Abstract
Thalamic ventrobasal (VB) relay neurons receive information via two major types of glutamatergic synapses, that is, from the medial lemniscus (lemniscal synapses) and primary somatosensory cortex (corticothalamic synapses). These two synapses influence and coordinate firing responses of VB neurons, but their precise operational mechanisms are not yet well understood. In this study, we compared the composition of glutamate receptors and synaptic properties of corticothalamic and lemniscal synapses. We found that the relative contribution of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) to non-NMDA receptor-mediated EPSCs was significantly greater in corticothalamic synapses than in lemniscal synapses. Furthermore, NMDA receptor 2B-containing NMDA receptor- and kainate receptor-mediated currents were observed only in corticothalamic synapses, but not in lemniscal synapses. EPSCs in corticothalamic synapses displayed the postsynaptic summation in a frequency-dependent manner, in which the summation of the NMDA receptor-mediated component was largely involved. The summation of kainate receptor-mediated currents also partially contributed to the postsynaptic summation in corticothalamic synapses. In contrast, the contribution of NMDA receptor-mediated currents to the postsynaptic summation of lemniscal EPSCs was relatively minor. Furthermore, our results indicated that the prominent NMDA receptor-mediated component in corticothalamic synapses was the key determinant for the late-persistent firing of VB neurons in response to corticothalamic stimuli. In lemniscal synapses, in contrast, the onset-transient firing in response to lemniscal stimuli was regulated mainly by AMPA receptors.
The VB thalamic nucleus is a key relay station of somatosensory processing. VB neurons receive somatosensory information via medial lemniscal synapses and convey it to the somatosensory cortex (for review see Diamond, 1995). VB neurons also receive massive feedback projections of corticothalamic fibres from cortical neurons in layer VI (Zhang & Deschenes, 1997). Since both lemniscal and corticothalamic projections display a strict topographic organization, a sensory-driven and interconnected thalamo-cortico-thalamic network is thought to constitute the functional unit for processing somatosensory information (Jones & Powell, 1969; Williams et al. 1994; Bourassa et al. 1995; Alloway et al. 2003).
Corticothalamic projections, in association with primary sensory inputs, coordinate firing responses of thalamic neurons in sensory processing in vivo (Krupa et al. 1999; Fanselow et al. 2001; Temereanca & Simons, 2004). Since both types of synapses are glutamatergic, glutamate receptors are a key molecule in sensory-driven firing responses of thalamic neurons (Salt & Eaton, 1989, 1991; Sillito et al. 1990; Eaton & Salt, 1996). In in vivo experiments, it has been reported that distinct types of glutamate receptors temporally integrate the firing responses of VB neurons to sensory stimulation. Non-NMDA receptors are responsible for the initial firing responses induced by somatosensory stimulation, but NMDA receptors are involved in delayed firing responses of VB neurons (Salt & Eaton, 1989). However, it remains unknown how these glutamate receptors are associated with corticothalamic and/or lemniscal synapses during the firing of VB neurons.
Corticothalamic synapses are distinguished from the primary sensory synapses by their properties, kinetics of synaptic transmissions and short-term plasticity in vitro (McCormick & von Krosigk, 1992; Castro-Alamancos, 2002; Blitz & Regehr, 2003), but the composition of glutamate receptors in both corticothalamic and lemniscal synapses remains unclear. We therefore compared glutamate receptor composition, temporal properties of synaptic transmission, and their impacts on respective firing responses of corticothalamic and lemniscal synapses in the mouse VB. Our results demonstrated that prominent NMDA receptors (NMDARs) in corticothalamic synapses play a crucial role in determining the temporal signature of firing responses of VB neurons to corticothalamic stimuli, whereas firing responses to lemniscal stimuli are mainly regulated by AMPA receptors (AMPARs).
Methods
C57BL/6 Cr mice of both sexes (postnatal day P12–17) were used in this study. All animal procedures were in accordance with the institutional guidelines for animal experimentation, and approved by the animal research committee of the National Institute for Physiological Sciences. The animals were deeply anaesthetized with halothane and decapitated, and brains were dissected rapidly. Horizontal slices 350 μm thick (Castro-Alamancos, 2002) were cut in ice-cold artificial cerebrospinal fluid (ACSF) using a vibratome (VT1000S; Leica, Nussloch, Germany) and kept in a submerged chamber for more than 1 h with 95% O2–5% CO2-saturated ACSF at room temperature. The ACSF contained (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3, and 20 glucose. The ACSF was equilibrated with 95% O2–5% CO2 (pH 7.3) and infused at a rate of 3.0 ml min−1. During recordings, ACSF containing 10 μm (–)-bicuculline methobromide (Tocris Cookson, Avonmouth, UK) and 10 μm CGP 55845 (Tocris Cookson) was used for blocking GABAA and GABAB receptors, respectively. All experiments were performed at 30–32°C.
VB neurons were visualized under a microscope (BX50WI; Olympus) with an infrared differential interference contrast video system (C2400-79H; Hamamatsu Photonics, Hamamatsu, Japan). Whole-cell recordings were made with 2–4.5 MΩ recording pipettes containing (mm): 120 caesium methane-sulphonate, 10 Hepes, 1 EGTA, 2 MgCl2, 0.1 CaCl2, 20 NaCl, 5 QX314, 2 ATP-Na2, 0.5 GTP-Na (pH 7.3 with CsOH, 298–310 mosmol l−1) for voltage-clamp recordings. In the case of current-clamp and loose-patch recordings, the internal solution contained (mm): 120 potassium gluconate, 10 Hepes, 1.0 EGTA, 2 MgCl2, 0.1 CaCl2, 10 NaCl, 2 Na2ATP, 0.5 Na2GTP (pH 7.3 with KOH, 295–310 mosmol l−1).
An EPC9 patch-clamp amplifier (HEKA, Lambrecht, Germany) was used for voltage-clamp whole-cell recordings. EPSCs were mainly recorded at holding potentials of −70 mV with filtering at 2–10 kHz and digitized at 50 kHz. Series resistances were monitored on-line and the uncompensated series resistance was typically less than 9 MΩ. A series resistance compensation of 75% was used during recordings from VB neurons. Liquid-junction potentials between the internal solutions and ACSF were corrected. The chord conductance mediated by non-NMDARs was measured by the peak amplitudes of non-NMDAR-mediated EPSCs at holding potentials from −90 to 0 mV. The conductance mediated by NMDARs was measured by amplitude of NMDAR-mediated currents at holding potentials between +40 and +5 mV. For current-clamp recordings, Axoclamp 2B (Molecular Devices, Sunnyvale, CA, USA) or EPC9 was used. We recorded from VB neurons at approximately their resting membrane potentials (see Results). When a cell became depolarized within 5 mV during a recording, we applied a small negative current through the pipette to hold the potential at around −70 mV. Loose-patch recording was performed as previously described (Kondo & Marty, 1998). Synaptic responses were evoked using a concentric electrode (tip diameter, 25 μm; Inter Medical, Nagoya, Japan) placed on the internal capsule for recording corticothalamic responses and on the medial lemniscus for recording lemniscal responses as reported previously (Castro-Alamancos & Calcagnotto, 2001; Castro-Alamancos, 2002). The stimulus consisted of a 100 μs-duration bipolar pulse of constant current steps (< 100 μA) using a biphasic isolator (BAK Electronics, Oxford, UK). The stimulus was delivered at 0.05 Hz. Data acquisition was performed using the PULSE program (HEKA, version 8.54). Pulse Fit (HEKA, version 8.54) and Igor Pro (Wavemetrics, Lake Oswego, OR, USA) were used to analyse the data. The statistical significance was determined using a t test (unpaired or paired) and ANOVA depending on the experimental design.
Drugs
dl-2-amino-5-phosphonopentanoic acid (DL-AP5), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX), 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX), 7-(hydroxyimino)cyclo-propa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) (RS)-α-methyl-4-carboxyphenylglycine (MCPG), 2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemitartrate (ifenprodil hemitartrate), SYM 2206 and SYM 2081 were purchased from Tocris Cookson (Avonmouth, UK). GYKI 53655 (Sigma-Aldrich) was gifted from Drs. H. Kamiya and T. Momiyama.
