Abstract
A key event in tRNA gene (tDNA) transcription by RNA polymerase (Pol) III is the TFIIIC-dependent assembly of TFIIIB upstream of the transcription start site. Different tDNA upstream sequences bind TFIIIB with different affinities, thereby modulating tDNA transcription. We found that in the absence of Nhp6 proteins, the influence of the 5′-flanking region on tRNA gene transcription is dramatically enhanced in Saccharomyces cerevisiae. Expression of a tDNA bearing a suboptimal TFIIIB binding site, but not of a tDNA preceded by a strong TFIIIB binding region, was strongly dependent on Nhp6 in vivo. Upstream sequence-dependent stimulation of tRNA gene transcription by Nhp6 could be reproduced in vitro, and Nhp6 proteins were found associated with tRNA genes in yeast cells. We also show that both transcription and silencing barrier activity of a tDNAThr at the HMR locus are compromised in the absence of Nhp6. Our data suggest that Nhp6 proteins are important components of Pol III chromatin templates that contribute both to the robustness of tRNA gene expression and to positional effects of Pol III transcription complexes.
Transcription of tRNA genes (tDNAs) by RNA polymerase (Pol) III in yeast involves multistep assembly of transcription factors into a preinitiation complex that recruits Pol III (14, 27). Two highly conserved internal control regions, the A and B blocks, together form a specific binding site for multisubunit transcription factor IIIC (TFIIIC). Promoter-bound TFIIIC provides an interaction platform for the productive assembly of TFIIIB in an ∼50-bp region upstream of the transcription start site (TSS). The TFIIIB-DNA complex is by itself capable of productively recruiting Pol III and supporting multiple rounds of transcription in vitro (36). Transcription then proceeds through a facilitated reinitiation pathway that involves Pol recapture after transcription termination (19, 20, 25). Accumulating evidence suggests that the transcription complexes assembled on class III genes may positionally influence other genomic transactions, such as Ty element retroposition (2, 16, 40) and the expression of neighboring genes. In the latter case, tDNAs can act as repressor elements (9, 34) or as barriers to the spread of silencing (22, 59). Despite the remarkable stability of the TFIIIB-DNA complex and its centrality in the transcription mechanism, TFIIIB contacts with its upstream DNA binding region are not based on simple sequence specificity rules. The 5′-flanking region of tRNA genes has long been known to modulate the efficiency of tRNA gene transcription (61). We have recently shown that the transcriptional strength of tRNA gene upstream regions correlates, at least in yeast, with the occurrence of a composite sequence pattern within the TFIIIB binding region and that degenerated yet recognizable sequence patterns also occur upstream of tDNAs in many eukaryotic genomes (29). Yeast tDNA upstream regions also display a remarkable bending propensity, a feature that might facilitate TFIIIB binding (29, 30). In Saccharomyces cerevisiae, tDNA upstream regions whose sequence conforms to the conserved pattern were found to enhance TFIIIB binding and tRNA gene transcription in vitro. In wild-type (WT) yeast cells, however, tRNA gene constructs that have identical internal promoters but are flanked by upstream regions of different transcriptional strengths were found to be transcribed at the same efficiency. The transcriptional difference observed in vitro only became apparent in vivo in yeast strains suffering from a deficit in the key TFIIIB component Brf1 (29), which has been shown to be a limiting factor for tRNA gene transcription in vivo (57). To explain the higher-than-expected in vivo expression levels of tRNA genes with suboptimal 5′-flanking regions, we postulated the existence in yeast cells of stimulatory factors that confer robustness to the expression of tRNA gene copies characterized by intrinsically weak upstream regions. The abundant HMG1 and HMG2 proteins are DNA architectural proteins that act on eukaryotic genomes and bend DNA and in so doing may facilitate DNA binding by transcription proteins (13). In yeast, the HMG-like protein Nhp6 has previously been shown to cooperate with the Pol III machinery in the transcription of SNR6, a Pol III-transcribed gene coding for the U6 snRNA (44, 47). Moreover, Nhp6 has been shown to promote transcription complex assembly on SNR6 by acting in concert with an upstream sequence element (48) and, more recently, to act as a transcriptional initiation fidelity factor for a subset of tRNA genes (39). We reasoned that this abundant, chromatin-associated protein might act as a transcriptional stimulator for at least some tRNA genes and that its ability to generate distorted DNA structures might also contribute to the positional roles of class III genes. By in vivo and in vitro analyses, we show that Nhp6 does indeed selectively activate the transcription of some tRNA genes and participate in the heterochromatin barrier function of HMR-tDNAThr.
MATERIALS AND METHODS
Strains and plasmids.
The S. cerevisiae strains used in this study are listed in Table 1. The N(GTT)CR and N(GTT)NR tDNAs (MIPS nomenclature) were in the pBlueScript-KS plasmid (29). The SNR6 gene was contained in the pB6 plasmid (12). The tRNALeu3 template used for the experiments in Fig. 2B and C is a shortened variant (Leu-45) of the L(CAA)CL gene (3). The SUP4 tRNATyr template in Fig. 3 [Y(GTA)JR] was carried by the pRS316 plasmid (18). To test the Nhp6 requirement for tRNA gene transcription in vivo, the previously described tDNASyn2 fusions ([5′CR]Syn2 and [5′NR]Syn2), carrying the 5′-flanking regions of either N(GTT)CR or N(GTT)NR (29), were subcloned into high-copy-number vector pFL46S (10) in order to be transformed into strains Y869 and Y865. For the experiment in Fig. 1C, the NHP6A open reading frame (ORF), plus 381 bp of the 5′-flanking sequence and 253 bp of the 3′-flanking sequence, was inserted into the pFL39 centromeric vector (10). For the experiment in Fig. 5, the BRF1 ORF, plus 194 bp and 619 bp of the 5′- and 3′-flanking sequences, respectively, was inserted into high-copy-number plasmid pFL45S (10). Plasmid pDD371 carried the SacI-SalI HMR fragment with the HMR-I silencer deleted cloned into pRS406 (58) and containing a synthetic polylinker at the EcoNI