Abstract
In microbial infections polymorphnuclear neutrophils (PMN) constitute a major part of the innate host defence, based upon their ability to rapidly accumulate in inflamed tissues and clear the site of infection from microbial pathogens by their potent effector mechanisms. The recently described transmembrane receptor herpes virus entry mediator (HVEM) is a member of the tumour necrosis factor receptor super family and is expressed on many haematopoietic cells, including T cells, B cells, natural killer cells, monocytes and PMN. Interaction of HVEM with the natural ligand LIGHT on T cells has a costimulatory effect, and increases the bactericidal activity of PMN. To further characterize the function of HVEM on PMN, we evaluated the effect of receptor ligation on human PMN effector functions using an agonistic monoclonal antibody. Here we demonstrate that activation of HVEM causes activation of neutrophil effector functions, including respiratory burst, degranulation and release of interleukin-8 in synergy with ligands for Toll-like receptors or GM-CSF. In addition, stimulation via HVEM enhanced neutrophil phagocytic activity of complement opsonized, but not of non-opsonized, particles. In conclusion, these results indicate a new, as yet unknown, participation of HVEM in the innate immune response and points to a new link between innate and adaptive immunity.
Keywords: cell surface molecules, herpes virus entry mediator, inflammation, neutrophils, TLR ligands
Introduction
Polymorphonuclear neutrophils (PMN) are of crucial importance in the innate host defence against microbial pathogens because of their ability to rapidly invade and clear a site of infection by ingesting and killing micro-organisms and releasing inflammatory mediators for the further recruitment of inflammatory cells.1–5 Activation of PMN may be mediated by host-derived soluble factors such as inflammatory cytokines, chemokines or heat-shock proteins.2,4,6 In addition, the recognition of antibody or complement opsonized particles or the direct detection of microbial products, i.e. via pathogen recognition receptors or Toll-like receptors (TLR), can trigger neutrophil effector functions.5,7–11
Receptors of the tumour necrosis factor (TNF) superfamily are important in the regulation of inflammatory processes by regulating activation status and cell survival.12–14 Ligation of CD95 or TNF receptor 1/2 on the plasma membrane is well known to induce the programmed death of cells.15–18 The intracellular signalling of TNF receptor family members is mediated by TNF receptor-associated factors (TRAF) and so-called death domain (DD) molecules and results in the induction of rapid and extremely specific signalling events.13,19 Receptors containing these DD typically associate with adaptors, such as Fas-associated protein with DD and the TNF receptor-associated DD, ultimately leading to the activation of effector caspases responsible for the induction of apoptosis.20–22 TNF receptor family members lacking these DD, and therefore the ability to activate caspases, regularly have activating functions such as CD137 (4-1BB),23–27 CD134 (OX40),28–30 or CD27.31 All of these TNF receptors are well known to act as costimulatory molecules in the context of antigen presentation by professional antigen-presenting cells to T cells. However, the expression of many TNF receptors is not restricted to antigen-presenting cells, but frequently is also detectable on PMN where they exhibit pleotropic functions. Whereas CD95 and TNF receptors directly induce apoptosis in PMN,15,17,18 ligation of CD137 on this cell type neutralizes specifically G-CSF-mediated survival.32 On the other hand, OX40 ligation directly rescues PMN from apoptosis by blocking activation of Bid and Bax while maintaining Mcl-1 levels.33 The exact mechanisms by which TNF receptors mediate activation downstream of the receptors complexes is currently not completely understood, but it involves the activation of nuclear factor-κB, mitogen-activated protein kinases, p38 and Jun N-terminal kinase, and others.34,35
The herpes virus entry mediator (HVEM) is a recently identified member of the TNF receptor family expressed on a variety of haematopoietic cells,36,37 including B and T lymphocytes as well as monocytes and dendritic cells, where this receptor has been decribed as a costimulatory molecule upon engagement with its natural ligands LIGHT (lymphotoxins, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for herpesvirus entry mediator) or lymphotoxin α.38–42 Beyond this, platelets are able to release biologically active LIGHT that is able to activate monocytes and endothelial cells, and thereby potentially contribute to inflammatory responses in thrombotic vessels, i.e. after myocardial infarction.43 In addition, the B- and T-lymphocyte attenuator has been recently demonstrated as an inhibitory ligand of HVEM suppressing T-cell proliferation.44 Beyond this, HVEM is also constitutively expressed on PMN at high levels.37 A recent report suggests that ligation of HVEM increases the bactericidal activity of PMN.36 However, it is largely unknown how HVEM influences the effects of inflammatory mediators such as cytokines or ligands of TLR on PMN. To address this issue, we investigated the activation and survival of PMN using an HVEM-specific agonistic monoclonal antibody. We describe that ligation of HVEM induces the activation of neutrophil effector mechanisms, i.e. degranulation of neutrophilic granules, respiratory burst as well as the release of the neutrophil chemokine interleukin-8 (IL-8) and phagocytosis of complement opsonized particles, whereas the phagocytosis of non-opsonized particles was not enhanced. The activation of these effector functions was synergistic with various TLR ligands, suggesting that cellular interactions via HVEM are able to augment the innate immune response against microbial pathogens. Our results suggest that the putative interaction of PMN with T cells via HVEM constitutes a new feedback loop between innate and adaptive immunity to sustain acute inflammatory reactions.
