Abstract
Our previous studies found that nerve growth factor (NGF), via ceramide, enhanced the number of action potentials (APs) evoked by a ramp of depolarizing current in capsaicin-sensitive sensory neurons. Ceramide can be metabolized by ceramidase to sphingosine (Sph), and Sph to sphingosine 1-phosphate (S1P) by sphingosine kinase. It is well established that each of these products of sphingomyelin metabolism can act as intracellular signalling molecules. This raises the question as to whether the enhanced excitability produced by NGF was mediated directly by ceramide or required additional metabolism to Sph and/or S1P. Sph applied externally did not affect the neuronal excitability, whereas internally perfused Sph augmented the number of APs evoked by the depolarizing ramp. Furthermore, internally perfused S1P enhanced the number of evoked APs. This sensitizing action of NGF, ceramide and internally perfused Sph was abolished by dimethylsphingosine (DMS), an inhibitor of sphingosine kinase. In contrast, internally perfused S1P enhanced the number of evoked APs in the presence of DMS. These observations support the idea that the metabolism of ceramide/Sph to S1P is critical for the sphingolipid-induced modulation of excitability. Both internally perfused Sph and S1P inhibited the outward K+ current by 25–35% for the step to +60 mV. The Sph- and S1P-sensitive currents had very similar current–voltage relations, suggesting that they were likely to be the same. In addition, the Sph-induced suppression of the K+ current was blocked by pretreatment with DMS. These findings demonstrate that intracellular S1P derived from ceramide acts as an internal second messenger to regulate membrane excitability; however, the effector system whereby S1P modulates excitability remains undetermined.
Ceramide has been shown to be an important second messenger molecule in a multitude of cellular processes such as apoptosis and cell growth (Schütze et al. 1994; Ballou et al. 1996; Mathias et al. 1998; Kolesnick et al. 2000). Ceramide can be further metabolized by the enzyme ceramidase to sphingosine (Sph), and Sph then can be phosphorylated by Sph kinase to form sphingosine 1-phosphate (S1P). Interestingly, both Sph and S1P can act as intracellular signalling molecules (Hannun & Bell, 1989; Hla et al. 1999; Pyne & Pyne, 2000; Spiegel & Milstien, 2002, 2003). For example, vascular endothelial growth factor increases intracellular S1P, which then mediates the stimulation of Ras, with subsequent activation of downstream signalling pathways (Shu et al. 2002). In addition to its action as an intracellular second messenger, S1P has been established as a primary first messenger. This sphingolipid is released from immunocompetent cells, after which S1P binding leads to the activation of G-protein-coupled receptors known as EDG or S1P receptors (reviewed by Hla et al. 1999; Spiegel & Milstien, 2002, 2003; Rosen & Goetzl, 2005). Thus, activation of sphingomyelinase could give rise to three different intracellular second messenger molecules: ceramide, Sph and/or S1P.
It is well established that a family of receptors, such as the p75 neurotrophin receptor (p75NTR) and the p55 TNF-α receptor, are coupled to the activation of a sphingomyelinase that cleaves ceramide from membrane sphingomyelin (Dobrowsky et al. 1994, 1995; Dobrowsky & Carter, 1998; Brann et al. 1999). Nerve growth factor (NGF), which can activate p75NTR and the tyrosine kinase receptor TrkA when injected into the paw of a rat, produces hyperalgesia to thermal and mechanical stimulation (Lewin et al. 1993). Similar sensitizing actions of NGF are observed in an isolated skin–nerve-type preparation wherein NGF increases the firing frequency of isolated saphenous nerve in response to thermal stimulation (Rueff & Mendell, 1996). The effect of NGF is directly on the neuron because NGF augments the capsaicin-evoked current in small-diameter sensory neurons (Shu & Mendell, 1999, 2001). Acute exposure to NGF also enhances AP firing evoked by a ramp of depolarizing current in adult rat sensory neurons maintained in culture. This effect of NGF appears to result from activation of the sphingomyelin signalling cascade via p75NTR to liberate ceramide (Zhang et al. 2002; Zhang & Nicol, 2004). These findings raise the question as to whether this increased excitability is a direct result of the action of ceramide or secondary to the metabolism of ceramide to Sph and/or S1P. In this report, we show that internally perfused Sph or S1P augments the number of APs evoked by a ramp of current in small-diameter capsaicin-sensitive sensory neurons. Inhibition of Sph kinase blocks the capacity of NGF, ceramide and Sph to augment AP firing; however, the sensitization produced by internal S1P is not affected. These results indicate that S1P is the active second messenger by which the liberation of ceramide augments the firing of sensory neurons.
Methods
Isolation and maintenance of rat sensory neurons
Primary cultures of dissociated adult rat dorsal root ganglion (DRG) neurons were prepared as previously described (Lindsay, 1988) with slight modification (Jiang et al. 2003). Briefly, male Sprague-Dawley rats (100–150 g) were killed by placing them in a chamber that was then filled with CO2. DRGs were removed and collected in a culture dish filled with sterilized Puck's solution. The ganglia were transferred to a conical tube filled with Puck's solution containing 10 U ml−1 of papain II, and incubated for 10 min at 37°C. The tube was centrifuged for 30 s at low speed (approximately 1850 g) and the pellet was resuspended in Puck's solution containing collagenase (1 mg ml−1, type 1A) and dispase II (2.5 mg ml−1). After 10 min incubation at 37°C, the tube was centrifuged for 30 s before the enzyme-containing supernatant was removed. The pellet was resuspended in F-12 medium supplemented with 250 ng ml−1 7S nerve growth factor (Harlan Bioproducts, Indianapolis, IN, USA), and mechanically dissociated with fire-polished pipettes until all obvious chunks of tissues were gone. Isolated cells were plated onto plastic coverslips that had been previously coated with poly d-lysine and laminin. The cells were maintained in F-12 medium containing nerve growth factor at 37°C and 3% CO2 and used within 6–24 h for electrophysiological recordings. All procedures have been approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Electrophysiology
Recordings were made using the whole-cell patch-clamp technique as previously described (Hamill et al. 1981; Zhang et al. 2002). Briefly, a coverslip with the sensory neurons was placed in a recording chamber where the neurons were bathed in normal Ringer solution containing (mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes and 10 glucose, pH was adjusted to 7.4 with NaOH. Patch pipettes were pulled from borosilicate glass tubing and fire-polished. Whole-cell voltages or currents were recorded with an Axopatch 200 patch-clamp amplifier (Axon Instruments, Union City, CA, USA). The data were acquired and analysed using pCLAMP 6.04 or pCLAMP 8.2 (Axon Instruments). The whole-cell recording configuration was established in normal Ringer solution. Both capacitance and series resistance compensation (typically 80%) were used. The mean series resistance before compensation was 2.1 ± 0.04 MΩ (n = 106). Leak subtraction was not used for the measurement of the K+ current (IK) so that any effects of these agents on the holding current could be determined. To assess excitability in the current-clamp experiments, neurons were held at their resting potentials (range −50 to −65 mV) and a depolarizing ramp of current (1 s in duration) was applied. The amplitude of the ramp was adjusted to produce two to four action potentials (APs) under control conditions then the same ramp was used throughout the recording period for each individual neuron.