site of the a2 gene. pDD442 contains a 320-bp fragment of the HMR-tDNA cloned into the BamHI site of the synthetic linker of pDD371. pDD570, containing the marked HMR-tDNA, was constructed by site-directed mutagenesis of pDD442 with the Quick-Change kit from Stratagene. Strains with genomically integrated 5′CR-Syn2 and 5′NR-Syn2 reporter tDNAs were constructed by first deleting tDNAAsn(GUU)CR or tDNAAsn(GUU)NR with URA3 amplified from pRS406 (58) by direct PCR-mediated homologous recombination in an ADE2 lys2Δ version of S. cerevisiae W303-1A (JRY4012 MATa ADE2 his3 leu2 lys2Δ trp1 ura3). The resulting tDNAΔ::URA3 strains were then transformed with chimeric PCR products of the respective tDNA 5′CR-Syn2 or tDNA 5′NR-Syn2 constructs containing approximately 100 bp of upstream homology and 350 bp of downstream homology flanking the deleted tDNAs. The chimeric PCR constructs were made by amplifying each tDNA Syn2 construct (29) with an upstream oligonucleotide containing an additional 50 bp upstream of tDNAAsn(GUU)CR or tDNAAsn (GUU)NR and a downstream oligonucleotide homologous to the end of each Syn2 construct. Each of these PCR products was then mixed with a second 30-bp overlapping PCR product corresponding to the regions approximately 350 bp downstream of each tDNA and was fused by PCR of the mixture with 20-mer primers complementary to the extreme upstream and downstream ends of each fragment. The fused fragments (∼620 bp) were directly used to transform the respective tDNAΔ::URA3 strains, and recombinants were selected on 5-fluoroorotic acid medium. Integrated constructs were verified by PCR amplification of the integrated region and DNA sequencing of the PCR product. Each resulting strain essentially has the coding sequence and terminator of the tDNA deleted and replaced with the Syn2 coding sequence and terminator. All primers used and further details on the endpoints and construction of the integrated Syn2 strains are available on request from D.D. Resulting strains containing the integrated Syn2 tDNAs were then crossed to DY2381 (MATα ade2 can1 his3 leu2 LYS2 trp1 ura3 nhp6a::URA3 nhp6b::HIS3) and sporulated, and Syn2 nhp6 null strains were identified.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| Y865 | MATα ura3-52 trp1-289 his3-Δ1 leu2-3,112 gal2 gal10 | 15 |
| Y869 | MATα ura3-52 trp1-289 his3-Δ1 leu2-3,112 gal2 gal10 nhp6A-Δ3::URA3 nhp6B-Δ3::HIS3 | 15 |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| BY4741-Nhp6A-TAP | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 NHP6A::TAP-HIS3MX6 | Open Biosystems; 28 |
| BY4741-Nhp6B-TAP | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 NHP6A::TAP-HIS3MX6 | Open Biosystems; 28 |
| BY4741-BRF1-TAP | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 BRF1::TAP-HIS3MX6 | Open Biosystems; 28 |
| DDY156 | MATα ade2-1 his3-11 leu2-3,112 LYS2 trp1-1 ura3-1 hmr::bgl-bclD | This work |
| DDY171 | MATα ADE2-1 his3-11 leu2-3,112 LYS2 trp1-1 ura3-1 hmr::bgl-bclD | This work |
| DDY618 | MATα ade2 can1 his3 leu2 LYS2 trp1 ura3 hmrΔ::bgl-bcl nhp6b::ADE2 | This work |
| DDY604 | MATα ade2 can1 his3 leu2 LYS2 trp1 ura3 hmrΔ::bgl-bcl nhp6a::KanMX, nhp6b::ADE2 | This work |
| DDY610 | MATα ade2 can1 his3 leu2 LYS2 trp1 ura3 hmrΔ::bgl-bcl nhp6a::KanMX, nhp6b::ADE2 sir4Δ::TRP1 | This work |
| DDY591 | MATα ade2 his3 leu2 LYS2 trp1 ura3 nhp6a::KanMX nhp6b::ADE2 | This work |
| DDY689 | MATα ADE2 his3 leu2 lys2Δ trp1 ura3 HMR-E-tDNA-a1 | This work |
| DDY714 | MATα ADE2 his3 leu2 lys2Δ trp1 ura3 HMR-E-tDNA-a1 nhp6a::KanMX | This work |
| DDY669 | MATα ade2 his3 leu2 lys2Δ trp1 ura3 HMR-E-tDNA-a1 nhp6b::ADE2 | This work |
| DDY671 | MATα ade2 his3 leu2 lys2Δ trp1 ura3 HMR-E-tDNA-a1 nhp6a::KanMX nhp6b::ADE2 | This work |
| DDY705 | MATα ade2 his3 leu2 LYS2 trp1 ura3 HMR-E-tDNA-a1 nhp6a::KanMX nhp6b::ADE2 sir2Δ::TRP1 | This work |
| JRY4012 | MATaADE2 his3 leu2 lys2Δ trp1 ura3 | Jasper Rine |
| DY2381 | MATα ade2 can1 his3 leu2 LYS2 trp1 ura3 nhp6a::URA3 nhp6b:HIS3 | David Stillman |
| DDY3524 | MATaADE2 his3 leu2 lys2Δ trp1 ura3 tn(guu)crΔ::5′CR-Syn2 | This work |
| DDY3529 | MATaADE2 his3 leu2 lys2Δ trp1 ura3 tn(guu)nrΔ::5′NR-Syn2 | This work |
| DDY3532 | MATα ade2 his3 leu2 lys2Δ trp1 ura3 tn(guu)crΔ::5′CR-Syn2 nhp6a::URA3 nhp6b::HIS3 | This work |
| DDY3534 | MATaade2 his3 leu2 LYS2 trp1 ura3 tn(guu)crΔ::5′CR-Syn2 nhp6a::URA3 nhp6b::HIS3 | This work |
| DDY3535 | MATα ADE2 his3 leu2 LYS2 trp1 ura3 tn(guu)nrΔ::5′NR-Syn2 nhp6a::URA3 nhp6b::HIS3 | This work |
| DDY3536 | MATaade2 his3 leu2 LYS2 trp1 ura3 tn(guu)nrΔ::5′NR-Syn2 nhp6a::URA3 nhp6b::HIS3 | This work |
FIG. 2.
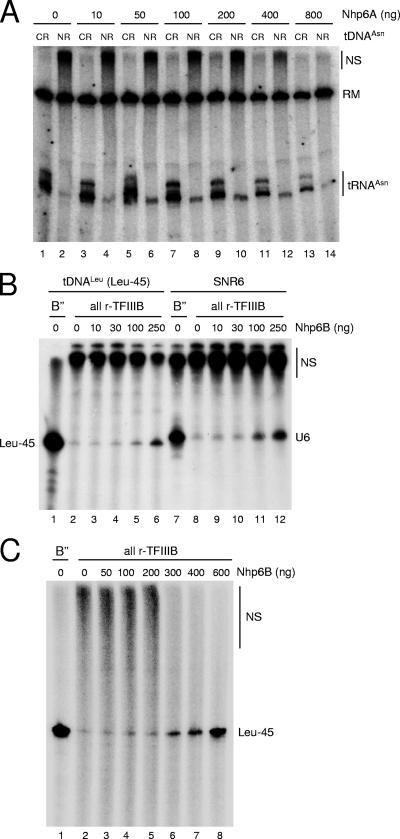
Stimulation of tRNA gene transcription in vitro by recombinant Nhp6. (A) In vitro transcription of either N(GTT)CR (odd-numbered lanes) or N(GTT)NR (even-numbered lanes) was carried out in a reconstituted system containing recombinant Bdp1 protein from baculovirus-infected insect cells and in the presence of the indicated amounts of recombinant Nhp6A protein. The migration position of tRNAAsn transcripts is indicated on the right, together with the position of a radiolabeled DNA fragment used as a recovery marker (RM). The migration position of large-size nonspecific transcription products is also indicated on the right (NS). (B) In vitro transcription of either a shortened variant (Leu-45) of the L(CAA)CL tDNA (lanes 1 to 6) or the SNR6 template (lanes 7 to 12) was carried out in a reconstituted system containing either the crude B" fraction (lanes 1 and 7) or recombinant Bdp1 protein purified from overexpressing E. coli cells (lanes 2 to 6 and 8 to 12) and supplemented with the indicated amount of recombinant Nhp6B protein. The migration position of the shortened tRNALeu transcript is indicated on the left (Leu-45). The migration position of the SNR6 transcript is indicated on the right (U6), as is the position of large-size nonspecific transcription products (NS). (C) In vitro transcription of the Leu-45 template was carried out in a reconstituted system containing either the crude B" fraction (lane 1) or recombinant Bdp1 protein purified from overexpressing E. coli cells (lanes 2 to 8) and supplemented with the indicated amounts of recombinant Nhp6B protein. The migration position of the shortened tRNALeu transcript is indicated on the left (Leu-45), as is the position of large-size nonspecific transcription products (NS).