Materials and methods
Materials
Monoclonal antibodies (mAbs) against HVEM (clone 122) have been described previously.37 A control mouse immunoglobulin G1 (IgG1) was purchased from Sigma-Aldrich (Taufkirchen, Germany) or a monoclonal mouse IgG1 (clone 4C9, specific for the Fc fragment of human IgG1) raised by fusion of SP2/0 myeloma cells (from American Type Culture Collection, Manassas, VA, USA) with splenocytes from a BALB/c mouse immunized with a recombinant TREM-1::IgG1 fusion protein and screened against human IgG (Sigma-Aldrich) by enzyme-linked immunosorbent assay (ELISA).45 All mAbs were endotoxin-free (< 0·1 EU/μg protein) determined by a limulus amoebocyte lysate assay (QCL-1000; BioWhittaker, Verviers, Belgium). Lipopolysaccharide (LPS) from Salmonella typhimurium was from Sigma-Aldrich, palmitoyl-3-Cys-Ser-(Lys)4 (Pam3Cys) from EMC Microcollections (Tübingen, Germany), and imiquimod from Invivogen (San Diego, CA). Granulocyte–macrophage colony-stimulating factor (GM-CSF) was from Immunex (Seattle, WA). The following mAbs were used for analysis by fluorescence-activated cell sorter (FACS): Fluorescein isothiocyanate (FITC)-labelled mAbs against CD66b (Coulter Immunotech, Hamburg, Germany), R-phycoerythrin (PE)-labelled mAbs against CD11b and respective isotype controls (all purchased from BD Pharmingen). Polyclonal rabbit antibodies against TLR2 and TLR4 were from Santa Cruz Biotechnologies (Santa Cruz, CA). Complement activated serum was obtained by incubation of autologous human serum in the presence of dry yeast (1 g/10 ml, Dr Oetker Nahrungmittel KG, Germany) for 1 hr at 37°, and subsequent centrifugation 10 min at 10 000 g. The supernatant was either used as complement-activated serum immediately or frozen at − 20° until required. All human studies were performed after obtaining informed consent from healthy volunteer donors and were approved by the local ethics committee according to the institutional guidelines.
Purification of cells
PMN were separated from heparinized blood of healthy volunteer donors by dextran sedimentation using Polymorphprep™ (Nycomed, Oslo, Norway) as described previously.46 Residual red blood cells were removed by a hypotonic lysis step [150 mm ammonium chloride, 1 mm potassium bicarbonate, 0·1 mm ethylenediaminetetraacetatic acid (all from Sigma-Aldrich) in water, pH 7·3 for 5 min]. Purity of cell preparation was assessed by FACS with CD66b as the marker for PMN. In general, 92–98% of cells were CD66b+ PMN.
Stimulation of cells
To study HVEM-mediated activation, 96-well plates (Corning Costar, Schiphol-Rijk, The Netherlands) were coated with the indicated amount of the respective mAbs for 3 hr at 37° and washed twice with phosphate-buffered saline (PBS). Then purified PMN (2 × 106/ml; in RPMI-1640 with 10% (v/v) fetal bovine serum; all from PAN Biotech, Aidenbach, Germany) and additional stimuli as indicated were added. Cells were cultured at 37° with 7·5% CO2.