To isolate IK, neurons were superfused with a Ringer solution wherein NaCl was substituted with equimolar N-methyl-glucamine chloride (NMG-Cl, 140 mm); pH was adjusted to 7.4 with KOH. Recording pipettes typically had resistances of 2–4 MΩ when filled with the following solution (mm): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 2.5 CaCl2, 5 EGTA (calculated free Ca2+ concentration of ∼100 nm; MaxChelator) and 10 Hepes; pH was adjusted to 7.3 with KOH. This pipette solution was used in the current-clamp recordings as well. The membrane was held at −60 mV; this value was chosen so that current measurements could be ascertained at a voltage that reflected the normal resting potential in these sensory neurons. Activation of IK was determined by voltage steps of 350 ms, which were applied at 5 s intervals in +10 mV increments to +60 mV. After obtaining the control response, the bath solution was changed to the appropriate Ringer solution, and cells were superfused continuously for the appropriate times. In a separate series of time control experiments, the peak amplitudes for IK did not vary significantly over a 20 min time period indicating that there was little run-down of this current over this time. At the end of each recording, the neuron was exposed to 100 nm capsaicin. This neurotoxin was used to distinguish capsaicin-sensitive sensory neurons, as these neurons are believed to transmit nociceptive information (Holzer, 1991). The results reported below were obtained from capsaicin-sensitive neurons only. All experiments were performed at room temperature (∼22°C).
Data analysis
Data are presented as the means ± s.e.m. The excitability parameters described in Tables 1 and 2 were determined, in part, by differentiating the voltage trace (dV/dt) in the current-clamp recordings (sampling frequency of 250 Hz). The voltage and time at which the first AP was fired were taken as the point that exceeded the baseline value of dV/dt by >20-fold. The baseline value of dV/dt was determined by averaging the points over 100 ms that began with the onset of the current ramp (134–234 ms). The rheobase was measured as the amount of ramp current at the firing threshold. The membrane resistance was calculated as the difference between the firing threshold and the resting membrane potential divided by the rheobase current. The voltage dependence for activation IK was fitted with the Boltzmann equation where G/Gmax = 1/[1 + exp(V0.5−Vm)/k], where G is the conductance (G = I/(Vm−EK)), Gmax is the maximal conductance obtained from the Boltzmann fit, V0.5 is the voltage for half-maximal activation, Vm is the membrane potential, and k is a slope factor. The Boltzmann parameters were determined for each individual neuron and were used to calculate the mean ± s.e.m. Statistical differences between the control recordings and those obtained under various treatment conditions were determined by using a t test, a paired t test, an analysis of variance (ANOVA), or a repeated measures ANOVA (RMANOVA). When a significant difference was obtained with an ANOVA, post hoc analyses were performed using a Tukey test. Values of P < 0.05 were judged to be statistically significant.
Table 1.
Effects of internal sphingosine (1 μm) on current clamp parameters
| Resting Vm | Latency | Threshold | Rheobase | Rheo Sph | Rm Sph | |
|---|---|---|---|---|---|---|
| (mv) | (ms) | (mV) | (pA) | Rheo Cont | Rm Cont | |
| Control | −58.7 ± 2.5 | 1315 ± 121 | −13.5 ± 3.3 | 378 ± 103 | 1.0 | 1.0 |
| 2 min | −57.7 ± 2.6 | 929 ± 127* | −18.9 ± 4.6 | 308 ± 111 | 0.71 ± 0.13 | 1.36 ± 0.18 |
| 6 min | −57.8 ± 2.4 | 899 ± 109* | −18.3 ± 5.0 | 279 ± 99 | 0.67 ± 0.10* | 1.46 ± 0.18 |
| 10 min | −56.6 ± 2.3 | 811 ± 124* | −16.3 ± 6.7 | 279 ± 119 | 0.60 ± 0.14* | 1.74 ± 0.26* |
| 20 min | −57.3 ± 2.6 | 642 ± 89* | −14.0 ± 4.0 | 191 ± 76* | 0.43 ± 0.07* | 2.53 ± 0.45* |
Sph, sphingosine; Vm, membrane potential; Rheo, rheobase; Rm, membrane resistance; Cont, control.
P < 0.05 (RM ANOVA), n = 7.
Table 2.
Effects of internal sphingosine 1-phosphate (1 μm) on current clamp parameters
| Resting Vm | Latency | Threshold | Rheobase | Rheo S1P | Rm S1P | |
|---|---|---|---|---|---|---|
| (mv) | (ms) | (mV) | (pA) | Rheo Cont | Rm Cont | |
| Control | −56.9 ± 2.7 | 446 ± 60 | −16.0 ± 3.6 | 241 ± 56 | 1.0 | 1.0 |
| 2 min | −55.3 ± 2.6 | 363 ± 47* | −18.3 ± 3.2 | 170 ± 34 | 0.76 ± 0.05* | 1.21 ± 0.08 |
| 6 min | −54.2 ± 2.4 | 343 ± 47* | −17.6 ± 1.9 | 150 ± 27* | 0.71 ± 0.08* | 1.36 ± 0.14 |
| 10 min | −54.1 ± 2.3 | 334 ± 42* | −19.1 ± 1.9 | 156 ± 33 | 0.72 ± 0.10* | 1.35 ± 0.18 |
| 20 min | −52.0 ± 1.8* | 313 ± 41* | −17.2 ± 2.3 | 126 ± 20* | 0.60 ± 0.08* | 1.59 ± 0.21* |
S1P, sphingosine 1-phosphate.
P < 0.05 (RM ANOVA), n = 7.