Results
Properties of corticothalamic and lemniscal EPSCs
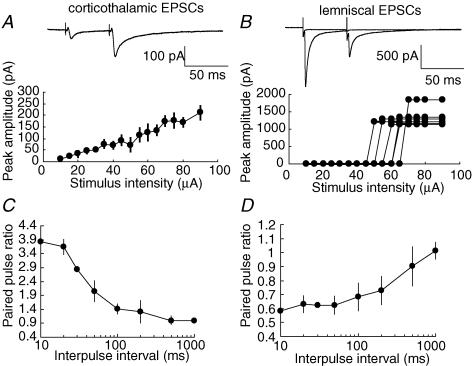
Whole-cell recordings were taken from 241 VB neurons. We recorded corticothalamic EPSCs evoked by the stimulation of the internal capsule in horizontal brain slices. We also recorded lemniscal EPSCs evoked by the stimulation of lemniscal fibres in the same slices. In 16% of the recorded VB neurons, both corticothalamic and lemniscal EPSCs were recorded from the same VB neuron (Fig. 1). We distinguished the two synaptic responses by their characteristics. First, corticothalamic EPSCs increased monotonically with increasing stimulus intensity, indicating that they were composed of a large number of small unitary events (Fig. 1A). In contrast, lemniscal EPSCs in the vast majority of the neurons (27 out of 30 neurons) showed all-or-none responses (Fig. 1B). A few neurons (3 out of 30 neurons) displayed two steps of amplitudes with increasing stimulus intensity. The threshold stimulation resulted in a unitary response that always had the same amplitude. Thus, lemniscal EPSCs were composed of one or two large unitary events. Second, the two types of synaptic responses were different in short-term plasticity. Corticothalamic EPSCs showed the paired-pulse facilitation when the same stimulus was delivered twice with a short interval (Fig. 1A). At the 50 ms interval, the amplitude of the second EPSC, on average, was 195.1 ± 18.2% (mean ± s.e.m., n = 20) greater than that of the first EPSC amplitude in corticothalamic synapses (Fig. 1C). The facilitation was observed up to the 200 ms intervals, although the facilitated amplitude was smaller toward longer intervals. In contrast, all lemniscal EPSCs led to the paired-pulse depression (Fig. 1B). At the 50 ms interval, the amplitude of the second EPSC was reduced to 71.7 ± 9.0% (n = 20) of that of the first EPSC in lemniscal synapses (Fig. 1D). The amplitude remained depressed at still longer intervals up to 1000 ms. These synaptic properties were consistent with those found in a previous study that examined EPSPs (Castro-Alamancos, 2002).
Figure 1. Characteristics of EPSCs elicited by stimulation of corticothalamic fibres and lemniscal fibres in VB neurons.
A, paired-pulse facilitation of corticothalamic EPSCs. The graph summarizes the peak amplitude of corticothalamic EPSCs plotted against the stimulus intensity (mean ± s.e.m., n = 30). B, paired-pulse depression of lemniscal EPSCs. The lemniscal synapse shows large EPSCs in an all-or-none fashion. The graph plots the peak amplitude of lemniscal EPSCs against the stimulus intensity (n = 6). The two traces in A and B were obtained from the same VB neuron. The trace in A was averaged from 5 to 6 consecutive EPSCs. C, summary of the paired-pulse facilitation (n = 20). The ratio of the peak amplitude in the second response to that in the first response is plotted as a function of the interpulse interval. D, summary of the paired-pulse depression (n = 20). The ratio of the peak amplitude in the second response to that in the first response is plotted as a function of the interpulse interval.
NMDAR-mediated and non-NMDAR-mediated components of corticothalamic and lemniscal EPSCs
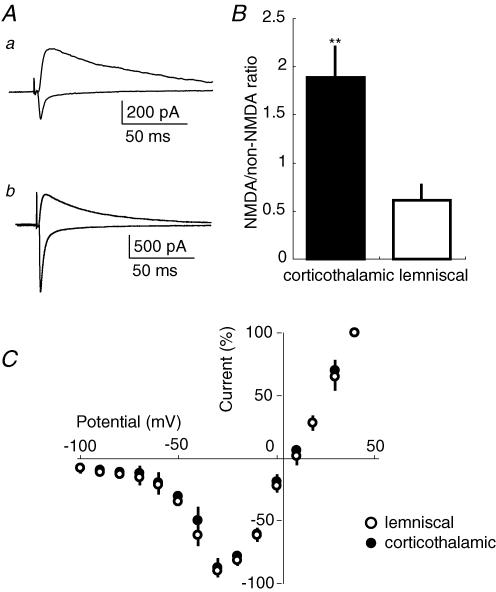
We next investigated whether there is any difference in the composition of NMDAR-mediated and non-NMDAR-mediated components between corticothalamic and lemniscal EPSCs. Non-NMDAR-mediated (i.e. AMPAR- and kainate receptor-mediated) EPSCs were recorded at a membrane potential of −90 mV (Fig. 2A). The stimulus strength for corticothalamic synapses was adjusted to evoke EPSCs with a peak amplitude of ∼200 pA (206.0 ± 21.5 pA, mean ± s.d., n = 10). The stimulus strength for lemniscal synapses was fixed at the suprathreshold level that produced a unitary response. The peak amplitude of the unitary response was 1420.1 ± 432.1 pA (n = 10). The peak conductances of non-NMDARs were 2.3 ± 0.2 nS (mean ± s.d., n = 8) and 15.7 ± 4.5 nS (n = 8) in corticothalamic and lemniscal synapses, respectively. Corticothalamic EPSCs showed large rise-time and decay-time constants in comparison with lemniscal EPSCs. NMDAR-mediated EPSCs were evoked at the same stimulus strength in the presence of NBQX (50 μm), a non-NMDAR antagonist, at a holding potential of +40 mV to relieve the voltage-dependent Mg2+ block of the NMDAR channel (Mayer et al. 1984; Nowak et al. 1984) (Fig. 2A). The peak amplitudes of NMDAR-mediated EPSCs were 395.4 ± 99.9 pA (mean ± s.d.) and 853.6 ± 221.4 pA in corticothalamic and lemniscal synapses, respectively. The NMDAR-mediated conductances were 10.8 ± 2.2 nS (mean ± s.d.) and 22.3 ± 7.3 nS in corticothalamic and lemniscal synapses, respectively. The NMDAR-mediated EPSCs were completely abolished by dl-AP5, an NMDAR antagonist, at 100 μm (data not shown). These results indicated that both corticothalamic and lemniscal EPSCs consisted of NMDAR- and non-NMDAR-mediated currents. The peak current ratio of NMDAR-mediated EPSCs at +40 mV to non-NMDAR-mediated EPSCs at −90 mV was significantly larger in corticothalamic synapses (Fig. 2B and 191.9 ± 31.0%, mean ± s.d., n = 10) than in lemniscal synapses (Fig. 2B and 60.1 ± 16.2%, P < 0.01, unpaired t test, n = 10). The ratios of NMDAR-mediated conductance to non-NMDAR-mediated conductance were 470.0 ± 72.5% (mean ± s.d.) and 142.0 ± 25.6% in the corticothalamic and lemniscal synapses, respectively, indicating that the relative contribution of the NMDAR-mediated component was significantly greater in corticothalamic synapses than that in lemniscal synapses (P < 0.01, unpaired t test).
Figure 2. Larger ratio of NMDAR component to non-NMDAR component in corticothalamic EPSCs than in lemniscal EPSCs.