Flow cytometry
PMN (2 × 105) were incubated with the indicated mAbs in PBS with 1% (w/v) bovine serum albumin and 0·05% sodium azide (all from Sigma-Aldrich; FACS buffer) on ice, then washed twice with FACS buffer and resuspended in FACS buffer. All analyses were performed using FACScanto™ (Becton Dickinson, Heidelberg, Germany) and FACSdiva software (Becton Dickinson).
Detection of IL-8 release
For analysis by ELISA, supernatants were derived from stimulation with purified PMNs after 6 hr and frozen at − 20° until required. Supernatants were analysed by ELISA for IL-8 (R & D Systems, Wiesbaden, Germany) according to the manufacturer's instructions.
Detection of respiratory burst
The presence of hydrogen peroxide was detected by oxidation of dichloro-fluorescein diacetate (DCFH-DA from Sigma-Aldrich) into green fluorescent DCF.8 Briefly, PMN (2 × 105) were stimulated as indicated in the presence of 25 μm DCFH-DA for 5 min at 37° and 5% CO2 in air. For the kinetic analysis cells were kept in a fluorescence reader (SpectraFluor 4; Tecan, Crailsheim, Germany). Relative fluorescence units were measured at excitation wavelength 485 nm and emission wavelength 520 nm for 145 min in 5-min intervals. Specific fluorescence index of stimulated cells was obtained by subtraction of the background fluorescence of labelled cells incubated in medium alone at the corresponding time-points.
Detection of phagocytosis
The phagocytic activity was evaluated by ingestion of PE-labelled polystyrene microspheres (diameter 1 μm, Fluoresbrite Plain Microspheres PCRed, Polysciences, Warrington, PA) as described previously.6 Briefly, PMN (2 × 105) were stimulated as indicated in the presence of 5 × 106 microbeads for 15–90 min at 37°, then kept on ice, washed twice in FACS buffer, and fixed in 1% paraformaldehyde in PBS. Analysis was performed using FACS. The amount of phagocytosis was quantified as percentage of PE-positive events in the indicated region. Where indicated, microbeads were complement-opsonized by preincubation with yeast activated serum at the indicated dilution for 1 hr at 37°, and subsequently washed three times in medium before adding them to PMN.
Detection of apoptosis
The amount of apoptotic cells was evaluated by detection of DNA fragmentation, quantifying hypodiploid nuclei according to a modified protocol described by Nicoletti et al. (22). Briefly, PMN (2 × 105) were resuspended in staining buffer (50 μg/ml propidium iodide (PI) in 0·1% sodium citrate plus 0·1% Triton X-100, all from Sigma-Aldrich), incubated at 4° for 2 hr and analysed by FACS.
Results
Ligation of HVEM initiates the neutrophil effector functions
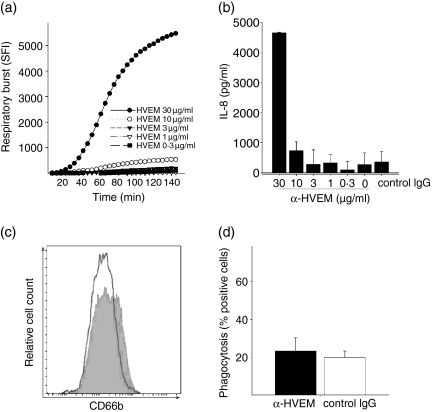
It has been demonstrated previously that HVEM is constitutively expressed on human PMN37 and receptor ligation with the activating ligand LIGHT increases the bactericidal activity of PMN against Listeria monocytogenes and Staphylococcus aureus.36 To establish an experimental setting independent of the various known ligands, we used the previously described agonistic mAb (clone 122) against HVEM.37 As depicted in Fig. 1, receptor ligation resulted in the initiation of the respiratory burst (Fig. 1a) and the release of IL-8 (Fig. 1b) in a concentration-dependent manner. After incubation with an isyotype-matched mAb against an irrelevant antigen no such effect was observed. Notably, using this mAb against HVEM activation was only achieved by immobilized antibody after coating on plastic but not by adding soluble antibody to the medium (not shown).
Figure 1.