Chemicals
C2-ceramide (N-acetyl sphingosine, hereafter referred to as ceramide), Sph and S1P were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). All other chemicals were obtained from Sigma Chemical Corp. (St Louis, MO, USA). Tissue culture supplies were purchased from Invitrogen (Carlsbad, CA, USA). Ceramide, Sph and capsaicin were dissolved in 1-methyl-2-pyrrolidinone to obtain concentrated stock solutions. S1P was dissolved according to instructions provided by the supplier (www.avantilipids.com/SyntheticSphingosine-1-phosphate.asp). These stock solutions were then diluted with Ringer solution or the pipette solution to yield the appropriate concentration. We have demonstrated previously that the vehicle 1-methyl-2-pyrrolidinone has no effect on AP firing or the activation of IK (Zhang et al. 2002).
Results
Sph and S1P enhance the excitability of sensory neurons
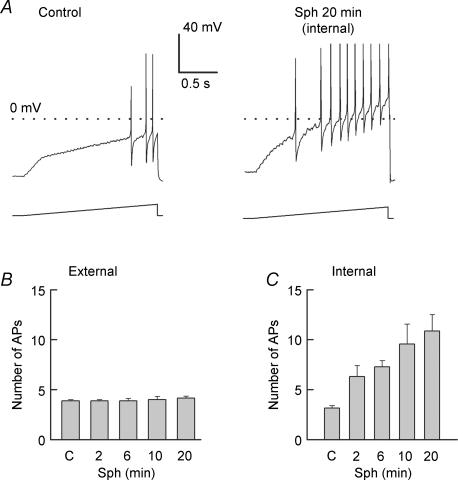
Our previous work demonstrated that ceramide, a product of sphingomyelin hydrolysis, enhanced the excitability of small-diameter capsaicin-sensitive sensory neurons (Zhang et al. 2002). However, it is well established that ceramide can be further metabolized to Sph through the action of ceramidase and, in turn, Sph can be phosphorylated by Sph kinase to yield S1P (Spiegel et al. 1996; Pyne & Pyne, 2000; Hannun et al. 2001). This raises the question whether the ceramide-induced augmentation of excitability was due directly to ceramide or to its metabolic products. To address this question, the effect of Sph on excitability was investigated by using a ramp of depolarizing current to evoke APs in the absence or presence of Sph. An extracellular application of 10 μm Sph to isolated sensory neurons had no effect on the number of APs, even after a 20 min exposure (summarized in Fig 1B, 3.9 ± 0.1 control versus 4.1 ± 0.2 after a 20 min treatment, n = 7). Because Sph is a polar lipid and a sufficient quantity may not cross the neuronal membrane over this 20 min time period, in another series of experiments Sph (1 μm) was perfused internally into sensory neurons by diffusion from the recording pipette. As shown in a recording from a representative neuron (see Fig. 1A), the number of APs evoked by the ramp increased from a control value of 3 APs to 10 APs after 20 min of internal perfusion. The results obtained from seven sensory neurons under these conditions are summarized in Fig. 1C and show that the number of APs increased in a time-dependent manner from an average control value of 3.1 ± 0.3 to a value 10.9 ± 1.6 (RMANOVA) after a 20 min exposure to internal Sph. The effects of internal Sph on the parameters of excitability for seven neurons are summarized in Table 1. The increased excitability resulting from the 20 min exposure to Sph was not accompanied by depolarization of the membrane or a change in the firing threshold of the AP. However, in these same neurons, the latency for the generation of the first AP was significantly decreased by 2.2 ± 0.8 fold (n = 7, RM ANOVA), the rheobase was reduced by 2.8 ± 0.5 fold, and the membrane resistance was increased by 2.5 ± 0.5 fold. These results demonstrate that elevations in internal Sph significantly enhance the excitability of these sensory neurons without altering the resting Vm or the firing threshold. These observations are similar to those reported previously by our laboratory for the effects of NGF and ceramide in augmenting the excitability in capsaicin-sensitive sensory neurons (Zhang et al. 2002).
Figure 1. Internally perfused, but not externally applied, sphingosine increases the excitability of sensory neurons.
A, left, representative response from an adult sensory neuron (resting membrane potential (Vm) −56 mV) to the application of a depolarizing ramp of current (300 pA, the peak amplitude of the ramp) obtained under ‘control’ conditions. A, right, response to the same ramp after 20 min of internal perfusion with 1 μm sphingosine (Sph) in the same neuron (resting Vm−58 mV). B, summary of the lack of effect of externally applied Sph (1 μm) on the excitability in seven adult neurons. The peak amplitudes of the ramps for individual neurons ranged from 250 to 800 pA; the same ramp was used prior to and throughout the treatment with sphingolipid for each individual neuron. C, the ability of internally perfused Sph (1 μm) to increase the number of action potentials (APs) evoked by the current ramp in seven adult sensory neurons. The peak amplitudes of the ramps ranged from 130 to 1500 pA. *Significant (P < 0.05) increase compared with the control values using an RM ANOVA.
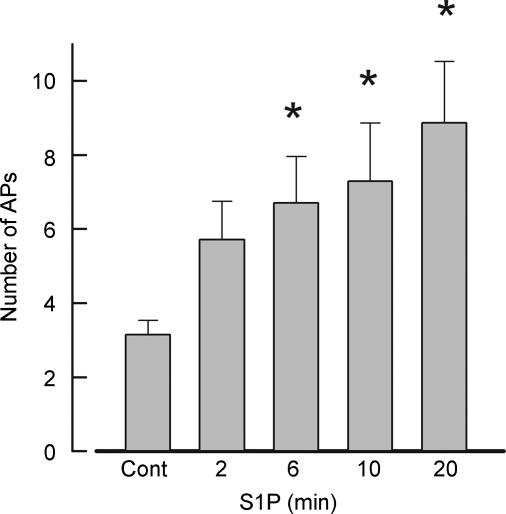
Because S1P is derived from the phosphorylation of Sph by Sph kinase, we determined whether internally perfused S1P had effects that were similar to those of ceramide and sphingosine. Indeed, S1P augmented the excitability of adult sensory neurons. These results are summarized for seven neurons in Fig. 2. Internal perfusion with 1 μm S1P significantly increased the number of APs elicited by the ramp in a time-dependent manner (RM ANOVA) from an average control value of 3.1 ± 0.4 to a value 8.9 ± 1.7 after a 20 min exposure to this sphingolipid. In recordings from a total of eight neurons, seven neurons exhibited an increase in the firing of evoked APs after S1P and are described above, whereas the excitability of one neuron remained unaffected by S1P. It is difficult to know whether this cell was insensitive to S1P or whether S1P did not diffuse from the recording pipette. The effects of S1P on resting Vm, firing latency, threshold, rheobase and membrane resistance for these seven neurons that responded are summarized in Table 2. Internal perfusion with S1P significantly decreased the latency of AP generation by 1.50 ± 0.15 fold, the rheobase by 1.89 ± 0.31 fold, and significantly increased the membrane resistance by 1.59 ± 0.21 fold after 20 min. Similar to Sph, the increased excitability produced by internal S1P was not accompanied by depolarization of the membrane or a change in the threshold for AP firing. Taken together, these results indicate that, like ceramide, both internal Sph and S1P enhance the excitability of sensory neurons in adult sensory neurons.