A, examples of non-NMDAR-mediated EPSCs (inward current traces) and NMDAR-mediated EPSCs (outward current traces) recorded at membrane potentials of −90 and +40 mV, respectively. NMDAR-mediated EPSCs were recorded in the presence of 50 μm NBQX to block non-NMDAR EPSCs. The two traces in a (corticothalamic EPSCs) and the two in b (lemniscal EPSCs) were obtained from the same VB neuron. Each trace was averaged from 5 to 6 consecutive EPSCs. B, ratio of the NMDAR-mediated EPSC amplitude to the non-NMDAR EPSC amplitude for corticothalamic synapses (filled bar, n = 10) and lemniscal synapses (open bar, n = 10). The ratio was first calculated for each neuron, and then averaged across all neurons. **P ≤ 0.01, unpaired t test. C, current–voltage relationships of NMDAR-mediated EPSCs in corticothalamic (•, mean ± s.e.m., n = 10) and lemniscal synapses (○, n = 10). The current amplitude was normalized to the amplitude at +40 mV.
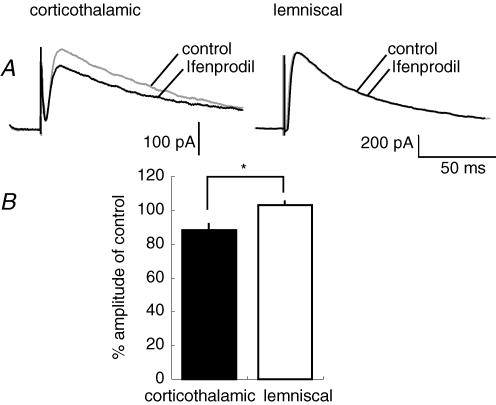
It was notable that the decay-time constant of NMDAR-mediated currents was evidently larger in corticothalamic synapses (104.4 ± 37.8 ms, mean ± s.d., n = 10) than in lemniscal synapses (45.1 ± 4.2 ms, P < 0.05, unpaired t test, n = 10; Table 1). However, the current–voltage (I–V) curves of NMDAR-mediated currents in corticothalamic and lemniscal synapses were almost superimposable (Fig. 2C). Since NMDA receptor 2B (NR2B)-containing NMDAR currents show a similar I–V relationship to NR2A-containing NMDAR currents, but have a longer decay time course (Monyer et al. 1994), we also examined the NR2B component in the corticothalamic and lemniscal synapses. Ifenprodil hemitartrate (ifenprodil, 10 μm), an NR2B subunit-specific antagonist of NMDARs (Williams, 1993), selectively reduced the amplitude of corticothalamic NMDAR-mediated currents to 88.4 ± 3.8% (mean ± s.d.) of that in control (P < 0.05, n = 7), but left lemniscal NMDAR-mediated currents virtually unchanged (Fig. 3A and B, and 103.0 ± 2.4% of that in control, P > 0.5, n = 7). The decay-time constant of NMDAR-mediated currents was still larger in corticothalamic synapses (80.3 ± 4.0 ms, n = 7) than in lemniscal synapses even in the presence of the drug (P < 0.05).
Table 1.
Summary of basic properties of EPSCs evoked by stimulating corticothalamic and lemniscal fibres
| Synaptic property | Lemniscal synapse | Corticothalamic synapse |
|---|---|---|
| 1. 10–90% rise time of non-NMDARs (ms) | 0.93 ± 0.25 (n = 20) | 1.97 ± 0.61(n = 20)* |
| 2. Decay time constant of non-NMDARs (ms) | ||
| τ1 | 4.07 ± 2.11 (n = 20) | 5.14 ± 1.29 (n = 16) |
| τ2 | — | 87.9 ± 24.5 |
| 3. NMDA/non-NMDA peak current ratio | 0.60 ± 0.16 (n = 10) | 1.91 ± 0.31 (n = 10)** |
| 4. NMDARs decay time constant (ms) τ | 45.1 ± 4.2 (n = 10) | 104.4 ± 37.8 (n = 10)** |
The amplitude of evoked EPSPs with a stimulus intensity of 50 μA was analysed in corticothalamic fibres. Stimulus intensities in lemniscal fibres were fixed at the suprathreshold level. The rise-time and decay-time constant were measured in the presence of 100 μm dl-AP5, and the decay-time constant was obtained by fitting EPSCs with a single or a double exponential decay curve. All values are given as mean ± s.d.
P ≤ 0.01
P ≤ 0.05, unpaired t test.
Figure 3. NR2B-containing NMDAR currents detected in corticothalamic synapses.
A, NMDAR-mediated EPSCs in corticothalamic synapses were decreased by the bath application of ifenprodil (10 μm) in the presence of NBQX (50 μm) (traces on the left), whereas those in lemniscal synapses were not altered (traces on the right). B, percentage of the peak amplitude of ifenprodil-resistant NMDAR-mediated EPSCs in corticothalamic (filled bar) and lemniscal (open bar) synapses (*P ≤ 0.05, n = 7, unpaired t test).
Kainate receptor-mediated component
To further investigate the properties of the non-NMDAR-mediated component in the two types of synapses, we recorded corticothalamic and lemniscal EPSCs in the presence of 100 μm dl-AP5. We confirmed that non-NMDAR-mediated currents were completely blocked by the AMPA and kainate receptor antagonists NBQX (50 μm; Fig. 4A and B) and CNQX (50 μm, n = 5). The basic properties of non-NMDAR-mediated corticothalamic and lemniscal EPSCs are summarized in Table 1. The 10–90% rise time of corticothalamic EPSCs was 1.97 ± 0.61 ms (mean ± s.d., n = 20), which was significantly longer than that of lemniscal EPSCs (0.93 ± 0.25 ms, P < 0.01, unpaired t test, n = 16). The longer rise time may result from more distal locations of corticothalamic synapses than lemniscal synapses (Jones & Powell, 1969; Bourassa et al. 1995; Zhang & Deschenes, 1997), or from asynchronous glutamate release from corticothalamic fibres.
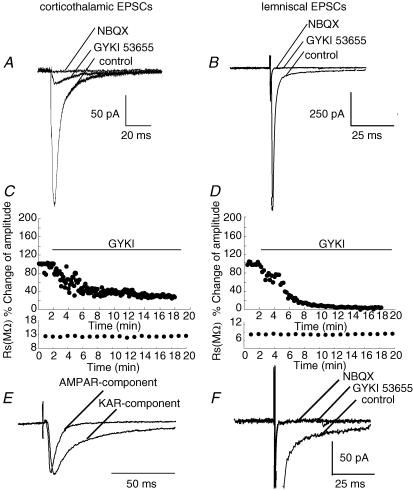
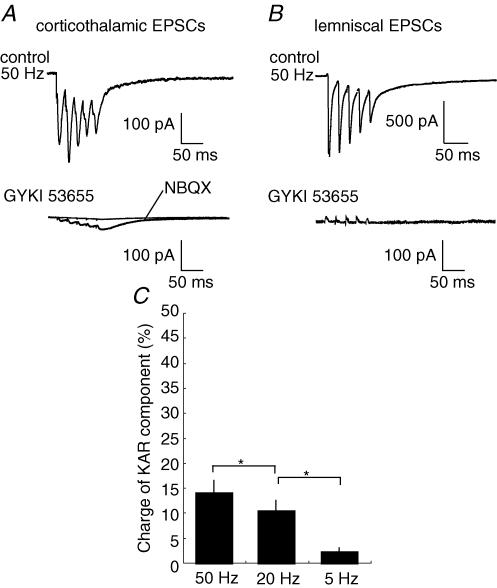
Figure 4. Pharmacologically isolated Kainate receptor (KAR)-mediated currents in corticothalamic synapses, but not in lemniscal synapses.
A, corticothalamic EPSCs were decreased by the bath application of GYKI 53655 (50 μm) in the presence of dl-AP5 (100 μm). Small GYKI 53655-resistant EPSCs were completely blocked by an additional application of 50 μm NBQX. B, lemniscal EPSCs were completely blocked by the bath application of GYKI 53655 in the presence of dl-AP5. Additional NBQX application did not change lemniscal EPSCs. C, effect of GYKI 53655 application on corticothalamic EPSCs. The normalized EPSC amplitude is plotted against time. The bottom graph shows corresponding series resistances. D, effect of GYKI 53655 application on lemniscal EPSCs. Conventions are the same as in C. E, GYKI 53655-resistant EPSCs are scaled to the peak of AMPAR-mediated EPSCs in corticothalamic synapses. AMPAR-mediated EPSCs were calculated by subtracting GYKI 53655-resistant EPSCs from those in the control. Each trace was averaged from 5 to 6 consecutive EPSCs. F, traces shown at an expanded amplitude scale of traces in B.