HVEM activates the respiratory burst, release of IL-8 and degranulation, but not the phagocytosis of PMN. PMN (2 × 105/well) were cultured on titrated amounts of anti-HVEM-coated 96-well culture plates. (a) PMN were cultured for 145 min in the presence of DCFH-DA for the measurement of respiratory burst. The mean + SD of one representative experiment performed in triplicate wells is shown. (b) Supernatants of PMN activated with the indicated stimuli were collected after 18 hr and analysed for the release of IL-8 by ELISA. Mean + SD of one representative experiment performed in triplicate wells is shown. (c) Surface expression of CD66b was analysed by flow cytometry after a 60-min incubation on anti-HVEM (10 μg/ml) coated (filled histogram) or control mouse IgG1 (10 μg/ml) coated plates (open histogram). (d) Phagocytosis of phycoerythrene-labelled polystyrene beads after 1 hr incubation on anti-HVEM (10 μg/ml) coated (filled) or control mouse IgG1 (10 μg/ml) coated plates (open). All results shown are representative of at least three independent experiments with different donors.
To further characterize HVEM-mediated activation of PMN, we investigated the degranulation of neutrophil granules by monitoring surface up-regulation of CD66b as surrogate marker for the release of specific granules.47–49 We found that cross-linking of HVEM resulted in a detectable surface increase of CD66b (two-fold; Fig. 1c) after 1 hr of stimulation.
In addition to the release of hydrolytic enzymes and cytotoxic substances from granules, the activation of PMN effector functions is characterized by enhanced phagocytosis,5 which is of crucial importance in the clearance of bacterial pathogens. HVEM has been demonstrated to enhance the bactericidal activity of PMN. To explore the contribution of HVEM in a situation excluding possibly contributing factors derived from microbial pathogens (such as TLR ligands), we studied PMN phagocytosis of inert fluorescence-labelled polystyrene microspheres. Using this system, we were unable to detect an enhancing effect of the antibody on phagocytosis by HVEM (Fig. 1d). These results indicate that the HVEM ligation results in the partial activation of neutrophil effector mechanisms with an apparent focus on the respiratory burst and degranulation.
Activation via HVEM is synergistic with inflammatory mediators
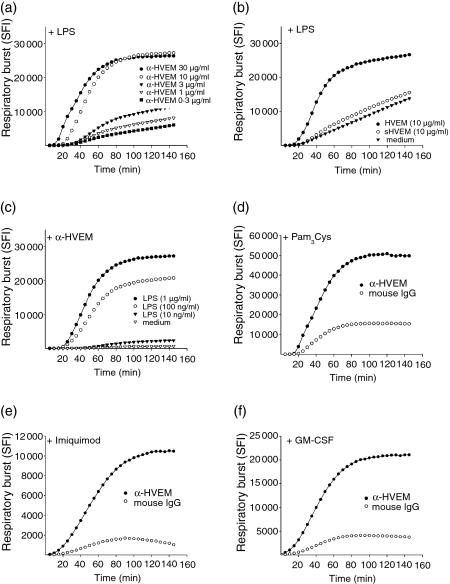
PMN are important effector cells in the combatting microbial infections caused by bacteria and fungi. Therefore, we were interested whether or not there might be a functional interaction between HVEM-mediated activation and inflammatory stimuli such as TLR ligands or inflammatory cytokines. To address this, LPS was added to titrated amounts of coated anti-HVEM and the respiratory burst kinetics were analysed subsequently (Fig. 2a). We found a strong and persistent increase in hydrogen peroxide production in anti-HVEM stimulated cells in the presence of LPS, but not in cells incubated with control mAb against an irrelevant antigen or in the the absence of LPS. The synergistic effect with LPS was only present when the mAb was plastic coated but not when it was added to the medium in soluble form (Fig. 2b). Likewise, when titrated amounts of LPS were added to a fixed amount of coated anti-HVEM the synergistic effect was evident (Fig. 2c). The synergistic effect of HVEM-mediated activation was not only detectable for the TLR4 ligand LPS, but also for the synthetic TLR2 ligand palmitoyl-3-Cys-Ser-(Lys)4 (Pam3Cys) (Fig. 2d) and the TLR7 ligand imiquimod (Fig. 2e) as well as the inflammatory cytokine GM-CSF (Fig. 2f).
Figure 2.