Figure 2. Sphingosine 1-phosphate (S1P) augments the excitability of sensory neurons.
Summary of the effects of internally perfused S1P (1 μm) on the number of APs evoked over the indicated times in seven adult sensory neurons. The peak amplitudes of the ramps ranged from 200 to 6000 pA. *Significant (P < 0.05) increase compared with the control values using an RM ANOVA.
An inhibitor of sphingosine kinase prevents the sensitization produced by NGF and Sph, but not S1P
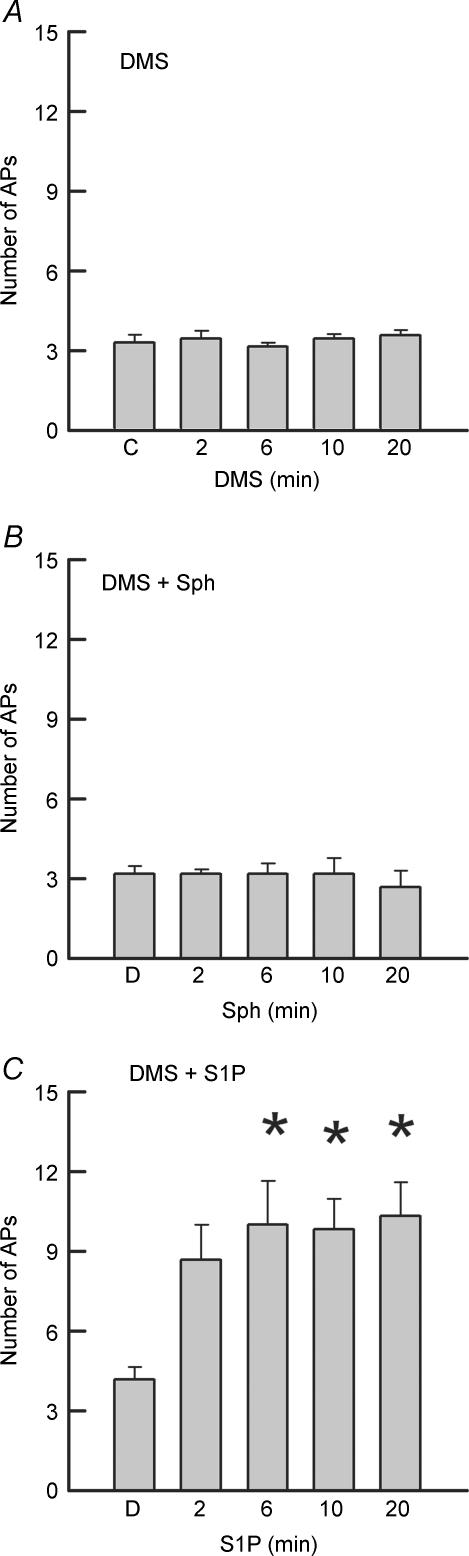
Although all three sphingolipids, ceramide, Sph and S1P, augment the excitability of sensory neurons, ceramide and Sph can be converted to S1P by a series of enzymatic reactions. This raises the question as to which sphingolipids are effective second messengers that enhance the excitability of sensory neurons. To examine this idea, dimethylsphingosine (DMS), a specific competitive inhibitor of Sph kinase, was used to block the conversion of ceramide and Sph to S1P (Olivera & Spiegel, 1993; Yatomi et al. 1996; Edsall et al. 1998). Figure 3A demonstrates that treatment with 20 μm DMS alone for 20 min had no significant impact on the excitability of sensory neurons (n = 7). In a separate series of experiments, 20 min pretreatment with 20 μm DMS blocked the capacity of internally perfused Sph (1 μm, Fig. 3B) to augment the number of APs evoked by the depolarizing ramp. Pretreatment with DMS also blocked the ability of ceramide to increase AP firing in five sensory neurons (data not shown). In contrast to the above, pretreatment with DMS did not alter the capacity of internal S1P to augment the number of evoked APs in six neurons and increased the number from a control value of 4.2 ± 0.5 to 10.3 ± 1.3 after 20 min (see Fig. 3C). In the presence of DMS, S1P decreased the rheobase and the latency, increased the membrane resistance, and did not alter the firing threshold. This excitatory effect of S1P in the presence of DMS is similar to the results described above for S1P alone. These results support the idea that the sensitizing action of Sph is secondary to its metabolism to S1P.
Figure 3. The augmented excitability produced by internal Sph, but not internal S1P, is blocked by dimethylsphingosine.
A, a bath application of 20 μm dimethylsphingosine (DMS) alone over a 20 min period has no effect on excitability in seven adult sensory neurons. The ramps ranged in peak amplitudes from 125 to 1000 pA. B, pretreatment with 20 μm DMS for 20 min blocked the capacity of internally perfused Sph (1 μm) to enhance the number of APs evoked by the ramp (range 90–250 pA). C, pretreatment with 20 μm DMS did not affect the augmented excitability produced by internally perfused S1P (1 μm) in six adult sensory neurons. The ramps ranged in peak amplitudes from 60 to 800 pA. *Significant (P < 0.05) increase compared to the control values using an RM ANOVA.
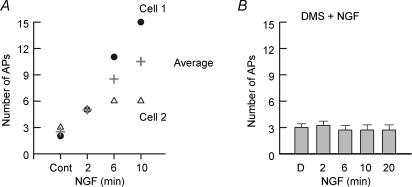
We have demonstrated previously that NGF augments the number of APs evoked by a ramp of depolarizing current (Zhang et al. 2002; Zhang & Nicol, 2004) and that this action is dependent on the activation of sphingomyelinase. To determine whether the NGF-induced increase in excitability is dependent on the conversion of ceramide to S1P, we examined the sensitizing effects of this neurotrophin in the absence or presence of DMS. As shown in Fig. 4A, extracellular treatment with 100 ng ml−1 NGF increased the number of evoked APs in a time-dependent manner wherein after a 10 min exposure to NGF the average number of APs was increased to 10.5 (15 and 6 APs, n = 2) from an average control value of 2.5 (2 and 3 APs, respectively), and is similar to our previous report where the number of APs was increased from 3.4 ± 0.2 to 13.9 ± 2.7 (n = 7) after a 10 min exposure to NGF (Zhang et al. 2002). Isolated sensory neurons obtained from the same tissue harvest were pretreated with 20 μm DMS. In these neurons exposed to DMS, NGF failed to increase the number of APs evoked by the ramp of current (Fig. 4B). Thus, these results support the idea that S1P is the intracellular second messenger mediating the NGF-induced enhancement of excitability.