FIG. 3.
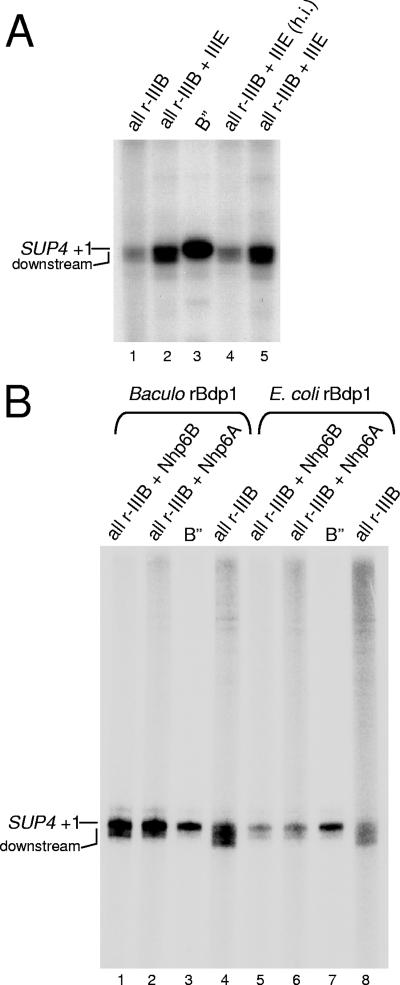
TFIIIE stimulates SUP4 transcription without influencing TSS selection. (A) In vitro transcription of the SUP4 tRNATyr template was carried out in a reconstituted system containing either TFIIIB reconstituted with the crude B" fraction (lane 3) or all-recombinant TFIIIB containing E. coli-expressed Bdp1 protein (lanes 1, 2, 4, and 5). The reaction mixtures in lanes 2 and 5 were supplemented with the TFIIIE fraction; the reaction mixture in lane 4 was supplemented with the heat-inactivated (h.i.) TFIIIE fraction. The migration positions of SUP4 transcripts resulting from initiation at +1 and from initiation events at downstream sites are indicated on the left. (B) In vitro transcription of the SUP4 tRNATyr template was carried out in a reconstituted system containing the crude B" fraction (lanes 3 and 7), baculovirus-expressed rBdp1 (lanes 1, 2, and 4), or E. coli-expressed rBdp1 (lanes 5, 6, and 8). The reaction mixtures in lanes 1 and 5 were supplemented with rNhp6B protein (100 ng); the reaction mixtures in lanes 2 and 6 were supplemented with rNhp6A protein. Only one-fifth of the total reaction products were loaded in lanes 3 and 7 to improve the resolution of the SUP4 tRNA signal and thus more easily compare transcript sizes in the different lanes.
FIG. 1.
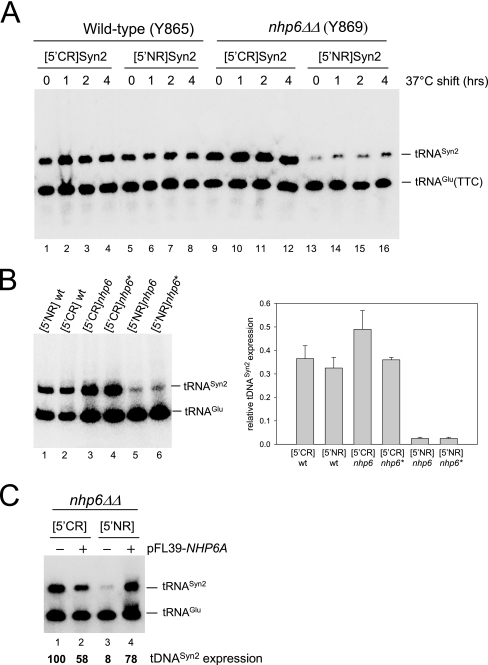
Nhp6 stimulates tRNA gene transcription in vivo in a context-dependent manner. (A) WT (lanes 1 to 8) and nhp6ΔΔ strains (lanes 9 to 16) were transformed with pFL46S containing either [5′CR]Syn2 (lanes 1 to 4 and 9 to 12) or [5′NR]Syn2 (lanes 5 to 8 and 13 to 16) tDNA, grown on selective medium at 30°C, and then shifted to 37°C for the indicated periods of time. Total RNA was isolated and subjected to Northern blot analysis with a probe complementary to both the tDNASyn2 transcript and the endogenous tRNAGlu(TTC). The migration positions of the two RNA species are indicated on the right. (B) Total RNA was extracted from NHP6A NHP6B strains carrying an integrated copy of either the [5′NR]Syn2 (lane 1) or the [5′CR]Syn2 (lane 2) reporter tDNA (strains DDY3529 and DDY3524, respectively; see Table 1) and from two different nhp6ΔΔ strains carrying either an integrated copy of [5′CR]Syn2 (lanes 3 and 4; strains DDY3532 and DDY3534, respectively) or an integrated copy of [5′NR]Syn2 (lanes 5 and 6; strains DDY3535 and DDY3536, respectively. The bar graph on the right reports the results of phosphorimager quantification of the gel image shown and of an image derived from an identical Northern blotting experiment conducted in parallel. tDNASyn2 transcript levels are expressed as ratios of each tRNASyn2 signal to the tRNAGlu(TTC) signal in the same lane. (C) nhp6ΔΔ strain Y869 containing plasmid-borne [5′CR]Syn2 (lanes 1 and 2) or [5′NR]Syn2 (lanes 3 and 4) was transformed with either the empty pFL39 vector (lanes 1 and 3) or pFL39 carrying the NHP6A gene (lanes 2 and 4). Total RNA was extracted and subjected to Northern blot analysis as for panel A. The migration positions of the tDNASyn2 transcript and the endogenous tRNAGlu(TTC) are indicated on the right. The values reported below each lane derive from phosphorimager quantification of the gel image after normalization with the tRNAGlu(TTC) signal as an internal standard and are relative to the value measured in lane 1, which was arbitrarily set to 100.
FIG. 5.