HVEM-mediated activation is synergistic with TLR agonists or GM-CSF. (a) PMN (2 ×× 105/well) were added to titrated anti-HVEM in the presence of LPS (0·5 μg/ml) and the respiratory burst activity was analysed as before. (b) Analysis of the respiratory burst of PMN (2 × 105/well) in the presence of LPS (0·5 μg/ml) and anti-HVEM (10 μg/ml) either coated (filled circles), added in soluble form (sHVEM, 10 μg/ml) or in absence of anti-HVEM. (c) Respiratory burst of PMN (2 × 105/well) on anti-HVEM- (10 μg/ml) or control IgG- (10 μg/ml) coated plates in the presence of titrated amounts of LPS. (d–f) The respiratory burst of PMN (2 × 105/well) stimulated on anti-HVEM- or control IgG-coated plates in the presence of: (d) TLR2 agonist Pam3Cys (500 ng/ml), (e) TLR7 agonist imiquimod (10 μg/ml) or (f) GM-CSF (100 U/ml). All data are depicted as mean + SD from triplicates and are representative of three independent experiments with different donors.
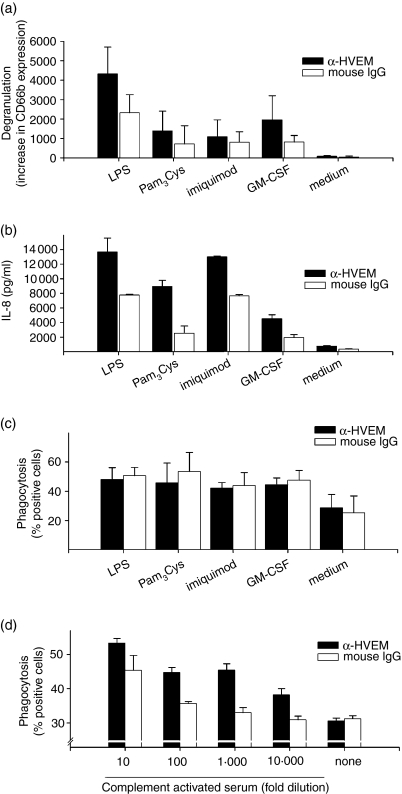
The synergy of HVEM with TLR ligand was detected for the degranulation (Fig. 3a), the shedding of l-selectin (CD62L, not shown) and the release of IL-8 (Fig. 3b). For the phagocytosis, however, we were unable to detect an enhancing effect beyond the activation mediated by the TLR agonist or GM-CSF (Fig. 3c) alone. However, using complement-opsonized particles as targets, we observed that the ligation of HVEM strongly enhanced phagocytosis in a concentration-dependent manner (Fig. 3d). This suggests that the impact of HVEM on this particular effector function is differently regulated than the respiratory burst or the release of IL-8.
Figure 3.
Synergistic effects of HVEM with TLR ligands or GM-CSF for degranulation, IL-8 release and phagocytosis of complement-opsonized particles. PMN (2 × 105/well) were activated with 10 μg/ml anti-HVEM or anti-mouse IgG1 coated on 96-well plates in the presence of LPS 500 ng/ml, Pam3Cys 500 ng/ml, imiquimod 10 μg/ml or GM-CSF 100 U/ml. Degranulation (a) was measured after 1 hr with FITC-labelled α-CD66b. Supernatants for the analysis of IL-8 release by ELISA were taken after 18 hr (b). (c) Amount of phagocytic activity was analysed after 1 hr of incubation with the indicated stimuli. (d) PE-labelled polystyrene beads were preincubated for 30 min with the indicated amount of yeast-activated autologous serum, washed and then added to PMN stimulated with anti-HVEM or control mAb, respectively. The amount of phagocytic activity was analysed after 1 hr of incubation. (a,c) show mean and standard deviation for a compiled analysis from three independent experiments. (b,d) show one representative experiment out of three performed in triplicates.
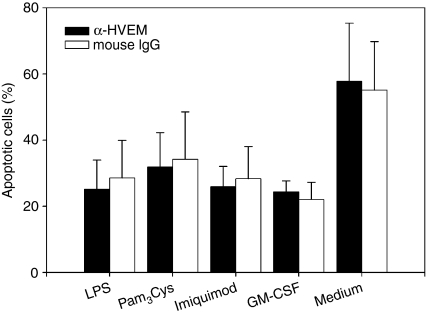
HVEM does not influence neutrophil survival in association with TLR ligands or GM-CSF
The duration of the neutrophil inflammatory response is regulated by the modulation of apoptosis.50 Members of the TNF family may have a versatile impact on PMN survival, i.e. TNF-α or CD95 inducing PMN apoptosis17,18 or CD134 enhancing PMN survival.33 To explore whether HVEM influences PMN survival, we analysed the effect of HVEM ligation on PMN survival by analysing DNA fragmentation. As shown in Fig. 4, stimulation of PMN for 24 hr with anti-HVEM did not alter the amount of apoptotic cells compared to cells incubated with an isotype matched control mAb or left unstimulated (Fig. 4). As shown previously, the inflammatory cytokine GM-CSF and the TLR ligands LPS, Pam3Cys or R-848 rescued the majority of PMN from constitutive apoptosis when incubated alone or in the presence of the HVEM-specific or control mAb, respectively, indicating that PMN survival that is mediated by inflammatory mediators is not affected by HVEM.