The decay of corticothalamic EPSCs was well fitted with a double-exponential function with the fast (τ1: 5.14 ± 1.29 ms; 88.4 ± 5.7%) and slow (τ2: 87.9 ± 14.5 ms) time constants, whereas the decay of lemniscal EPSCs was fitted with a single exponential (4.1 ± 2.1 ms), suggesting the difference in the composition of non-NMDARs between corticothalamic and lemniscal synapses. Since AMPA and kainate receptors have fast and slow gating kinetics, respectively (Lerma et al. 2001), we examined the kainate receptor-mediated component of corticothalamic EPSCs. The change in the amplitude of corticothalamic EPSCs was investigated after the application of GYKI 53655 (50 μm) and SYM 2206 (100 μm), both of which are selective AMPAR antagonists (Pelletier et al. 1996; Bleakman et al. 1996; Li et al. 1999). In the presence of dl-AP5, corticothalamic EPSCs were partially blocked by the AMPAR antagonists, but a resistant component remained (Fig. 4A and C; GYKI 53655: 13.9 ± 5.1%, mean ± s.d., n = 12; SYM 2206: 15.9 ± 6.5%, n = 12). The decay of resistant currents was best fitted with a single exponential (Fig. 4E; τ1: 59.6 ± 23.8 ms). The resistant component was completely blocked by 50 μm NBQX (n = 10; Fig. 4A) and/or 20 μm SYM 2081, a kainate receptor antagonist (Jones et al. 1997; DeVries, 2000; Cho et al. 2003), indicating that the component was mediated by kainate receptors. In contrast, a unitary lemniscal EPSC was completely blocked by GYKI 53655 (50 μm; Fig. 4B and D) and SYM 2206 (100 μm). In addition to GYKI 53655 or SYM 2206, the subsequent application of NBQX did not change the EPSC amplitude in lemniscal synapses (Fig. 4F). These results indicated that corticothalamic synapses contained a significant amount of kainate receptor-mediated EPSCs. In contrast, no kainate receptor-mediated currents were detected and AMPAR-mediated currents were dominant in lemniscal EPSCs.
Postsynaptic summation of corticothalamic and lemniscal EPSCs induced by repetitive stimulation and their contribution by glutamate receptors
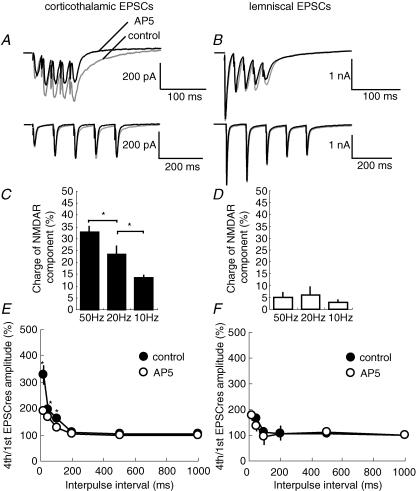
Due to the different composition of NMDA, kainate and AMPA receptors between corticothalamic and lemniscal synapses and the fact that NMDA and kainate receptors have slower kinetics than those of AMPARs, we next examined how these differences would affect the postsynaptic summation of EPSCs by repetitive stimulation. We first stimulated corticothalamic or lemniscal fibres using 5-pulse trains at 1–50 Hz and compared compound EPSCs before and after the bath application of 100 μm dl-AP5. In this experiment, holding potentials were kept at −60 mV in order to observe NMDAR-mediated currents. Compound EPSCs in corticothalamic synapses decreased after the application of dl-AP5 in a frequency-dependent manner (Fig. 5A). Percentage charges of NMDAR components of compound EPSCs were 32.8 ± 4.93 and 13.5 ± 8.6% (mean ± s.e.m., n = 5) at 50 and 10 Hz, in corticothalamic synapses (Fig. 5C). On the other hand, percentage charges of NMDAR components of compound EPSCs in lemniscal synapses were much smaller than those in corticothalamic synapses (Fig. 5B and D, and 4.93 ± 2.2 and 2.86 ± 1.14 at 50 and 10 Hz, respectively, n = 5).
Figure 5. Significant contribution of NMDAR-mediated EPSCs to the postsynaptic summation of EPSCs in corticothalamic synapses.
A, traces of compound EPSCs of corticothalamic synapses during the repetitive stimulation (with a 5-pulse train) at 50 Hz (upper traces) and 10 Hz (lower traces). Grey lines indicate the control traces and black lines indicate the EPSCs in the presence of 100 μm dl-AP5. B, traces of compound EPSCs of lemniscal synapses during the repetitive stimulation at 50 Hz (upper traces) and 10 Hz (lower traces). Conventions are the same as in A. C, per cent charges of NMDAR-mediated components normalized to the total charge of control corticothalamic EPSCs (n = 5) at different stimulation frequencies. D, per cent charges of NMDAR-mediated components normalized to total charge of control lemniscal EPSCs (n = 5) at different frequencies. E, degree of the postsynaptic summation of corticothalamic EPSC responses to the repetitive stimulation in various interpulse intervals before and after the bath application of dl-AP5 (n = 10). The amplitude of the fourth corticothalamic EPSCres (measured 1 ms before the fifth stimulus) was expressed as a function of the amplitude of the first pulse (measured 1 ms before the second stimulation) without (•) and with (○) dl-AP5 (mean ± s.e.m., n = 10). F, the same analyses were performed for lemniscal EPSCs (n = 10). All pooled data are plotted against the interpulse interval. *P ≤ 0.05, paired t test.
The postsynaptic summation of corticothalamic EPSCs by NMDARs was additionally assessed by measuring the amplitude of the residual EPSC at 1 ms prior to the next stimulation (EPSCres) before and after the application of dl-AP5. The current level before the train stimulation was used as the baseline. Before the application of dl-AP5, the EPSCres increased progressively in a frequency-dependent manner during the series of stimulation between 5 and 50 Hz (Fig. 5E, filled circles). The ratios of the fourth EPSCres (measured 1 ms before the fifth stimulus) to the first EPSCres, were 327.0 ± 35.4% (mean ± s.e.m.) and 159.4 ± 11.7% at 50 and 10 Hz, respectively (n = 10; Fig. 5A and E, filled circles). After the application of dl-AP5, EPSCsres decreased overall but still summated during the pulse train. The ratios of the fourth EPSCres to the first EPSCres were 187.8 ± 8.5 and 126.7 ± 12.4% at 50 and 10 Hz, respectively (n = 10; Fig. 5A and E, open circles). To distinguish presynaptic short-term facilitation from the postsynaptic summation of EPSCs, we further analysed the absolute peak amplitude of EPSC (EPSCab), measured from the current immediately preceding the stimulus artifact to the peak of corticothalamic EPSCs. It was evident that the corticothalamic EPSCab increased following the second or third pulse, but a large increase was not observed following the remaining two stimuli. At 50 Hz, for example, the second EPSCab was 164.3 ± 11.3% of the first EPSCab, whereas the fourth EPSCab was only 97.1 ± 9.6% of the first EPSCab. In addition, we did not observe any significant change of EPSCsab before and after the application of dl-AP5. These results indicated that NMDARs-mediated currents in corticothalamic synapses were mainly responsible for the frequency-dependent postsynaptic summation, but not for the presynaptic short-term facilitation.
In lemniscal synapses, postsynaptic summations of EPSCs were not significantly different before and after the bath application of dl-AP5. At 50 Hz, for example, the fourth to the first EPSCres ratio was 178.2 ± 13.5% without dl-AP5, and 174 .0 ± 15.1% (n = 10) in the presence of the drug. Overall these values were much smaller than the corresponding ones of corticothalamic EPSCs (Fig. 5F, open circles). No summation was observed during low-frequency (≤≤10 Hz) repetitive stimulation in lemniscal EPSCs either with or without dl-AP5 (Fig. 5B and F).
