. J Pharm Sci. 1974 Nov;63(11):1812–1812. doi: 10.1002/jps.2600631140. [DOI] [PubMed] [Google Scholar]
- MILNE M. D., SCRIBNER B. H., CRAWFORD M. A. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med. 1958 May;24(5):709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]