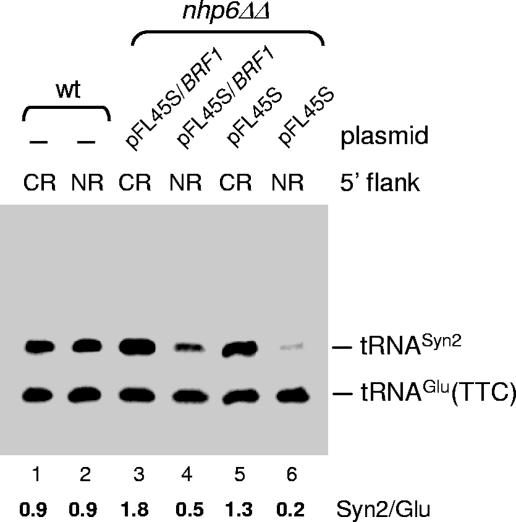
BRF1 overexpression partially rescues the tDNA transcriptional defect in the nhp6ΔΔ strain. WT (lanes 1 and 2) and nhp6ΔΔ (lanes 3 to 6) strains were transformed with pFL46S containing either [5′CR]Syn2 (lanes 1, 3, and 5) or [5′NR]Syn2 (lanes 2, 4, and 6) tDNA together with the empty pFL45S vector (lanes 5 and 6), pFL45S carrying the BRF1 gene (lanes 3 and 4), or no additional plasmid (lanes 1 and 2). Total RNA was isolated and subjected to Northern blot analysis with a probe complementary to both the tDNASyn2 transcript and the endogenous tRNAGlu(TTC). The migration positions of the two RNA species are indicated on the right.
In vivo RNA analyses.
Northern blot analysis of tDNASyn2 expression in vivo was carried out as previously described (29). In order to distinguish the HMR-tRNA transcript from transcripts from the other seven copies of tDNAThr(AGT), WT and nhp6 mutant strains were transformed with plasmid pDD570, which contains the HMR-tDNA mutagenized to contain an extra 19 bp at the end of the transcript (5′-GCCGCAGTAATCTTGCGGA-3′), and selected on plates lacking uracil. Single-colony Ura+ isolates were grown to mid-log phase in minimal medium lacking uracil, and total RNA was isolated. Three micrograms of total RNA from each strain was resolved on a sequencing minigel (10% polyacrylamide-8 M urea) and electroblotted to Zeta-Probe (Bio-Rad) membrane. Duplicate blots were probed with either the pan-tRNAThr probe 5′-GATCTGCTTCCAATCGGATTTGAACCGATGATCTCCACATTACTAGTGTGGCGCCTTACCAACTTGGCCATAGAAGC or the 19-base extension-specific probe 5′-TCCGCAAGATTACTGCGGCTGCTTC. The probes were end labeled with 32P by using polynucleotide kinase, and hybridizations were performed according to the Zeta-Probe manual for oligonucleotide probes.
In vitro transcription.
Transcription of class III genes was reconstituted in vitro essentially as previously described (11, 25). All reaction mixtures contained 150 ng of TFIIIC purified up to the DEAE Sephadex A-25 step (33); 40 ng of recombinant TBP and 80 ng of recombinant Brf1, both purified from overexpressing E. coli cells (33); and 10 ng of highly purified RNA Pol III (25). As a source of Bdp1 protein, which is required to reconstitute TFIIIB activity, we used either 0.5 μg of the B" fraction, partially purified from chromatin pellets generated during yeast nuclear extract preparation (37), or recombinant 8His-Bdp1 protein purified from either E. coli (33) or baculovirus-infected cells (23, 25). The TFIIIE fraction was isolated from yeast nuclear extracts as previously described (17). The template (100 ng of a class III gene-containing plasmid) was preincubated at 20°C for 20 min in a 45-μl reaction mixture (25 mM Tris-HCl, pH 7.9, 100 mM KCl, 5 mM MgCl2, 8% [vol/vol] glycerol, 8 U of SUPERase-In RNase inhibitor [Ambion]) in the presence of transcription proteins (except Pol III). Pol III was then added together with unlabeled nucleoside triphosphates (500 μM ATP, CTP, and GTP; 25 μM UTP) and 10 μCi of [α-32P]UTP (800 Ci/mmol; Amersham Biosciences), and multiple rounds of transcription were allowed to take place for 20 min at 20°C. Radiolabeled transcripts were separated on 6% polyacrylamide-7 M urea gels and visualized and quantified by phosphorimaging with a Personal Imager FX (Bio-Rad). In the experiment in Fig. 3B, Nhp6 proteins were preincubated with template DNA (7 min at 25°C) before addition of the transcription components to favor Nhp6 action in TSS selection (39).
Nhp6p expression and purification.
The NHP6A ORF was cloned into a modified version of pET28b containing an engineered PmeI restriction site into the polylinker to facilitate the cloning of PCR products (8). The construct was transformed into E. coli BL21 Rosetta(DE3) cells (Novagen). Nhp6A expression was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) and incubating the mixture for 2 h at 37°C. The 6His-Nhp6A protein in the soluble fraction was purified by chromatography on Ni-nitrilotriacetic acid resin (QIAGEN) under nondenaturing conditions by following the manufacturer's instructions. Recombinant purified Nhp6B protein was a gift of M.-C. Marsolier (CEA-Saclay) (47).
Chromatin immunoprecipitation (ChiP).
Yeast strains expressing tandem affinity purification (TAP) protein-tagged versions of the Brf1, Nhp6A, and Nhp6B proteins were from the Yeast TAP Fusion Collection (Open Biosystems, Huntsville, AL) (28). BY4741 was used as a nontagged control strain. Yeast cultures (200 ml) were grown to an optical density at 600 nm of ∼0.5, formaldehyde was added to a final concentration of 1% (vol/vol), and the incubation was continued for 20 min at 20°C. Glycine was then added to 240 mM, and 5 min later, cells were washed twice with TBS (20 mM Tris/HCl, pH 7.5, 150 mM NaCl) and once with a lysis buffer containing 50 mM HEPES-KOH (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 1 mM phenylmethylsulfonyl fluoride. Cells were resuspended in 500 μl of lysis buffer containing 0.5% SDS and disrupted with glass beads. Pelleted cross-linked chromatin was sonicated to a fragment size of 200 to 700 bp, clarified by centrifugation, and stored in aliquots at −80°C. TAP protein-associated chromatin was purified by immunoglobulin G (IgG)-agarose chromatography. Sonicated chromatin (800 μl) was incubated with 10 μl of rabbit IgG-agarose overnight at 4°C. After high-salt washings, chromatin was recovered from beads by heating for 10 min at 65°C in 200 μl of 50 mM Tris/HCl (pH 7.5)-10 mM EDTA-1% SDS. After treatment with Pronase, DNA was phenol extracted, ethanol precipitated, resuspended in Tris-EDTA buffer, and used for PCR analysis. Conditions for PCRs were essentially as previously described (42). One-two hundred fiftieth of the total immunopurified DNA was used for each PCR, consisting of 25 cycles of amplification in the presence of [α-32P]dATP. For the input controls, 0.005% of the amount of chromatin used in the immunoprecipitations was added as the template to the PCR. PCR products were resolved on 6% polyacrylamide-1× Tris-borate-EDTA gels. PCR signals were quantified by phosphorimaging on a Personal Imager FX (Bio-Rad). Data in Fig. 4B were obtained by real-time PCR with the Corbett Rotorgene system. PCRs of 45 cycles were carried out with the QIAGEN QuantiTect SYBR Green PCR Master mix. In each reaction mixture, half the quantity of immunoprecipitate and input DNA used in Fig. 4A was used as the template; primers were used at a 1.2 μM concentration. All samples were run in triplicate in two independent PCRs. Primer sequences are available upon request.