Since corticothalamic EPSCs contained a significant kainate receptor-mediated component (Fig. 4), which also has slow kinetics, we next investigated how kainate receptor-mediated currents contribute to the summation of EPSCs during the repetitive stimulation. In the presence of dl-AP5 (100 μm), GYKI 53655- and SYM 2206-resistant currents that were evoked in response to the corticothalamic repetitive stimulation were measured (Fig. 6A). The kainate receptor-mediated currents also displayed the summation in a frequency-dependent manner. The percentage of kainate receptor-mediated charges of the total EPSC charge increased progressively in a frequency-dependent manner, being 13.8 ± 2.7% (mean ± s.e.m., n = 10) at 50 Hz, 10.2 ± 2.4% at 20 Hz, and 2.1 ± 0.85% at 5 Hz (Fig. 6C).
Figure 6. Postsynaptic summation of kainate receptor-mediated currents in corticothalamic synapses.
A, postsynaptic summation by kainate receptor-mediated currents in corticothalamic EPSCs. Recordings were performed in the presence of 100 μm dl-AP5. Summated EPSCs by the repetitive stimulation with a 5-pulse train at 50 Hz (upper traces) were partially inhibited by 50 μm GYKI 53655 and completely blocked by 50 μm NBQX (lower traces). B, absence of the summation of the kainate receptor component in lemniscal EPSCs. Summated EPSCs by the repetitive stimulation at 50 Hz (upper traces) were completely blocked by GYKI 53655 (lower traces). C, contribution of kainate receptor-mediated charge (normalized to the total charge of corticothalamic EPSCs) at different stimulation frequencies (n = 10). *P ≤ 0.05, paired t test.
It has been reported that metabotropic glutamate receptor (mGluR) 1 induces slow inward currents in response to a high-frequency stimulation in the mouse thalamus (Golshani et al. 1998; Liu et al. 1998). However, GYKI 53655- and SYM 2206-resistant currents were completely blocked by NBQX (50 μm), but not significantly affected by the application of MCPG (500 μm), an antagonist of mGluRs (101.2 ± 1.0% in comparison with control resistant currents, n = 5, P > 0.05, paired t test), even when the repetitive stimulation of corticothalamic fibres was applied at 50 Hz. The results indicated that the resistant current component was mediated by kainate receptors, but not induced by mGluRs (Fig. 6A).
In contrast to corticothalamic EPSCs, lemniscal EPSCs were almost completely abolished by the bath application of GYKI 53655 (50 μm) and/or SYM 2206 (100 μm) in the presence of dl-AP5, even when the repetitive stimulation was applied at 50 Hz (Fig. 6B). An additional application of NBQX (98.8 ± 2.0%, n = 8) and/or MCPG (101.2 ± 2.1%, n = 10) did not cause any significant change in the charge at various frequencies (P > 0.05, paired t test).
Contrasting temporal properties of firing responses in the two types of synapses
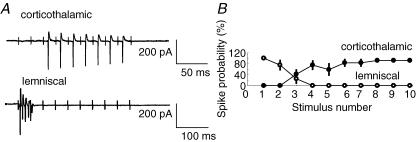
During the repetitive stimulation, the postsynaptic summation was significantly greater at high frequencies than at lower frequencies in corticothalamic synapses, but not in lemniscal synapses. The question at hand was how these different synaptic properties were transformed into different temporal firing patterns of VB neurons. To answer this question, we recorded firing responses (spikes), in the loose patch mode, from VB neurons in response to a 10-pulse train stimulation delivered to corticothalamic and lemniscal fibres. For corticothalamic fibres, we first increased the stimulation intensity gradually until the threshold for spike generation was reached in the 9th or 10th stimulus in the train at 10 Hz, the frequency at which the postsynaptic summation started to appear (Fig. 5E), and then applied the stimulation of the same intensity at 40 Hz, the frequency at which the summation was evidently larger (Fig. 5E). In the case of lemniscal fibres, we confirmed the appearance of spikes in an all-or-none fashion at the threshold of stimulus intensity and fixed the stimulus intensity at the suprathreshold level, and then applied the pulse train at 20 Hz.
The firing responses to these stimulation protocols allowed us to distinguish between corticothalamic and lemniscal synapses. To quantify the difference between the two types of synapses, we measured the success rate for spike generation in each set of 30 trials. The stimulation of corticothalamic fibres induced a late-persistent firing pattern (Fig. 7A, upper trace). The probability for the spike generation was the lowest for the first three stimuli and increased from the fourth stimulus on (Fig. 7B, filled circles; success rates: 0.0 ± 0.0% (mean ± s.e.m.) for the first stimulus, 91.6 ± 2.8% for the 9th stimulus, n = 15). In contrast, the stimulation of lemniscal fibres resulted in an onset-transient firing pattern (Fig. 7A, lower trace). The probability for the spike generation was the highest at the onset of the pulse train and decreased rapidly in subsequent stimuli (Fig. 7B, open circles; success rates: 100.0 ± 0.0% for the first stimulus, 0.0 ± 0.0% for the 9th stimulus, n = 15).
Figure 7. Contrasting temporal properties of firing responses in the two types of synapses.
A, late-persistent and onset-transient firings elicited by stimulating (with a 10-pulse train) corticothalamic (upper trace) and lemniscal (lower trace) fibres, respectively, were recorded from the same VB neuron in the loose patch mode. B, probability for the spike generation is plotted against the pulse number for corticothalamic (•, mean ± s.e.m., n = 15) and lemniscal (○, n = 15) synapses, respectively.
Contributions to firing responses by glutamate receptors in the two types of synapses
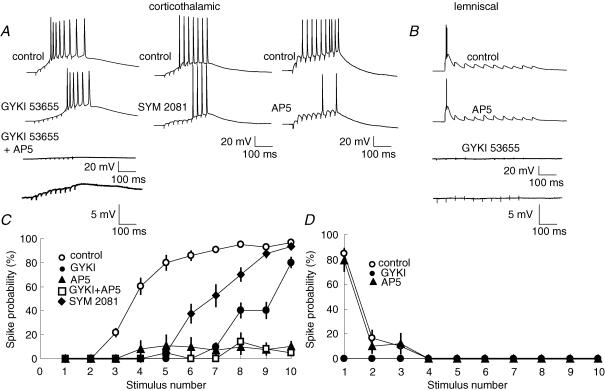
To elucidate different contributions to firing responses by AMPA, kainate and NMDA receptors in corticothalamic and lemniscal synapses, we recorded VB neurons (including a subset of neurons described in the preceding section, which were repatched) with and without the presence of respective receptor antagonists in a whole-cell current-clamp configuration. The resting membrane potential of these VB neurons was −67.9 ± 3.9 mV (n = 30) and the input resistance was 193.2 ± 52.3 MΩ (n = 30). We applied the same stimulation protocols to corticothalamic and lemniscal fibres as in the loose-patch experiment. In the control experiment, the stimulation to corticothalamic fibres induced EPSPs, which displayed the temporal summation and evoked action potentials between the fourth and tenth stimuli (late-persistent firing; Fig. 8A). The probability for the generation of action potentials was the lowest after the first three stimuli and increased between the fourth to tenth stimuli (success rates: 0.0 ± 0.0%, mean ± s.e.m., for the first stimulus, 86.2 ± 5.6% for the 9th stimulus, n = 34; Fig. 8C, open circles). In contrast, after the stimulation to lemniscal fibres, action potentials appeared at the onset of each pulse train (onset-transient firing) and EPSPs weakened drastically in the course of the stimulation (Fig. 8B). The probability for the generation of action potentials was the highest at the onset of the stimulation and decreased rapidly in subsequent stimuli (Fig. 8D, open circles; success rates: 85.0 ± 3.8% for the first stimulus, 0.0 ± 0.0% for the 9th stimulus, n = 12).