Figure 4. The enhanced excitability produced by nerve growth factor is blocked by DMS.
A, exposure to 100 ng ml−1 nerve growth factor (NGF) increases the number of APs evoked by the depolarizing ramp of current in a time-dependent manner. The results shown were obtained from two sensory neurons (cell 1, 2000 pA; cell 2, 240 pA) and the plus symbol represents their average value. B, in other sensory neurons from the same tissue harvest as in A, a 20 min pretreatment with 20 μm DMS completely blocked the increased excitability caused by bath-applied NGF (100 ng ml−1) in six adult sensory neurons. The ramps ranged in peak amplitudes from 50 to 6000 pA.
Sph and S1P suppress IK
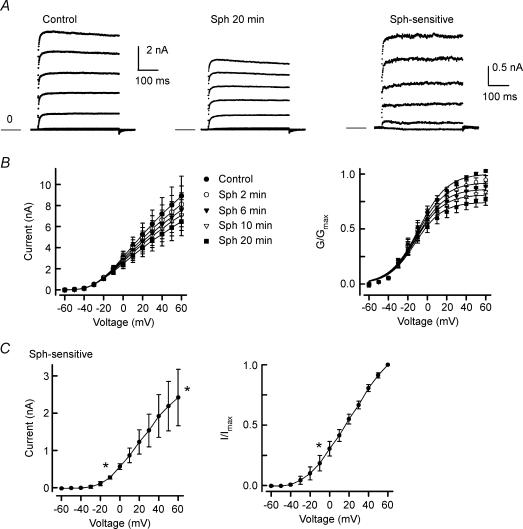
It is well established that a reduction in K+ currents enhances the excitability of sensory neurons (Weinreich & Wonderlin, 1987; Gold et al. 1996; Nicol et al. 1997; Cordoba-Rodriguez et al. 1999; Zhang et al. 2002). Therefore, we explored the idea that the sensitizing action of Sph or S1P observed in the current-clamp experiments results from the inhibition of an outward K+ current (IK). Internal perfusion with Sph (1 μm) produced a time-dependent decrease in IK in capsaicin-sensitive adult sensory neurons (see Fig. 5A). As shown for a representative neuron, under control conditions (left panel) the peak IK obtained for the step to +60 mV was 8.43 nA. After internal perfusion with 1 μm Sph for 20 min, IK was reduced to 6.02 nA (middle panel) and corresponds to ∼30% inhibition. The Sph-sensitive IK (right panel) was obtained by subtraction of the traces in the middle panel from those in the left panel. This Sph-sensitive current exhibits little time-dependent decrease in amplitude during the voltage step suggesting that it may be a delayed rectifier type of IK. The Sph-induced inhibition of IK obtained from five neurons is summarized in Fig. 5B. The left panel of Fig. 5B shows the current–voltage relations for the time-dependent suppression of IK where Sph significantly reduced IK from an average control value of 8.90 ± 1.88 nA to a value of 6.47 ± 1.35 nA (step to +60 mV) 20 min after attaining the whole-cell configuration. The inhibition of IK measured at +60 mV after 20 min corresponded to a reduction by 26 ± 5% (range 17–42% inhibition). In another series of experiments, internal perfusion with 50 μm Sph reduced IK by 57 ± 2% (n = 4, data not shown) after a 20 min exposure. The effects of Sph on the conductance–voltage relation are summarized in the right panel of Fig. 5B, and the Boltzmann fitting parameters are summarized in Table 3. The values of G /Gmax were reduced in a time-dependent manner; however, there was no significant change in the average values for V0.5 and k. In a separate series of studies, the inhibition of IK produced by internally perfused Sph (1 μm) was blocked by pretreatment with 20 μm DMS (5.83 ± 0.53 for the control versus 5.97 ± 0.60 nA after 20 min perfusion with Sph, n = 5). In addition, IK was recorded from four small-diameter sensory neurons before and after exposure to 20 μm DMS. Under control conditions, the peak IK measured at +60 mV was 7.62 ± 1.18 nA; these values were not significantly different (P = 0.07, t test) from the control IK for those experiments examining the internal perfusion of Sph (as shown in Fig. 5B). After 5 and 10 min exposures to 20 μm DMS, the peak IK values at +60 mV were 7.71 ± 1.11 and 7.58 ± 0.95 nA, respectively. These results suggest that DMS by itself does not inhibit IK, and that the low values of IK for that particular series of experiments wherein DMS blocked the Sph-induced decrease in IK were within biological variation. Figure 5C illustrates the current–voltage relations for the Sph-sensitive IK (left panel) and their normalization to the maximal peak current obtained at +60 mV (I/Imax, right panel). It appears that this current begins to activate at about −10 mV because the values of the Sph-sensitive IK were significantly different at this voltage compared with those obtained at −60 mV (ANOVA). These results indicate that internally perfused Sph can suppress IK without significantly shifting V0.5 and that this inhibition depends on the conversion of Sph to S1P.
Figure 5. Internally perfused Sph suppresses K+ current.
A, current traces from a representative neuron under control conditions (left) and after a 20 min application of 1 μm Sph (middle). The Sph-sensitive K+ current (IK) was obtained by subtraction of the traces after treatment with Sph from the control (right panel). Currents were obtained with voltage steps in 10 mV increments from −80 to +60 mV from a holding voltage of −60 mV. The current traces presented in these panels are in 20 mV increments (−80 to +60 mV) for purposes of clarity. The calibration bar on the left refers to the left and middle panels, whereas the calibration bar on the right refers to the right panel. The line labelled zero marks the level for zero current. B, left, current–voltage relation for the time-dependent suppression of IK by Sph in five neurons. The decreases in the currents measured at 6, 10 and 20 min were significantly different from the control for voltages between −10 and +60 mV. B, right, conductance–voltage relation, wherein the conductances at each time were normalized to the fitted value of maximal conductance (Gmax) obtained for each neuron under the control condition. The decreases in the values for G/Gmax measured at 6, 10 and 20 min were significantly different to the control for voltages between −10 and +60 mV, −10 and +60 mV, and −20 and +60 mV, respectively. C, left, current–voltage relation for the Sph-sensitive IK, as determined from the subtraction of the current traces obtained after the 20 min exposures to Sph from their respective controls. C, right, normalized current–voltage relation for the Sph-sensitive IK. Currents were normalized to the peak IK obtained for the step to +60 mV under control conditions. *Significant difference between the currents obtained for voltages between −10 and +60 mV compared with that at −60 mV (P < 0.05, ANOVA).