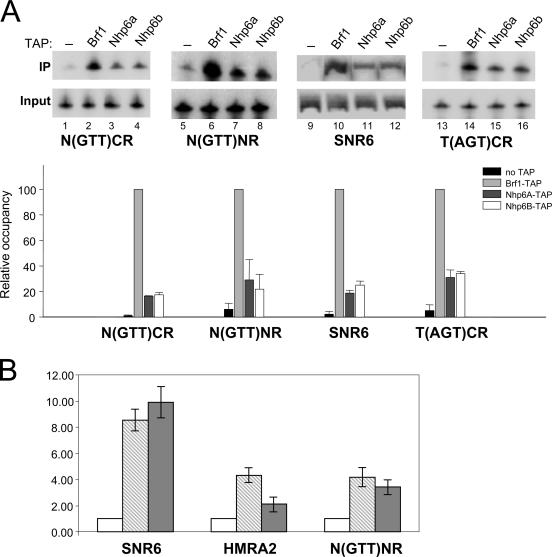
FIG. 4.
Nhp6 association with SNR6 and tRNA genes in vivo. (A) ChIP analysis was performed with an untagged reference strain (lanes 1, 5, 9, and 13) and with BRF1-TAP-, NHP6A-TAP-, and NHP6B-TAP-tagged strains, as indicated above the lanes. The extent of association of each of the three tagged proteins with the N(GTT)CR, N(GTT)NR, SNR6, and T(AGT)CR loci was assessed by PCR in the presence of radiolabeled dATP. The phosphorimager quantification of gel images is reported below as a bar graph of data derived from three independent experiments (error bars indicate standard deviations). PCR signals from immunoprecipitated (IP) DNA were normalized to the PCR signals obtained in the input DNA reaction mixture. The calculated values were then expressed relative to the values obtained with BRF1-TAP, which were arbitrarily set to 100. (B) The extent of association of Nhp6A and Nhp6B with the SNR6, HMRA2, and N(GTT)NR loci was quantitatively evaluated by real-time PCR. Signals obtained with immunoprecipitated DNA were normalized to the input, and the calculated values are expressed relative to the background signal obtained with the untagged strain, which was arbitrarily set to 1. Two independent PCRs were conducted in triplicate, and error bars indicate standard deviations of average values obtained in each experiment. NHP6A-TAP, light gray bars; NHP6B-TAP, dark gray bars; untagged reference strain, open bars.
Heterochromatin barrier assays.
The HMR-tRNA boundary element was cloned between the HMR-E silencer and the a1 gene as previously described (21, 22, 35). Briefly, his3 test strains were grown as patches and then replica plated to a MATa his4 mating lawn on glucose minimal medium. Only diploid cells resulting from mating are able to grow in the absence of histidine. Barrier activity results in an essentially nonmating phenotype, as the blocking of silencing results in expression of the a1 gene in MATα cells. Mutations that weaken the ability of the barrier to block silencing result in a significant increase in the percentage of cells able to mate, as silencing spreads through the barrier and represses the a1 gene.
RESULTS
Nhp6 stimulates tRNA gene transcription in vivo in a context-dependent manner.
The combination of different 5′-flanking regions with a synthetic reporter tRNA gene offers an easy way to evaluate the in vivo transcriptional effect of the upstream regions of individual members of tDNA multigene families (29). A reporter tRNA gene fruitfully used in this strategy, tDNASyn2, contains the S. cerevisiae tRNAGly(TTC) coding sequence tagged by the insertion of an intron-like sequence that cannot be spliced out from the tRNA precursor. Pseudointron tagging allows the identification and quantification of transcripts synthesized from upstream region-modified tDNASyn2 by Northern hybridization analysis with endogenous, intronless tRNAGlu(TTC) as an internal standard (43). We have previously analyzed the in vivo expression of two gene fusions, called [5′CR]Syn2 and [5′NR]Syn2, in which the 5′-flanking region of either N(GTT)CR or N(GTT)NR, a member of the tRNAAsn(GTT) gene family, is fused to tDNASyn2. In vitro, in the presence of limiting TFIIIB concentrations, N(GTT)CR is transcribed much more efficiently than N(GTT)NR, and this transcriptional difference is abolished at saturating TFIIIB concentrations. Accordingly, it was found that [5′CR]Syn2 is expressed 10-fold more than [5′NR]Syn2 in yeast cells suffering from a deficit of the TFIIIB component Brf1 while the two templates are expressed to roughly similar levels in WT cells (29). The upstream region of N(GTT)NR can thus be classified as a suboptimal TFIIIB assembly site. To test whether Nhp6 affects the expression of tRNA genes in vivo and whether its action depends on the quality of the TFIIIB assembly region, the [5′CR]Syn2 and [5′NR]Syn2 templates carried by the multicopy pFL46 vector were transformed into an nhp6ΔΔ strain and into an otherwise isogenic WT strain (15). The levels of tRNASyn2 expression were measured by Northern blotting before and after a shift to 37°C (a temperature at which the SNR6 transcriptional defect of nhp6ΔΔ cells is exacerbated (44). As shown in Fig. 1A, in the WT strain [5′NR]Syn2 expression was only slightly lower than [5′CR]Syn2 expression (cf. lanes 1 to 4 with lanes 5 to 8). In contrast, in the nhp6ΔΔ strain, [5′NR]Syn2 expression was decreased three- to fivefold with respect to that in the WT strain (lanes 13 to 16) while [5′CR]Syn2 expression was about twofold higher in the mutant than in the WT strain (cf. lanes 9 to 12 with lanes 1 to 4). As a consequence, [5′CR]Syn2 was expressed ∼10-fold more than [5′CR]Syn2 in the nhp6ΔΔ strain. The [5′CR]Syn2 and [5′NR]Syn2 reporters were also integrated at the original N(GTT)CR and N(GTT)NR genomic loci in both the NHP6 and nhp6ΔΔ genetic backgrounds, and their expression was tested by Northern blotting. Figure 1B shows that the two integrated reporters were expressed at similar levels in the WT background (lanes 1 and 2), while [5′NR]Syn2 expression was specifically and dramatically (10- to 20-fold) decreased with respect to [5′CR]Syn2 in the nhp6ΔΔ background (cf. lanes 5 and 6 with lanes 3 and 4). As shown in Fig. 1C, reintroduction of NHP6A (carried by the centromeric pFL39 vector) into nhp6ΔΔ resulted in strong (∼10-fold) activation of [5′NR]Syn2 transcription (cf. lanes 3 and 4). In contrast, reintroduction of NHP6A into nhp6ΔΔ produced a 1.7-fold inhibition of the expression of [5′CR]Syn2 (cf. lanes 1 and 2). We conclude from these results that Nhp6 acts as a stimulator of the expression of at least some tRNA genes in yeast cells and that its action becomes determinant with a tDNA carrying a suboptimal TFIIIB binding site. At variance with the SNR6 transcriptional defect in nhp6ΔΔ, reported to be much more evident at the nonpermissive temperature of 37°C (44), the tDNASyn2 expression defect was not exacerbated upon a temperature shift. This is in agreement with the previously reported observation that the temperature sensitivity of the nhp6ΔΔ strain is largely due to a specific defect in SNR6 transcription (44).