Figure 8. Contributions to firing responses by glutamate receptors in the two types of synapses.
A, properties of firing responses to the pulse train stimulation of corticothalamic synapses under various pharmacological conditions. Recordings were performed in the current-clamp mode with the same stimulus protocol as in the loose-patch mode. The upper traces show the control responses. The bath application of 50 μm GYKI 53655 decreased EPSP amplitudes and firing response (middle-left trace). An additional application of dl-AP5 (100 μm) significantly decreased peak EPSP amplitudes and almost abolished firing responses, but the summation of corticothalamic EPSPs remained (lower-left trace; enlarged at the bottom). The lower-middle trace displays decreased firing responses by the bath application of 20 μm SYM 2081. The lower-right trace displays firing responses by the bath application of 100 μm dl-AP5. B, properties of firing responses to train stimulation of lemniscal synapses. The bath application of 100 μm dl-AP5 did not significantly affect peak EPSP amplitudes and firing response (middle trace). GYKI 53655 abolished EPSCs and firing response completely (lower traces). C, probabilities for the spike generation are plotted against the stimulus number under various conditions for corticothalamic synapses (○: control, n = 34; •: application of GYKI 53655, n = 10; ▴: application of dl-AP5, n = 10; □: co-application of dl-AP5 and GYKI 53655, n = 10; ♦: application of SYM 2081, n = 10). Error bars indicate the mean ± s.e.m. D, probabilities for the spike generation for lemniscal synapses (○: control, n = 12; •: application of GYKI 53655, n = 7; ▴: application of dl-AP5, n = 5).
To determine the contribution of AMPARs to the late-persistent firing, we first bath-applied GYKI 53655 (50 μm) and stimulated corticothalamic fibres. Although spike generation was attenuated following the application of GYKI 53655, EPSPs were still summated in the course of stimulation and reached the threshold to generate the late-persistent firing under this condition. The mean success rates for spike generation were 0.0 ± 0.0% for the first stimulus and 40.0 ± 21.0% for the 9th stimulus (Fig. 8C, filled circles). The co-application of dl-AP5 (100 μm) and GYKI 53655 (50 μm) markedly reduced the summation of EPSPs and probability of spike generation: the success rates for spike generation were 0.0 ± 0.0% for the first stimulus and 8.0 ± 2.5% for the 9th stimulus (significantly different from the corresponding value in the control experiment, P < 0.01, one-way repeated ANOVA, n = 10; Fig. 8A, left traces and Fig. 8C, open squares). However, the temporal summation of corticothalamic EPSPs was still observed (Fig. 8A, left-bottom trace). In addition, summated EPSPs were completely blocked by the bath application of 50 μm NBQX (data not shown), indicating that they were mediated by kainate receptors.
Next, we bath-applied SYM 2801 (20 μm) to determine the contribution of kainate receptors to the late-persistent firing. After 10 min application of SYM 2081, the spike generation was attenuated but the late-persistent pattern was maintained (Fig. 8A, middle traces). The mean success rates for spike generation were 5.00 ± 4.33% for the 5th stimulus (significantly lower than the corresponding value in the control experiment, P < 0.01, n = 10) and 87.5 ± 6.25% for the 9th stimulus (Fig. 8C, filled diamonds). The result from this experiment suggested that kainate receptors, similar to AMPARs, were only partially involved in the late-persistent firing.
Finally, we examined the contribution of NMDARs to the late-persistent firing. The bath application of dl-AP5 (100 μm) disturbed the stability of the late-persistent firing and resulted in a marked decrease in the probability of the spike generation in all stimuli in the pulse train (Fig. 8A, right traces). The mean success rates for spike generation were 8.33 ± 6.45% for the 4th stimulus and 7.78 ± 3.75% for the 9th stimulus, both of which were significantly lower than those in the control experiment (P < 0.01, one-way repeated ANOVA, n = 10; Fig. 8C, filled triangles). Taken together, these data demonstrated that while AMPA, kainate and NMDA receptors were all necessary for the spike generation in response to the corticothalamic stimulation, NMDARs were the determinant contributor to the late-persistent firing response.
In contrast to corticothalamic synapses, the bath application of 100 μm dl-AP5 did not significantly affect the probability of spike generation in lemniscal responses, with the success rates for spike generation being 79.3 ± 8.8% for the first stimulus and 0.0 ± 0.0% for the 9th stimulus (P > 0.05, in comparison with the corresponding values in the control experiment, n = 5; Fig. 8B and D, filled triangles). The bath application of GYKI 53655 (50 μm), on the other hand, completely blocked lemniscal EPSPs and spike generation throughout the pulse train (Fig. 8D, filled circles; success rates for spike generation: 0.0 ± 0.0% for the first stimulus; 0.0 ± 0.0% for the 9th stimulus, n = 7). This result indicated that AMPARs were essential for the onset-transient firing response in lemniscal synapses.
Discussion
In the present study, we investigated and determined the composition of glutamate receptors and synaptic properties in corticothalamic and lemniscal synapses. We also demonstrated how the different composition of glutamate receptors in the two synapses was translated into the different firing of VB neurons. Our findings are summarized as follows. (1) The relative contribution of NMDAR-mediated EPSCs was significantly greater than that of non-NMDAR-mediated EPSCs in corticothalamic synapses, but not in lemniscal synapses. (2) NR2B-containing NMDAR-mediated currents were observed in corticothalamic synapses, but not in lemniscal synapses. (3) Kainate receptor-mediated currents were observed only in corticothalamic synapses. (4) Corticothalamic EPSCs summated postsynaptically during the repetitive stimulation in a frequency-dependent manner. The summation of NMDAR-mediated EPSCs was the primary contributor, whereas kainate receptor-mediated EPSCs were partially involved. In contrast, the contribution of NMDA receptor-mediated EPSCs to the summation of lemniscal EPSCs was relatively minor. (5) The stimulation of corticothalamic fibres with the pulse train resulted in the late-persistent firing, whereas the stimulation of lemniscal fibres led to the onset-transient firing. NMDARs were found to be prominently involved in the late-persistent firing in corticothalamic synapses, whereas the onset-transient firing in lemniscal synapses was primarily attributed to the activation of AMPARs.
Larger ratio of NMDAR component to non-NMDAR component in corticothalamic EPSCs than in lemniscal EPSCs
NMDARs are responsible for somatosensory-driven responses in VB neurons in vivo (Salt, 1986; Salt & Eaton, 1989), yet their synaptic origin in VB neurons remains undetermined. Primary sensory synapses activate NMDARs in the lateral geniculate nucleus (Scharfman et al. 1990), but the involvement of NMDARs has not been definitively demonstrated in lemniscal synapses. NMDARs are also activated by the corticothalamic stimulation both in vivo (Deschenes & Hu, 1990; Eaton & Salt, 1996) and in vitro (Scharfman et al. 1990; Kao & Coulter, 1997; von Krosigk et al. 1999). One of our primary findings was that the relative contribution of the NMDAR-mediated component, in comparison with non-NMDAR-mediated component, was much greater in corticothalamic EPSCs than in lemniscal EPSCs, suggesting that NMDARs in corticothalamic synapses play a principal role in evoking somatosensory-driven responses of VB neurons. Functional NMDARs are a heterometric complex composed of NR1 and NR2 subunits. It has been reported that NR2B-containing NMDAR-mediated currents shows a similar I–V relationship to NR2A-containing NMDAR-mediated currents, but has a longer decay time course (Monyer et al. 1994). We found that NR2B-mediated currents existed only in corticothalamic synapses, but not in lemniscal synapses, and the decay-time constant was much longer in corticothalamic synapses than that in lemniscal synapses even under ifenprodil. Presumably, it is because the electrotonic length from the soma to the site of corticothalamic synapses is larger than that from the soma to the site of lemniscal synapses.