Table 3.
Effects of internal Sph and S1P on the Boltzmann fitting parameters
| G/Gmax | V0.5 (mV) | k (mV) | |
|---|---|---|---|
| Internal Sph (n = 5) | |||
| Control | 1.03 ± 0.01 | −8.8 ± 3.8 | 13.7 ± 0.6 |
| 2 min | 0.95 ± 0.04 | −9.9 ± 3.6 | 13.5 ± 0.6 |
| 6 min | 0.89 ± 0.04* | −10.4 ± 3.5 | 13.6 ± 0.6 |
| 10 min | 0.84 ± 0.05* | −11.3 ± 3.2 | 13.5 ± 0.6 |
| 20 min | 0.77 ± 0.05* | −12.1 ± 2.8 | 13.4 ± 0.6 |
| Internal S1P (n = 7) | |||
| Control | 1.02 ± 0.01 | −1.6 ± 2.7 | 15.9 ± 1.5 |
| 2 min | 0.87 ± 0.05 | −3.9 ± 3.0 | 15.4 ± 1.2 |
| 6 min | 0.76 ± 0.07* | −5.7 ± 3.0 | 15.9 ± 1.0 |
| 10 min | 0.72 ± 0.07* | −3.7 ± 3.6 | 17.5 ± 1.2 |
| 20 min | 0.69 ± 0.09* | −2.9 ± 4.7 | 17.0 ± 1.5 |
P < 0.05 (RM ANOVA).
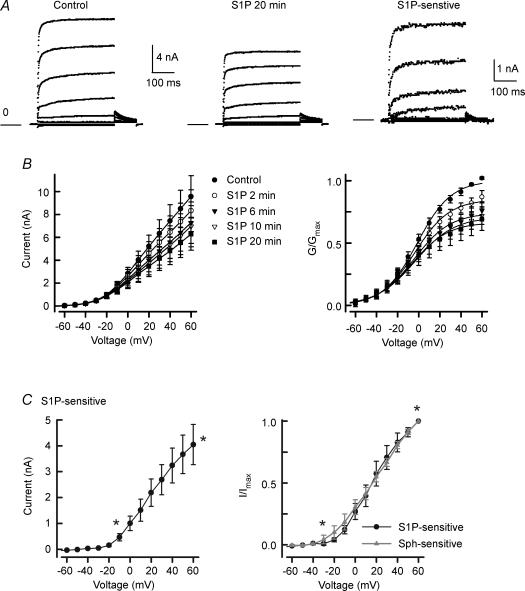
As indicated in the current-clamp studies, the enhanced excitability depends on the conversion of Sph to S1P. If the Sph-induced inhibition of IK is actually mediated by S1P, then the inhibition produced by S1P should be very similar to that observed for Sph. Indeed, internal perfusion with 1 μm S1P suppressed the outward IK in a time-dependent manner that was quite similar to that for Sph. Current traces obtained from a representative neuron are shown in Fig. 6A. Under control conditions (left panel) the peak IK obtained at +60 mV was 15.69 nA, whereas after a 20 min exposure to S1P (middle panel) the peak current was reduced to 10.85 nA (∼30% inhibition). The S1P-sensitive IK obtained by subtraction has a peak amplitude of 4.85 nA and is illustrated in the right panel. Figure 6B (left panel) summarizes the current–voltage relations for the suppression of IK by S1P observed in seven neurons at the different time points wherein IK was reduced significantly from a control value of 9.57 ± 1.81 nA to a value of 6.28 ± 1.41 nA after a 20 min exposure to S1P (RM ANOVA). This corresponds to a reduction of 32 ± 8% (range 0–56%) of the peak IK obtained at +60 mV and is similar to the 26 ± 5% inhibition by Sph. One of the seven neurons exposed to S1P exhibited a reversal of inhibition after 20 min, even though IK was suppressed by 16% at 10 min. If this neuron is excluded, then the suppression produced by S1P ranged from 15 to 56%. Figure 6B (right panel) demonstrates the conductance–voltage relation wherein the Boltzmann fitting parameters are summarized in Table 3. As for Sph, S1P reduced the value of G /Gmax in a time-dependent manner but did not alter V0.5 or k. For the control recordings, the values of V0.5 for Sph- and S1P-treated neurons were not different (P = 0.13, t test). The current–voltage relation for the S1P-sensitive IK is shown in Fig. 6C (left panel). This current begins to exhibit significant activation at about −10 mV (compared to −60 mV, ANOVA) and is the same voltage as determined for activation of the Sph-sensitive IK. The normalization of the S1P-sensitive IK to their respective maximal peak currents obtained at +60 mV (I/Imax) is shown in the right panel of Fig. 6C. Here, the S1P-sensitive current exhibits a significant increase at about −30 mV, perhaps resulting from the reduced variability upon normalization. The Sph-sensitive current–voltage relation is overlaid on that for the S1P-sensitive current and demonstrates that these two currents are essentially the same (P > 0.05, t test for each respective voltage). Taken together with the findings for DMS, these many points of similarity between the Sph- and S1P-sensitive currents would strongly indicate that S1P is the active agent that leads to the suppression of IK.
Figure 6. Internally perfused S1P suppresses IK.