Stimulation of tRNA gene transcription in vitro by recombinant Nhp6.
To test whether Nhp6 influences tDNA expression by direct involvement in the transcription process, we tried to reproduce the effect of Nhp6 on tDNA expression in in vitro assays using reconstituted Pol III transcription systems and recombinant Nhp6A protein. In preliminary experiments, the N(GTT)CR and N(GTT)NR templates were transcribed in a system reconstituted from purified Pol III, a partially purified TFIIIC fraction, recombinant TBP and Brf1 proteins, and the crude B" fraction (37) as a source of the Bdp1 component. In this system, addition of increasing amounts (up to 200 ng) of purified, recombinant Nhp6A protein had no effect on transcription levels of either tDNA (data not shown). Western blot analysis, however, revealed that the crude B" fraction contains substantial amounts of Nhp6 protein (data not shown). We thus repeated the in vitro transcription experiment with recombinant Bdp1 protein, produced in a baculovirus system, in place of the B" fraction (25). As shown in Fig. 2A, N(GTT)NR was transcribed 18-fold less than N(GTT)CR in the presence of all-recombinant TFIIIB (lanes 1 and 2) [the two bands of N(GTT)CR transcript correspond to two alternative sites of transcription termination; see reference 11]. Addition of increasing amounts of recombinant Nhp6A did not significantly affect N(GTT)CR transcription (cf. lanes 1, 3, 5, 7, and 9). In contrast, N(GTT)NR transcription was stimulated up to fivefold by Nhp6A (cf. lanes 2, 4, 6, 8, and 10). As a consequence, at the optimal Nhp6A concentration of 0.4 μM (lane 9 and 10), the N(GTT)CR and N(GTT)NR transcription levels differed by only three- to fourfold. Higher concentrations of Nhp6A recombinant protein started to inhibit the transcription of both tDNAs under these transcription conditions. At variance with the SUP4 tRNA gene, which requires Nhp6 for correct transcription initiation (39), primer extension analysis did not reveal significant effects of Nhp6 on TSS selection on the tDNAAsn(GTT) templates in vitro (data not shown). It has recently been reported that Nhp6 proteins can stimulate in vitro transcription of tRNA genes indirectly by reducing transcription factor and RNA Pol sequestration by imperfect TATA boxes on plasmid DNA. Such a nonspecific stimulatory effect of Nhp6A on tRNA gene transcription is readily identified by a concomitant, Nhp6-dependent reduction of the amount of slowly migrating, nonspecifically initiated transcripts (38). In the experiment in Fig. 2A, N(GTT)NR transcriptional stimulation occurred without any concomitant reduction of slowly migrating nonspecific transcripts (cf. lanes 2, 4, 6, 8, and 10), thus suggesting a direct mechanism of stimulation. Direct stimulation of preinitiation complex assembly by Nhp6 in vitro has previously been observed in the case of the SNR6 gene (44, 47). To better define nonspecific and specific effects in Nhp6-dependent transcriptional stimulation in vitro, in an independent series of experiments we analyzed the effects of a large range of Nhp6 concentrations on the in vitro transcription of SNR6 and of a tRNALeu3 gene previously reported to be unresponsive to Nhp6 (44). These templates were tested in the presence of all-recombinant TFIIIB reconstituted with E. coli-produced Bdp1 protein (instead of baculovirus-expressed Bdp1) and of recombinant Nhp6B protein. As shown in Fig. 2B and in agreement with previous analyses (44, 47), SNR6 transcription could be stimulated up to fivefold by Nhp6B (cf. lanes 8 and 12) with no concomitant decrease in nonspecific transcription, as revealed by the levels of low-mobility RNAs at the top of the gel. At concentrations higher than 0.5 μM, Nhp6B became inhibitory for SNR6 transcription (data not shown). Transcriptional stimulation by recombinant Nhp6B was also observed with the tRNALeu3 gene, but it was more modest than with SNR6 (only twofold stimulation at 0.5 μM Nhp6B and no stimulation at 0.2 μM; cf. lane 1 with lanes 5 and 6). We noted that the increase in specific tRNALeu3 transcription at 0.5 μM Nhp6B was accompanied by an approximately twofold reduction in nonspecific transcription (cf. lanes 2 and 6). Transcriptional stimulations of tDNALeu3 and SNR6 thus appear to differ both quantitatively and qualitatively. In support of this conclusion are the results in Fig. 2C, showing that tRNALeu3 gene transcription in the presence of all-recombinant TFIIIB could be stimulated up to sevenfold by further increasing the concentration of Nhp6B up to 1.2 μM (Fig. 2C, lanes 6 to 8). At this concentration of Nhp6 protein, however, nonspecific transcription was dramatically reduced (lane 8). The Nhp6-dependent decrease in nonspecific transcription was also observed with TFIIIB reconstituted with baculovirus-expressed rBdp1 protein (data not shown). We conclude from these results that Nhp6 can stimulate in vitro transcription of class III genes by at least two different mechanisms, i.e., (i) by reducing the availability of competing, nonspecific initiation sites (the case of tRNALeu3 in Fig. 2 and that of the SUP4 gene [39]) and (ii) by directly stimulating transcription complex assembly at the proper initiation sites [as observed for upstream region-defective N(GTT)NR in Fig. 2A]. We should mention, however, that the Nhp6-dependent decrease in nonspecific transcription was somehow influenced by the particular tDNA and tDNA-carrying plasmid used in the in vitro transcription assays, and it was more pronounced with Nhp6B than with the Nhp6A isoform (data not shown). Kassavetis and Steiner (39) have recently noted the possibility that Nhp6 may be a component of the previously described TFIIIE fraction that possesses Pol III-stimulatory activity in vitro (17, 56). The results reported in Fig. 3A argue against this possibility by showing that a protein component(s) in the TFIIIE fraction stimulates transcription of the SUP4 tRNA gene in the presence of all-recombinant TFIIIB (cf. lane 1 with lanes 2 and 5) yet does not correct the initiation fidelity defect typically observed with all-recombinant TFIIIB but not with the crude B" fraction (cf. lanes 1 and 3; see also reference 1). Figure 3B shows that, in contrast, recombinant Nhp6A and Nhp6B proteins, at the nonstimulatory concentration of 0.2 μM, are both able to restore initiation fidelity on the SUP4 gene in the presence of fully recombinant TFIIIB reconstituted with either baculovirus- or E. coli-expressed rBdp1 protein. These results are in perfect agreement with those of Kassavetis and Steiner (39). As expected on the basis of the results in Fig. 3, Nhp6 was not detected in the TFIIIE fraction by Western blot analysis with anti-Nhp6 antiserum (data not shown).
Nhp6 associates with tRNA genes in vivo.