Existence of kainate receptor component in corticothalamic EPSCs but not in lemniscal EPSCs
In the present study, we used two AMPAR-selective antagonists, SYM 2206 and GYKI 53655, the latter being the most potent antagonist available. GYKI 53655- and SYM 2206-resistant currents in corticothalamic synapses were completely blocked by NBQX and/or SYM 2081, indicating that they were kainate receptor-mediated currents. SYM 2081 is commonly used as an antagonist of kainate receptors because SYM 2081 rapidly desensitizes kainate receptor-mediated responses (Jones et al. 1997; DeVries, 2000; Cho et al. 2003). In contrast, kainate receptor-mediated currents were not detected in lemniscal synapses, a finding consistent with the results reported in a previous study (Binns et al. 2003).
It has been shown that kainate receptors are expressed in the mouse VB. Among five subunits of kainate receptors, GluR5, 6, 7, and KA2 exist in the rodent VB (Bahn et al. 1994). Furthermore, GluR5/6/7 immunolabelling in mouse VB neurons is seen at the postsynaptic sites of corticothalamic synapses and corticothalamic synapses are much more heavily labelled with the anti-GluR5/6/7 complex antibody than lemniscal synapses (Bolea et al. 2001). Thus, our electrophysiological results are supported by these anatomical findings. However, Bolea and colleagues reported no kainate receptor-mediated currents in corticothalamic EPSCs. This discrepancy may be attributed to the differences in experimental conditions between the two studies. They recorded EPSCs at a lower temperature (22–25°C), which is known to reduce kainate receptor-mediated currents (Kidd & Isaac, 2001). In addition, they used a different AMPA receptor antagonist, GYKI 52466, which has been shown to reduce kainate receptor-mediated currents by 20–30% (Paternain et al. 1995).
Relation to developmental changes of glutamate receptors in the two types of synapses
It has been revealed in developmental studies that major changes in the composition of glutamate receptors have already occurred before and around postnatal day 12 (P12) in the rodent thalamus. At corticothalamic synapses, for example, NMDAR-mediated currents are dominant during the first 12 postnatal days. However, after P12, other than the presence of the NMDAR component, there is also a substantial increase in non-NMDAR-mediated currents (Golshani et al. 1998). The expression of NR2B in the mouse thalamus is already high at birth, which remains at about the same level through the postnatal development, and starts to decline at around P7. In the meantime, the NR2A expression starts to appear and increases gradually during the next 2–3 weeks to reach the adult level. It is also reported that mRNA of kainate receptors is only weakly detected in the adult rat thalamus, but strongly expressed during the early postnatal development until around P12 (Bahn et al. 1994). In the mouse VB, the immunoreactivity of kainate receptors is detectable at least at P15–P19 (Bolea et al. 2001). However, it is still unclear whether these developmental changes occur simultaneously in both corticothalamic and lemniscal synapses. Our results were obtained from juvenile animals (P12–P17). Thus, our observation that corticothalamic and lemniscal synapses were composed of different components of AMPA, NMDA and kainate receptors reflected the distinct composition of glutamate receptors in the two types of synapses after their major changes in the postnatal development. It is unknown at the moment if the same composition is maintained in the adult.
Firing responses in corticothalamic and lemniscal synapses and their regulations by different composition of glutamate receptors
It is proposed that firing responses evoked by the synaptic stimulation with a train of pulses can be generally defined by such elements as membrane time constants, kinetics of EPSCs and short-term plastic properties (Zucker, 1989). The present study showed that the postsynaptic temporal summation of corticothalamic EPSCs and in part the short-term facilitation of synaptic transmission led to the late-persistent firing pattern. On the other hand, lemniscal EPSCs, which exhibited fast kinetics of EPSCs, and the short-term depression of synaptic transmission, resulted in the onset-transient firing pattern. The location of synaptic contacts is thought to contribute to the temporal summation of EPSCs and/or EPSPs in response to the repetitive stimulation. As corticothalamic and lemniscal synapses are located at the distal and proximal portion of the dendrite, respectively (Jones & Powell, 1969; Bourassa et al. 1995; Zhang & Deschenes, 1997), corticothalamic EPSCs, in comparison with lemniscal synapses, would be more strongly attenuated and exhibit slower kinetics when synaptic currents are measured at the soma (Bloomfield & Sherman, 1989; Hausser & Roth, 1997; Destexhe et al. 1998; Destexhe, 2000). Another determinant for the kinetics of EPSCs and/or EPSPs is the composition of glutamate receptors. The large NMDAR component in corticothalamic synapses induced marked postsynaptic summation at the high frequency stimulation because NMDARs have slower kinetics than AMPARs. Especially in current-clamp condition, activated NMDARs, which were relieved from the voltage-dependent Mg2+ block, led to large postsynaptic summation of EPSPs and finally reached the threshold to generate action potentials as the late-persistent firing response.
Kainate receptors with slow kinetics may also play a certain role on the firing response. Although kainate receptors in corticothalamic synapses were a minor contributor to the late-persistent firing, kainate receptor-mediated EPSCs showed the frequency-dependent summation because of the slow kinetics of kainate receptors, as have been reported for several types of neurons (Mulle et al. 1998; Bureau et al. 2000; Kidd & Isaac, 2001). This summation would lead to the generation of tonic depolarization in response to the repetitive stimulation in a frequency-dependent manner. Thus, in association with the AMPAR activation, the kainate receptor-mediated depolarization also helps to relieve the voltage-dependent Mg2+ block of NMDARs, which were essential for the late-persistent firing response (Fig. 7C). In addition, kainate receptors in corticothalamic synapses may also contribute to transmitting the average cortical activities (Frerking & Ohliger-Frerking, 2002). It is well known that depolarization switches the firing pattern from burst mode to tonic mode by activating low-threshold T-type calcium channels (Destexhe et al. 1998; Williams & Stuart, 2000; Zhuravleva et al. 2001). Taken together, we propose that prominent NMDA and kainate receptors with slow kinetics in corticothalamic synapses contribute to generate a prolonged depolarization and a fast switch between the burst and tonic modes, depending on the cortical activity. This would enable a more rapid response than those generated by conventional neuromodulators such as mGluRs, which operate on a time scale of a few hundreds of milliseconds (McCormick & von Krosigk, 1992).
On the contrary, the large AMPAR-component in lemniscal synapses displayed EPSCs with fast kinetics that did not induce a visible postsynaptic summation. It has been reported that NMDARs trigger additional action potentials with longer latencies and act as a modulator, whereas AMPARs evoke precisely timed action potentials with short latencies and detect the timing information (Blitz & Regehr, 2003). These different functions of glutamate receptors may be responsible for the differences in the temporal features of firing responses in the two synapses in vivo. For example, corticothalamic synapses facilitate and prolong firing responses of VB neurons to a whisker movement with a long latency (Krupa et al. 1999; Temereanca & Simons, 2004). Lemniscal inputs, in contrast, generate spikes in VB neurons with a short latency (Krupa et al. 1999; Fanselow et al. 2001).
Functional implications of corticothalamic and lemniscal synapses
As shown in the present study, lemniscal synaptic properties were transformed into the onset-transient firing in response to the stimulation with a train of pulses, because most of recorded cells were rather hyperpolarized like in the burst mode (Sherman & Guillery, 1998). In another sense, VB neurons did not faithfully transfer lemniscal inputs, even if repetitive inputs reached VB neurons in the burst mode. Castro-Alamancos (2002) reported that a VB neuron that was tonically depolarized closer to its firing threshold (tonic mode) is able to overcome the disturbance of transfer and relay lemniscal sensory information faithfully in response to high-frequency inputs. Therefore, the relay of sensory information is warranted when lemniscal inputs and depolarization coincide. We suggest that NMDAR-mediated currents may play the role of generating or maintaining the depolarized state in vivo.