Figure 4.
HVEM does not affect TLR- or GM-CSF-mediated survival of PMN. PMN (2 × 105/well) were cultured on anti-HVEM or control mAb-coated (10 μg/ml) plates in the presence of LPS 500 ng/ml, Pam3Cys 500 ng/ml, imiquimod 10 μg/ml or GM-CSF 100 U/ml. The amount of apoptotic cells was analysed by DNA fragmentation as described in the Materials and methods after 24 hr. A compiled analysis of three dependent experiments each performed in duplicates is depicted.
Discussion
In microbial infections, PMN have a crucial role in the recognition and clearance of pathogens.1–5 The rapid employment of their potent effector functions arms PMN to be highly effective in the clearance of bacteria or fungi. During the course of an inflammatory response, i.e. when PMN alone may be unable to cope with a microbial burden, immune cells other than PMN, such as B and T lymphocytes, will be recruited to a site of infection in a situation to initiate an adaptive immune response. In this situation, the interaction of HVEM on PMN with its natural ligand, LIGHT, on activated T cells may become important on the one hand as a costimulatory interaction41 for the cross-priming of T cells51 and on the other hand by reverse signalling also having additional effects on PMN. In addition, Otterdal and co-workers recently identified platelets as a novel source of LIGHT and showed that platelet-derived LIGHT can activate endocthelial cells and monocytes43 and thus be also involved in mediating inflammatory responses mediated by PMN.
A previous study shows that the LIGHT–HVEM interaction on PMN enhances bacterial clearance by increased phagocytosis of bacteria and bacterial killing.36 In our present study we have dissected the functional effects of HVEM on PMN using an agonistic mAb and found that receptor ligation on PMN results in the initiation of the respiratory burst and degranulation in a concentration-dependent manner. Compared to other agonists8 the effects of HVEM ligation alone are rather weak because high concentrations are needed to induce a response (Fig. 1). However, in combination with LPS activation via HVEM ligation results in a markedly increased respiratory burst (Fig. 2). This also holds true for degranulation, l-selectin shedding and the release of IL-8 (Fig. 3a,b). Interestingly, our results show no increase in phagocytic activity and no interference with the phagocytosis mediated by TLR agonists or GM-CSF (Figs 1f and 3c). However, HVEM enhances the phagocytic activity of complement-opsonized particles (Fig. 3d), suggesting that HVEM specifically synergizes with complement receptors, but not with TLR agonists or GM-CSF for this particular PMN effector function. Our results present a likely mechanism of action for the results obtained previously by Heo et al.36 who reported an enhanced phagocytic activity of fluorescence-labelled bacteria in the presence of HVEM ligation, but were unable to separate the effects mediated by HVEM from those of other activating agents like LPS or lipopeptides derived from the bacteria.
It is probable that the observed synergies are mediated by common signalling pathways that are involved in HVEM, TLR or complement receptor signalling, potentially at the level of mitogen-activated protein kinases and/or further downstream at the level of protein kinases B or C.5,34,35,52 However, the cross-talk among these receptors is obviously highly complex, and further investigations are needed to elucidate the differential mechanisms involved.
Members of the TNF receptor family, such as TNF receptor 1/2, CD95 or CD137, are often involved in the regulation of neutrophil survival, either by promoting cell death15,17,18,32 or by enhancing survival. This has recently been elegantly demonstrated for OX40.33 To investigate the role of HVEM in neutrophil survival, we assessed the impact of receptor ligation and found no significant change in the amount of constitutive apoptosis of PMN. TLR agonists like LPS, Pam3Cys and imiquimod or cytokines like GM-CSF are known to delay apoptosis of PMN in vitro.8,33 Therefore we investigated the possibility that HVEM might alter TLR- or GM-CSF-mediated survival and found no impact of HVEM in modulating the life span of PMN beyond the effects of the inflammatory stimuli alone. HVEM is an activating member of the TNF receptor family mediating activation of nuclear factor-κB and AP-1.53 This pathway is involved in promoting PMN survival.54,55 Nevertheless, we do not observe a pro- or anti-apoptotic effect on PMN as has been described for OX40 ligation.33 This underlines the highly specific mode of activation mediated by distinct activating TNF receptor family members as previously described for CD137.32 Despite the engagement of related signalling pathways, ligation of different TNF receptor family members results in diverse biological effects. Additional studies are required to elucidate the intracellular signalling events responsible for this.