If Nhp6 directly participates in tRNA gene transcription in vivo, it should be possible to detect Nhp6 association with tDNAs by ChIP. To address this point, we made use of S. cerevisiae strains producing TAP-tagged versions (28) of the Nhp6A, Nhp6B, and Brf1 proteins (the latter is known to strongly and specifically associate with class III genes [32, 49, 54]). Cultures of the TAP-tagged and control strains grown to logarithmic phase were subjected to formaldehyde cross-linking, and sheared chromatin was then affinity purified with IgG-agarose. The purified chromatin was analyzed by PCR with primers specific for the SNR6 gene (which requires Nhp6 for transcription) and for the tDNAs N(GTT)CR, N(GTT)NR, and T(AGT)CR. The latter tDNA is the one that has been shown to act as a barrier to heterochromatin spread at the HMR locus (22). As shown in Fig. 4A (lanes 9 to 12), both the Nhp6A and Nhp6B proteins were found associated with the SNR6 gene to a significant extent (cf. lanes 11 and 12 with lane 9, showing the result of ChIP with the nontagged parental strain). Quantification showed that the amount of precipitated SNR6 in the case of each TAP-tagged Nhp6 protein was about 20 to 30% of the amount of SNR6 DNA coprecipitated with TAP-Brf1 (cf. lanes 11 and 12 with lane 10 in Fig. 4A; see the bar plot below the gel image). Since each TAP-tagged isoform of Nhp6 protein represents only a fraction of the total Nhp6 (41), we argue from these data that the extent of Nhp6 association with SNR6 is roughly comparable to the extent of Brf1 association and is thus consistent with a direct role for Nhp6 in the SNR6 transcription process (44, 47). Lower but still significant extents of association were observed in the case of tRNA genes. The amount of precipitated N(GTT)CR tDNA (normalized to the input DNA) in the case of each TAP-Nhp6 protein was about fivefold lower than the amount of N(GTT)CR coprecipitated with TAP-Brf1 (cf. lanes 3 and 4 with lanes 1 and 2). Nhp6 association with the N(GTT)NR and T(AGT)CR tDNAs appeared to be slightly higher than to N(GTT)CR (lanes 5 to 8 and 13 to 16, respectively). In another series of ChIP experiments, we more carefully analyzed, by quantitative real-time PCR, the association of Nhp6 with SNR6, tDNAs, and non-Pol III-related loci. As expected on the basis of the abundance and nonspecific DNA binding activity of Nhp6, we found both Nhp6 proteins significantly associated with several tDNA-unrelated genomic segments (data not shown). As exemplified by the quantitative ChIP data in Fig. 4B, Nhp6 association with the tN(GTT)NR locus was comparable to association with the unrelated HMRA2 locus (with Nhp6B being represented only 1.7-fold more at the tDNA), while Nhp6 proteins were found to associate with the SNR6 locus to a two- to three-times-greater extent. Nhp6 proteins thus interact with both SNR6 and tRNA genes in yeast cells, but the extent of Nhp6 association with tDNAs could not be distinguished from its interaction with non-tDNA-related loci.
BRF1 overexpression partially rescues the tDNA transcriptional defect in the nhp6ΔΔ strain.
The BRF1 gene, coding for a component of the transcription initiation factor TFIIIB, has previously been shown to act as a multicopy suppressor of the nhp6ΔΔ temperature-sensitive phenotype and to restore U6 snRNA levels in the same strain (44). We thus asked whether BRF1 overexpression could correct the transcriptional defect of the [5′NR]Syn2 construct in the nhp6ΔΔ strain. As shown in Fig. 5, the [5′CR]Syn2 and [5′NR]Syn2 plasmid-borne constructs were expressed at identical levels in a WT strain (lanes 1 and 2) while [5′NR]Syn2 expression was 6.5-fold lower than [5′CR]Syn2 expression in nhp6ΔΔ cotransformed with the empty pFL45S multicopy vector (lanes 5 and 6). Cotransformation with pFL45S containing the BRF1 ORF with its own promoter regions resulted in a 2.5-fold increase in [5′NR]Syn2 expression (cf. lanes 6 and 4) but only in a 1.4-fold increase in [5′CR]Syn2 expression (cf. lane 5 with lane 3). As a consequence, [5′NR]Syn2 expression under these conditions was only 3.6-fold lower than [5′CR]Syn2 expression (cf. lanes 3 and 4). This result further supports the notion that Nhp6 exerts its effect on tRNA gene expression at the transcriptional level and suggests that it might do so by favoring TFIIIB assembly on tDNA upstream regions.
Loss of Nhp6 compromises both transcription and heterochromatin barrier function of the HMR-tDNA.
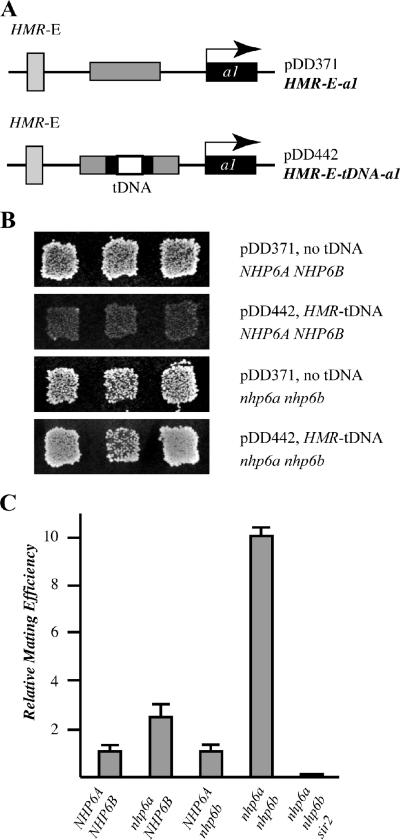
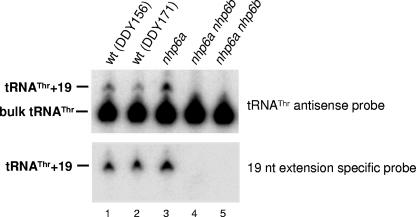
The transcription complexes assembled on class III genes may positionally influence the expression of neighboring Pol II-transcribed genes. In particular, a key element of the downstream heterochromatin boundary of the silenced HMR locus has been demonstrated to be the tDNAThr T(AGT)CR. In a plasmid-based silencing assay, this tDNA alone acts as an efficient barrier to silencing from the HMR-E silencer (22). Since ChIP analysis (Fig. 4) shows that T(AGT)CR tDNA is bound by Nhp6 proteins in vivo, we considered the possibility that Nhp6 might contribute to the silencing barrier function of this tDNA. To measure the barrier function, we used the reporter constructs depicted in Fig. 6A. The barrier assay was performed as described by Donze and Kamakaka (22) and is described briefly below. The HMR-E silencer alone completely represses the transcription of a downstream a1 gene. Insertion of a DNA fragment containing a barrier element between the E silencer and the a1 gene blocks the spreading of silenced chromatin, allowing expression of a1. Figure 6B shows that when it is transformed into a MATα hmrΔ strain, the a1 gene on plasmid pDD371 is silenced by HMR-E, allowing the cells to retain the α mating phenotype, as indicated by growth when cells are replica plated onto an appropriate mating lawn. When the tDNA barrier is inserted between HMR-E and the a1 gene (plasmid pDD442), the spreading of silencing is blocked, allowing a1 expression leading to a nonmating phenotype. However, in an nhp6ΔΔ background, the ability of the HMR-tDNA to block the spread of silencing is compromised, as indicated by an increase in mating by cells containing plasmid pDD442. Quantitative matings of strains containing the HMR-tDNA barrier integrated back into chromosome III of S. cerevisiae (Fig. 6C) show that the nhp6ΔΔ strain mates about 10 times more efficiently than the WT NHP6 strain and that the increased spread of silencing is Sir protein dependent. We then asked whether the loss of barrier function of T(AGT)CR in the nhp6ΔΔ strain correlates with a decrease in tDNA expression levels. To this end, WT and nhp6ΔΔ strains (each with HMR and the HMR-tDNA deleted) were transformed with a plasmid carrying marked T(AGT)CR, containing an additional 19 bp between the end of the coding sequence and the terminator, cloned into the HMR locus between the E silencer and the a1 gene. As shown by Northern analysis of RNAs extracted from these strains (Fig. 7), in the nhp6ΔΔ strain the expression of tagged T(AGT)CR dropped to undetectable levels (cf. lanes 4 and 5 with 1 to 3). An approximately threefold decrease in T(AGT)CR expression was also observed in strains in which the marked HMR-tDNA barrier was integrated back into chromosome III of S. cerevisiae (data not shown).