A, current traces from a representative neuron under control conditions (left) and after a 20 min application of 1 μm S1P (middle). The S1P-sensitive IK was obtained by subtraction of the traces after treatment with S1P from the control. Currents were obtained with voltage steps in 10 mV increments from −80 to +60 mV from a holding voltage of −60 mV. The current traces presented in these panels are in 20 mV increments (−80 to +60 mV) for purposes of clarity. The calibration bar on the left refers to the left and middle panels, whereas the calibration bar on the right refers to the right panel. The line labelled zero marks the level for zero current. B, left, current–voltage relation for the time-dependent suppression of IK by S1P in seven neurons. The decreases in the currents measured at 2, 6, 10 and 20 min were significantly different from the control for voltages between 40 and 60 mV, 0 and +60 mV, 0 and +60 mV, and −10 and +60 mV, respectively. B, right, conductance–voltage relation wherein the conductances at each time were normalized to the fitted value of Gmax obtained for each neuron under the control condition. The decreases in the values for G/Gmax measured at 6, 10 and 20 min were significantly different from the control for voltages between 20 and +60 mV, 10 and +60 mV, and 10 and +60 mV, respectively. C, left, current–voltage relation for the S1P-sensitive IK as determined from the subtraction of the current traces obtained after the 20 min exposures to S1P from their respective controls. C, right, normalized current–voltage relation for the S1P-sensitive IK. Currents were normalized to the peak IK obtained for the step to +60 mV under control conditions. *Significant difference between the S1P-sensitive currents obtained for those designated voltages compared with that obtained at −60 mV (P < 0.05, ANOVA). The Sph-sensitive IK from Fig. 4C is indicated by the triangles and line.
Discussion
These results demonstrate that internal perfusion with either Sph or S1P augments the excitability of capsaicin-sensitive sensory neurons in a manner analogous to that observed in previous studies with NGF and ceramide (Zhang et al. 2002; Zhang & Nicol, 2004). This enhanced excitability is associated with a lowering of the rheobase and an increase in the membrane resistance although there is no significant change in the resting Vm or the firing threshold of the AP. In addition, both Sph and S1P suppress an IK(s) by about 30% without any significant change in the V0.5 for activation. The Sph- and S1P-sensitive IKs exhibit the properties of a delayed rectifier, notably rapid activation with little time-dependent decrease in amplitude during the voltage step. This suppression is compatible with the observed sphingolipid-induced increase in the number of APs generated by a ramp of depolarizing current. Indeed, suppression of delayed rectifier K+ channels may not necessarily alter the resting Vm, but could significantly modify the excitability of neurons.
The idea that S1P acts as an intracellular signalling agent is supported by the observations that treatment with the Sph kinase inhibitor DMS blocked the ability of NGF, ceramide and internal Sph to augment excitability. In contrast, exposure to DMS did not affect the sensitization produced by internally applied S1P. We assume that DMS is preventing the conversion of exogenous or endogenous ceramide and Sph to S1P since previous work demonstrated that exposing cells to this sphingosine kinase inhibitor decreases the levels of cellular S1P and increases Sph and/or ceramide levels (Meyer zu Heringdorf et al. 1998; Hobson et al. 2001). Furthermore, our previous work suggests that a component of NGF-induced excitability in sensory neurons is mediated by the p75 neurotrophin receptor (Zhang & Nicol, 2004) and activation of this receptor initiates the sphingolipid signalling cascade (Dobrowsky et al. 1994; Brann et al. 1999). Thus, our current results, when taken with previous findings, indicate that NGF-induced modulation of excitability in sensory neurons involves activation of a signalling pathway that ultimately liberates S1P as an intracellular second messenger.
Previous studies by various investigators using different endpoints suggest that other signalling pathways also contribute to the NGF-induced peripheral sensitization. At the receptor level, much indirect evidence supports the notion that activation of the TrkA receptor mediates the sensitizing actions of NGF. Co-expression of TrkA and TRPV1 receptors in oocytes and HEK cells results in an increase in capsaicin-sensitivity after NGF treatment (Chuang et al. 2001), suggesting that TrkA activation is critical for NGF-induced increases in currents conducted by TRPV1. In addition, systemic injection of NGF results in hyperalgesia in transgenic mice with a presumed p75 receptor knock-out (Lee et al. 1992), suggesting that the nociceptive actions of NGF are mediated by TrkA (Bergmann et al. 1998). However, recent work with this transgenic strain shows that the animals express a protein isoform of the p75 receptor (von Schack et al. 2001; Paul et al. 2004), thus complicating the interpretation of these behavioural results. Activation of the TrkA receptor will increase the activity of a number of downstream signalling pathways (Kaplan & Miller, 2000) and inhibiting these cascades attenuates NGF-induced sensitization of sensory neurons. Although this implies that TrkA activation mediates NGF-induced sensitization, results vary depending on the preparation used and the endpoints examined. For example, NGF-induced augmentation of capsaicin-evoked currents in expression systems are attenuated by blocking phospholipase C (PLC) activity (Chuang et al. 2001), whereas NGF actions on capsaicin currents in isolated sensory neurons are reduced by inhibition of protein kinase A (PKA) and protein kinase C (PKC) (Shu & Mendell, 2001). Augmentation of the capsaicin-induced increase in intracellular Ca2+ concentration by NGF are reversed by inhibition of phosphatidylinositol 3 kinase and PKC, but not by inhibitors of MAP kinase or PKA (Bonnington & McNaughton, 2003). Thus, controversy remains as to which signalling cascades mediate the acute sensitizing actions of NGF. It is possible that different NGF signalling pathways mediate various effectors that can contribute to the sensitization of sensory neurons. Furthermore, both TrkA and p75 receptors may be necessary for the NGF-induced peripheral sensitization since there is precedent in the literature for an interaction between these TrkA and p75 receptors (Bilderback et al. 2001; Lad et al. 2003). Additional studies are warranted to determine which of these possibilities can account for the variable results seen by different investigators when examining the acute effects of NGF on sensory neurons.
Although our results suggest that S1P acts as an intracellular second messenger, it also is possible that in cells, S1P can be dephosphorylated to Sph, which in turn can be catabolized to ceramide, and ceramide can be phosphorylated by ceramide kinase to produce ceramide 1-phosphate (C1P), which recent evidence indicates can act as an intracellular second messenger much like S1P (Colombaioni & Garcia-Gil, 2004; Lamour & Chalfant, 2005). In non-neuronal cells, exposure to S1P increases COX2 expression (Pettus et al. 2003, 2005) and increases the accumulation of cAMP (Damirin et al. 2005), whereas C1P can increase phospholipase A2 activity that results in increased levels of arachidonic acid (Pettus et al. 2005). These results suggest that activation of the sphingolipid cascade could increase the production of prostaglandins in cells. This could, in part, account for the sensitizing actions of ceramide and S1P since the prostaglandins E2 and I2 increase intracellular cAMP and consequently sensitize sensory neurons (Hingtgen et al. 1995; Nicol et al. 1997). In addition, S1P can be released from a variety of cell types (see below) and acts as a first messenger by binding to G-protein-coupled receptors (S1P receptors; also known as EDG receptors; Taha et al. 2004; Rosen & Goetzl, 2005). The binding of S1P to its receptors activates a number of signal transduction cascades (Spiegel & Milstien, 2002, 2003; Rosen & Goetzl, 2005) and results in an increase in excitability of sensory neurons (Zhang et al. 2006). S1P increases the intracellular Ca2+ concentration independent of the production of inositol trisphoshate (Ghosh et al. 1990, 1994), and potentially could increase excitability as well. However, in sensory neurons this seems unlikely for two reasons. First, treatment with external S1P did not elevate intracellular Ca2+ levels in capsaicin-sensitive sensory neurons (Y. H. Zhang & G. D. Nicol, unpublished observations). Second, acute exposure to NGF did not increase intracellular Ca2+ levels (Bonnington & McNaughton, 2003). At present, other potential effectors or second messengers activated by S1P in neurons are unknown (see review by Colombaioni & Garcia-Gil, 2004) and this remains an area of future investigation.