Taken together, our results strongly suggest that the properties of corticothalamic synapses are useful in amplifying or gating lemniscal sensory information, depending on cortical activity. A large temporal summation of corticothalamic EPSCs was observed at high frequency including gamma frequency (20–50 Hz), which is considered to engage the cortex and thalamus during the arousal state (Steriade, 1993). This gain system in corticothalamic synapses may underlie the sensory processing (Murphy et al. 2000; Sillito & Jones, 2002; Miyata et al. 2003; Jung et al. 2004), spatiotemporal definition of receptive fields (Temereanca & Simons, 2004), and switching the firing modes during the arousal state (Guillery & Sherman, 2002). In sum, this type of facilitation can provide an elegant stimulus-specific transfer of somatosensory information through lemniscal synapses, depending on cortical activity.
Acknowledgments
We thank Drs H. Kamiya and T. Momiyama for providing pharmacological tools and T. Kodama for analysis programs. We also thank Drs K. Rockland, S. and K. Cheng for helpful comments on this study. This work was in part supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. M.M. is also grateful for the grant from Toyota Riken.
References
- Alloway KD, Hoffer ZS, Hoover JE. Quantitative comparisons of corticothalamic topography within the ventrobasal complex and the posterior nucleus of the rodent thalamus. Brain Res. 2003;968:54–68. doi: 10.1016/s0006-8993(02)04265-8. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns KE, Turner JP, Salt TE. Kainate receptor (GluR5) -mediated disinhibition of responses in rat ventrobasal thalamus allows a novel sensory processing mechanism. J Physiol. 2003;551:525–537. doi: 10.1113/jphysiol.2003.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, Lodge D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Regehr WG. Retinogeniculate synaptic properties controlling spike number and timing in relay neurons. J Neurophysiol. 2003;90:2438–2450. doi: 10.1152/jn.00562.2003. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Sherman SM. Dendritic current flow in relay cells and interneurons of the cat's lateral geniculate nucleus. Proc Natl Acad Sci U S A. 1989;86:3911–3914. doi: 10.1073/pnas.86.10.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Liu XB, Jones EG. Kainate receptors at corticothalamic synapses do not contribute to synaptic responses. Thalamus Related System. 2001;1:187–196. [Google Scholar]
- Bourassa J, Pinault D, Deschenes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A. 2000;97:6838–6843. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol. 2002;87:946–953. doi: 10.1152/jn.00426.2001. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: in vitro and in vivo. J Neurophysiol. 2001;85:1489–1497. doi: 10.1152/jn.2001.85.4.1489. [DOI] [PubMed] [Google Scholar]
- Cho K, Francis JC, Hirbec H, Dev K, Brown MW, Henley JM, Bashir ZI. Regulation of kainate receptors by protein kinase C and metabotropic glutamate receptors. J Physiol. 2003;548:723–730. doi: 10.1113/jphysiol.2003.040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Hu B. Membrane resistance increase induced in thalamic neurons by stimulation of brainstem cholinergic afferents. Brain Res. 1990;513:339–342. doi: 10.1016/0006-8993(90)90478-t. [DOI] [PubMed] [Google Scholar]
- Destexhe A. Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J Physiol(Paris) 2000;94:391–410. doi: 10.1016/s0928-4257(00)01093-7. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Diamond ME. Somatosensory thalamus of the rat. In: Jones EG, Diamond IT, editors. Cerebral Cortex, the Barrel Cortex of Rodents. Vol 11. New York: Plenum; 1995. pp. 189–220. [Google Scholar]
- Eaton SA, Salt TE. Role of N-methyl-D-aspartate and metabotropic glutamate receptors in corticothalamic excitatory postsynaptic potentials in vivo. Neuroscience. 1996;73:1–5. doi: 10.1016/0306-4522(96)00123-6. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci U S A. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P, Warren RA, Jones EG. Progression of change in NMDA, non-NMDA, and metabotropic glutamate receptor function at the developing corticothalamic synapse. J Neurophysiol. 1998;80:143–154. doi: 10.1152/jn.1998.80.1.143. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hausser M, Roth A. Estimating the time course of the excitatory synaptic conductance in neocortical pyramidal cells using a novel voltage jump method. J Neurosci. 1997;17:7606–7625. doi: 10.1523/JNEUROSCI.17-20-07606.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TP. The cortical projection of the ventroposterior nucleus of the thalamus in the cat. Brain Res. 1969;13:298–318. doi: 10.1016/0006-8993(69)90289-3. [DOI] [PubMed] [Google Scholar]
- Jones KA, Wilding TJ, Huettner JE, Costa AM. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Jung SC, Kim JH, Choi IS, Cho JH, Bae YC, Lee MG, Shin HC, Choi BJ. Corticothalamic modulation on formalin-induced change of VPM thalamic activities. Neuroreport. 2004;15:1405–1408. doi: 10.1097/01.wnr.0000131008.36444.2d. [DOI] [PubMed] [Google Scholar]
- Kao CQ, Coulter DA. Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro. J Neurophysiol. 1997;77:2661–2676. doi: 10.1152/jn.1997.77.5.2661. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J Neurophysiol. 2001;86:1139–1148. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- Kondo S, Marty A. Synaptic currents at individual connections among stellate cells in rat cerebellar slices. J Physiol. 1998;509:221–232. doi: 10.1111/j.1469-7793.1998.221bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci U S A. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- Liu XB, Munoz A, Jones EG. Changes in subcellular localization of metabotropic glutamate receptor subtypes during postnatal development of mouse thalamus. J Comp Neurol. 1998;395:450–465. doi: 10.1002/(sici)1096-9861(19980615)395:4<450::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate ‘metabotropic’ receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kashiwadani H, Fukaya M, Hayashi T, Wu D, Suzuki T, Watanabe M, Kawakami Y. Role of thalamic phospholipase Cβ4 mediated by metabotropic glutamate receptor type 1 in inflammatory pain. J Neurosci. 2003;23:8098–8108. doi: 10.1523/JNEUROSCI.23-22-08098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Duckett SG, Sillito AM. Comparison of the laminar distribution of input from areas 17 and 18 of the visual cortex to the lateral geniculate nucleus of the cat. J Neurosci. 2000;20:845–853. doi: 10.1523/JNEUROSCI.20-02-00845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Pelletier JC, Hesson DP, Jones KA, Costa AM. Substituted 1,2-dihydrophthalazines: potent, selective, and noncompetitive inhibitors of the AMPA receptor. J Med Chem. 1996;39:343–346. doi: 10.1021/jm950740w. [DOI] [PubMed] [Google Scholar]
- Salt TE. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986;322:263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Salt TE, Eaton SA. Function of non-NMDA receptors and NMDA receptors in synaptic responses to natural somatosensory stimulation in the ventrobasal thalamus. Exp Brain Res. 1989;77:646–652. doi: 10.1007/BF00249618. [DOI] [PubMed] [Google Scholar]
- Salt TE, Eaton SA. Sensory excitatory postsynaptic potentials mediated by NMDA and non-NMDA receptors in the thalamus in vivo. Eur J Neurosci. 1991;3:296–300. doi: 10.1111/j.1460-9568.1991.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Lu SM, Guido W, Adams PR, Sherman SM. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci U S A. 1990;87:4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing ‘drivers’ from ‘modulators’. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci. 2002;357:1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Murphy PC, Salt TE, Moody CI. Dependence of retinogeniculate transmission in cat on NMDA receptors. J Neurophysiol. 1990;63:347–355. doi: 10.1152/jn.1990.63.2.347. [DOI] [PubMed] [Google Scholar]
- Steriade M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Curr Opin Neurobiol. 1993;3:619–625. doi: 10.1016/0959-4388(93)90064-6. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron. 2004;41:639–651. doi: 10.1016/s0896-6273(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Von Krosigk M, Monckton JE, Reiner PB, Mccormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience. 1999;91:7–20. doi: 10.1016/s0306-4522(98)00557-0. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J Neurosci. 2000;20:1307–1317. doi: 10.1523/JNEUROSCI.20-04-01307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur J Neurosci. 1994;6:429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravleva SO, Kostyuk PG, Shuba YM. Subtypes of low voltage-activated Ca2+ channels in laterodorsal thalamic neurons: possible localization and physiological roles. Pflugers Arch. 2001;441:832–839. doi: 10.1007/s004240000490. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]