In conclusion, we demonstrate that HVEM expression on PMN is functionally relevant. On the one hand, HVEM ligation specifically enhances particular effector functions of PMN in co-operation with inflammatory stimuli. On the other hand it does not directly alter neutrophil survival or the ability to phagocytose non-opsonized particles. Our results indicate a potential role for HVEM at the interface of the innate and the adaptive immune responses. They suggest a so far unrecognized functional link between PMN and T cells. Further studies are needed to investigate impact of the LIGHT–HVEM interaction in this context.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (RA988/2–1 and RA988/3–1 to H.S. and M.P.R.), and KRF-2005–084-E00001, KRF-2005–201-E00008 and SRC Fund to IRC at the University of Ulsan (B.S.K.). The authors thank Andrea Drescher and Annekatrin Meinl for their excellent technical assistance.
Abbreviations
- DCFH-DA
dichlorofluorescein diacetate
- DD
death domain
- ELISA
enzyme linked immunosorbent assay
- FACS
fluorescence activated cell sorter
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HVEM
herpes virus entry mediator
- IL
interleukin
- LIGHT
lymphotoxins, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for herpesvirus entry mediator, a receptor expressed by T lymphocytes
- LPS
bacterial lipopolysaccharide
- mAb
monoclonal antibody
- NK
natural killer cells
- Pam3Cys
palmitoyl-3-Cys-Ser-(Lys)4
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PMN
polymorphonuclear neutrophil
- TLR
Toll-like receptor
- TRAF
TNF-receptor-associated factor
- TREM-1
triggering receptor expressed on myeloid cells 1
References
- 1.Ben Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–6. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 2.Livingston DH, Appel SH, Sonnenfeld G, Malangoni MA. The effect of tumor necrosis factor-alpha and interferon-gamma on neutrophil function. J Surg Res. 1989;46:322–6. doi: 10.1016/0022-4804(89)90195-9. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 4.van Furth R, Diesselhoff-den Dulk MC, Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973;138:1314–30. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–45. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- 6.Radsak MP, Hilf N, Singh-Jasuja H, Braedel S, Brossart P, Rammensee HG, Schild H. The heat shock protein Gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood. 2003;101:2810–15. doi: 10.1182/blood-2002-07-2261. [DOI] [PubMed] [Google Scholar]
- 7.Downey GP, Fukushima T, Fialkow L, Waddell TK. Intracellular signaling in neutrophil priming and activation. Semin Cell Biol. 1995;6:345–56. doi: 10.1016/s1043-4682(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 8.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells – 1. In neutrophil inflammatory responses. Differential regulation of activation and survival. J Immunol. 2004;172:4956–63. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 9.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 10.Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of toll-like receptor 2 (TLR2) in neutrophil activation. GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–8. [PubMed] [Google Scholar]
- 11.Neufert C, Pai RK, Noss EH, Berger M, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol. 2001;167:1542–9. doi: 10.4049/jimmunol.167.3.1542. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 13.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 15.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–8. [PubMed] [Google Scholar]
- 16.Raff M. Cell suicide for beginners. Nature. 1998;396:119–22. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 17.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–40. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray J, Barbara JA, Dunkley SA, Van Lopez AFOX, Condliffe AM, Dransfield I, Haslett C, Chilvers ER. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–83. [PubMed] [Google Scholar]
- 19.Fesik SW. Insights into programmed cell death through structural biology. Cell. 2000;103:273–82. doi: 10.1016/s0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 20.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–16. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 21.Garrone P, Neidhardt EM, Garcia E, Galibert L, Van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaffidi C, Kirchhoff S, Krammer PH, Peter ME. Apoptosis signaling in lymphocytes. Curr Opin Immunol. 1999;11:277–85. doi: 10.1016/s0952-7915(99)80045-4. [DOI] [PubMed] [Google Scholar]
- 23.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–90. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85:1043–52. [PubMed] [Google Scholar]