In contrast to our observations in sensory neurons demonstrating that external Sph is ineffective at altering the generation of APs, previous studies have shown that external Sph is capable of modulating membrane currents. When applied externally to rat ventricular myocytes, Sph reduced both INa and ICa (McDonough et al. 1994; Yasui & Palade, 1996). Similar results were reported for Sph-induced inhibition of ICa in GH4C1 cells (Titievsky et al. 1998). Whether the action of Sph on these cells was secondary to the generation of S1P by activation of Sph kinase was not determined. In this regard, exposing human umbilical vein endothelial cells to Sph leads to the formation of S1P in the external medium (Ancellin et al. 2002). Furthermore, Sph kinase activity is detected in conditioned media from these cells, demonstrating that Sph kinase can be secreted or released from cells. This could be a mechanism leading to increased levels of S1P in the plasma with consequent activation of the G-protein-coupled S1P receptors. Our negative results with externally applied Sph suggest that this lipid is not active on sensory neurons and that it is not metabolized to S1P under our experimental conditions.
In conclusion, our current observations, when taken together with our previous findings (Zhang et al. 2002), suggest that activation of sphingolipid metabolism in sensory neurons is an important signalling pathway in modulating excitability, and that NGF-mediated sensitization involves activation of this pathway. Ceramide can be metabolized to S1P, which then modulates the activity of different ion channels by an unknown mechanism to enhance AP firing in nociceptive sensory neurons. Thus, the sphingomyelin signalling cascade is likely to play an important role in controlling the sensitivity of sensory neurons after exposure to inflammatory or neuropathic conditions.
Acknowledgments
We would like to thank Drs Gary Strichartz and Ted Cummins for their useful comments. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481-01 from the National Center for Research Resources, NIH. This work was supported by NIH NINDS NS37951 and NS46084.
References
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Ballou LR, Laulederkind SJF, Rosloneic EF, Raghow R. Ceramide signalling and the immune response. Biochim Biophys Acta. 1996;1301:273–287. doi: 10.1016/0005-2760(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Bergmann I, Reiter R, Toyka KV, Koltzenburg M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci Lett. 1998;255:87–90. doi: 10.1016/s0304-3940(98)00713-7. [DOI] [PubMed] [Google Scholar]
- Bilderback TR, Gazula VR, Dobrowsky RT. Phosphoinositide 3-kinase regulates crosstalk between TrkA tyrosine kinase and p75NTR-dependent sphingolipid signaling pathways. J Neurochem. 2001;76:1540–1551. doi: 10.1046/j.1471-4159.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H-H, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AL, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from Ptdlns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Colombaioni L, Garcia-Gil M. Sphingolipid metabolites in neural signalling and function. Brain Res Brain Res Rev. 2004;46:328–355. doi: 10.1016/j.brainresrev.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Cordoba-Rodriguez R, Moore KA, Kao JP, Weinreich D. Calcium regulation of a slow post-spike hyperpolarization in vagal afferent neurons. Proc Natl Acad Sci U S A. 1999;96:7650–7657. doi: 10.1073/pnas.96.14.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damirin A, Tomura H, Komachi M, Tobo M, Sato K, Mogi C, Nochi H, Tamoto K, Okajima F. Sphingosine 1-phosphate receptors mediate the lipid-induced cAMP accumulation through cyclooxygenase-2/prostaglandin I2 pathway in human coronary artery smooth muscle cells. Mol Pharmacol. 2005;67:1177–1185. doi: 10.1124/mol.104.004317. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Carter BD. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann N Y Acad Sci. 1998;845:32–45. doi: 10.1111/j.1749-6632.1998.tb09660.x. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co-expression of p75NTR with Trk receptors. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J Biol Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca2+-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett. 1996;205:161–164. doi: 10.1016/0304-3940(96)12401-0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- Hingtgen CM, Waite KJ, Vasko MR. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3′,5′-cyclic monophosphate transduction cascade. J Neurosci. 1995;15:5411–5419. doi: 10.1523/JNEUROSCI.15-07-05411.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Jiang X, Zhang YH, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neuroscience. 2003;119:65–72. doi: 10.1016/s0306-4522(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lad CP, Peterson DA, Bradshaw RA, Neet KE. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J Biol Chem. 2003;278:24808–24817. doi: 10.1074/jbc.M212270200. [DOI] [PubMed] [Google Scholar]
- Lamour NF, Chalfant CE. Ceramide-1-phosphate: the ‘missing’ link in eicosanoid biosynthesis and inflammation. Mol Interv. 2005;5:358–367. doi: 10.1124/mi.5.6.8. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough PM, Yasui K, Betto R, Salviati G, Glembotski CC, Palade PT, Sabbadini RA. Control of cardiac Ca2+ levels. Inhibitory actions of sphingosine on Ca2+ transients and L-type Ca2+ channel conductance. Circ Res. 1994;75:981–989. doi: 10.1161/01.res.75.6.981. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Paul CE, Vereker E, Dickson KM, Barker PA. A proapoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci. 2004;24:1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Rueff A, Mendell LM. Nerve growth factor and NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nature Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukoc Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine 1-phosphate: a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Titievsky A, Titievskaya I, Pasternack M, Kaila K, Tornquist K. Sphingosine inhibits voltage-operated calcium channels in GH4C1 cells. J Biol Chem. 1998;273:242–247. doi: 10.1074/jbc.273.1.242. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Palade P. Sphingolipid actions on sodium and calcium currents of rat ventricular myocytes. Am J Physiol. 1996;270:C645–C649. doi: 10.1152/ajpcell.1996.270.2.C645. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-Dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine 1-phosphate via activation of a G protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol. 2006 doi: 10.1152/jn.00120.2006. in press. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–192. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]