- 25.Schwarz H, Blanco FJ, von Kempis J, Valbracht J, Lotz M. ILA, a member of the human nerve growth factor/tumor necrosis factor receptor family, regulates T-lymphocyte proliferation and survival. Blood. 1996;87:2839–45. [PubMed] [Google Scholar]
- 26.Schwarz H, Tuckwell J, Lotz M. A receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene. 1993;134:295–8. doi: 10.1016/0378-1119(93)90110-o. [DOI] [PubMed] [Google Scholar]
- 27.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 28.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 29.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes – a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calderhead DM, Buhlmann JE, van den Eertwegh AJ, Claassen E, Noelle RJ, Fell HP. Cloning of mouse Ox40: a T cell activation marker that may mediate T–B cell interactions. J Immunol. 1993;151:5261–71. [PubMed] [Google Scholar]
- 31.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–17. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 32.Heinisch IV, Daigle I, Knopfli B, Simon HU. CD137 activation abrogates granulocyte-macrophage colony-stimulating factor-mediated anti-apoptosis in neutrophils. Eur J Immunol. 2000;30:3441–6. doi: 10.1002/1521-4141(2000012)30:12<3441::AID-IMMU3441>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–75. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 34.Wajant H, Grell M, Scheurich P. TNF receptor associated factors in cytokine signaling. Cytokine Growth Factor Rev. 1999;10:15–26. doi: 10.1016/s1359-6101(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 35.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Techn. 2000;50:184–95. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Heo SK, Ju SA, Lee SC, Park SM, Choe SY, Kwon B, Kwon BS, Kim BS. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J Leukoc Biol. 2006;79:330–8. doi: 10.1189/jlb.1104694. [DOI] [PubMed] [Google Scholar]
- 37.Jung HW, La SJ, Kim JY, et al. High levels of soluble herpes virus entry mediator in sera of patients with allergic and autoimmune diseases. Exp Mol Med. 2003;35:501–8. doi: 10.1038/emm.2003.65. [DOI] [PubMed] [Google Scholar]
- 38.Zhai Y, Guo R, Hsu TL, et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142–51. doi: 10.1172/JCI3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarrias MR, Whitbeck JC, Rooney I, Ware CF, Eisenberg RJ, Cohen GH, Lambris JD. The three HveA receptor ligands, gD, LT-alpha and LIGHT bind to distinct sites on HveA. Mol Immunol. 2000;37:665–73. doi: 10.1016/s0161-5890(00)00089-4. [DOI] [PubMed] [Google Scholar]
- 40.Morel Y, Schiano de Colella JM, Harrop J, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 41.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–86. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 42.Morel Y, Truneh A, Costello RT, Olive D. LIGHT, a new TNF superfamily member, is essential for memory T helper cell-mediated activation of dendritic cells. Eur J Immunol. 2003;33:3213–19. doi: 10.1002/eji.200324410. [DOI] [PubMed] [Google Scholar]
- 43.Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108(3):928–35. doi: 10.1182/blood-2005-09-010629. Aug 1. [DOI] [PubMed] [Google Scholar]
- 44.Sedy JR, Gavrieli M, Potter KG, et al. 2005 B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2006;6:90–8. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 45.Salih HR, Rammensee HG, Steinle A. Cutting edge. down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 46.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation. major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubel C, Fernandez GC, Rosa FA, Gomez S, Bompadre MB, Coso OA, Isturiz MA, Palermo MS. Soluble fibrinogen modulates neutrophil functionality through the activation of an extracellular signal-regulated kinase-dependent pathway. J Immunol. 2002;168:3527–35. doi: 10.4049/jimmunol.168.7.3527. [DOI] [PubMed] [Google Scholar]
- 48.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 49.Borregaard N, Theilgaard-Monch K, Sorensen OE, Cowland JB. Regulation of human neutrophil granule protein expression. Curr Opin Hematol. 2001;8:23–7. doi: 10.1097/00062752-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–10. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 51.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167:2538–46. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–32. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 54.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kappaB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–67. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- 55.Kettritz R, Choi M, Rolle S, Wellner M, Luft FC. Integrins and cytokines activate nuclear transcription factor-kappaB in human neutrophils. J Biol Chem. 2004;279:2657–65. doi: 10.1074/jbc.M309778200. [DOI] [PubMed] [Google Scholar]