FIG. 6.
Nhp6 is required for the heterochromatin barrier function of the HMR-tDNA. (A) Plasmid pDD371 contains the HMR locus of S. cerevisiae lacking the I silencer. Plasmid pDD442 contains the HMR-tDNA cloned into the a2 gene between the E silencer and a1, which efficiently blocks silencing from repressing the transcription of a1. (B) Strains DDY171 and DDY591 (nhp6ΔΔ) were transformed with plasmid pDD371 or pDD442, and mating assays were performed as previously described (22). (C) Quantitative analysis of the effects of nhp6 mutations on mating efficiency. The strains used in this assay contain the HMR-E-tDNA constructs integrated back into chromosome III and are DDY689 (NHP6A NHP6B), DDY714 (nhp6a NHP6B), DDY669 (NHP6A nhp6b), DDY671 (nhp6a nhp6b), and DDY705 (nhp6a nhp6b sir2).
FIG. 7.
Nhp6 is required for transcription of HMR-tDNAThr. WT and nhp6 mutant strains (each with HMR and the HMR-tDNA deleted) were transformed with plasmid pDD570, which carries a marked HMR-tDNA boundary (containing an additional 19 bp between the end of the coding sequence and the terminator) cloned into the HMR locus between the E silencer and the a1 gene. Total RNA was isolated and subjected to Northern blot analysis with either a bulk tRNAThr antisense oligonucleotide (upper panel) or an oligonucleotide specific for the 19-base extension marking the HMR-tDNA (lower panel) as the probe.
DISCUSSION
We have presented in vitro and in vivo evidence that the HMG box Nhp6 proteins of S. cerevisiae positively modulate tRNA gene transcription in a promoter context-dependent manner and are required for the heterochromatin barrier function of the HMR-tDNA. Previous studies reported the involvement of Nhp6 in SNR6 transcription but failed to reveal a stimulatory role in tRNA gene transcription. We could unveil such a role by initially focusing on tRNA genes characterized by transcriptionally weak 5′-flanking regions. We previously reported that yeast tRNA genes coding for the same mature tRNA product, and thus having identical internal control regions, can nevertheless be transcribed at very different efficiencies in vitro in the presence of limiting TFIIIB concentrations because of the influence of the 5′-flanking sequence on TFIIIB binding and transcription complex assembly. The same transcriptional difference, however, could not be detected in vivo, except in TFIIIB-deficient strains (29). Our data indicate that the reason for the lack of an upstream sequence effect in WT yeast cells is that tRNA gene transcription in vivo is positively influenced by Nhp6 proteins that selectively exert a stimulatory action on upstream region-defective tDNAs. By ChIP analysis, we were able to demonstrate for the first time a selective enrichment of Nhp6 proteins at the SNR6 gene, the most relevant target of Nhp6 (44). Nhp6 proteins were also found to associate with tDNA loci, but to an extent similar to that of unrelated loci, a fact that was not completely unexpected, given the abundance and the lack of sequence specificity of these DNA binding proteins. The presence of Nhp6 proteins at tDNAs in vivo and their ability to gene specifically stimulate transcription in vitro are consistent with their direct involvement in tRNA gene transcription. Nhp6 proteins were also found to be required for in vivo expression of the HMR-tDNA T(AGT)CR and to enhance its ability to act as a barrier to heterochromatin spread, thus reinforcing the idea of HMG box family proteins as architectural facilitators in the assembly of nucleoprotein complexes involved in different DNA transactions, including the transcription by Pol II of many genes that are spread throughout the genome (50, 60).
The steady-state levels of tRNA were previously found to be unaltered in nhp6ΔΔ cells compared to WT cells (44). In the analysis by Kruppa et al., however, only bulk tRNA synthesis was investigated while our study was based on individual tDNA analysis made possible by the use of reporter tDNASyn2 fusion constructs. Yeast tRNAs are encoded by 274 different tDNAs, mostly organized in multigene families, and each tDNA family may contain members characterized by suboptimal 5′-flanking regions. According to our previous bioinformatic analysis (29), at least 32 tRNA genes (i.e., more than 10% of all tDNAs), among which is N(GGT)NR, have largely suboptimal upstream regions, scoring 0; i.e., they are completely devoid of conserved AT-rich sequence motifs (29). If 10% of all tDNAs are poorly active in the nhp6ΔΔ strain, we do not expect a detectable decrease in the steady-state levels of bulk tRNAs. However, in the case of small tDNA families comprising only two or three tDNAs, poor transcription of one of the members in the absence of Nhp6 would result in a significant decrease in the levels of the corresponding tRNA. Previous studies have also directly analyzed the effect of Nhp6 on tRNA gene transcription in vitro and found no evidence of tDNA transcriptional stimulation by Nhp6 (44, 47). All the tDNAs analyzed in these studies, however, have upstream sequence scores higher than 0, according to reference 29. It is thus reasonable to assume that their transcription is not strongly responsive to Nhp6. We also note, however, that the T(AGT)CR tDNA, whose transcription was found to require Nhp6 in vivo, has an upstream region that is not suboptimal according to reference 29. The sequence features of tDNA upstream regions revealed in that study are thus not to be taken as the sole criterion by which to predict the Nhp6 dependence of tRNA gene transcription.
A recent study has shown that under conditions of limiting Pol III with recombinant TFIIIB and highly purified TFIIIC, Nhp6 proteins can stimulate tRNA gene transcription in vitro indirectly by shielding nonspecific transcription initiation sites (38). Our data indicate that Nhp6 can stimulate class III gene transcription in vitro both by reducing transcription factor sequestration at nonspecific sites (indirect effect) and by directly enhancing transcription complex assembly on specific templates, such as SNR6 and tDNAs with weak upstream regions (direct effect). The indirect effect was observed in vitro at considerably higher Nhp6 concentrations than the direct effect. Nhp6 has also been found recently to be required for accurate TSS selection on some tDNAs both in vitro and in vivo (39). The tDNA transcriptional stimulation we observed, however, does not seem to be a consequence of TSS selection defects in the absence of Nhp6, as no TSS alterations were found to be induced by Nhp6 protein in vitro with N(GTT)CR and N(GTT)NR templates.
By which mechanism, then, does Nhp6 stimulate tRNA gene transcription? Studies of the role of Nhp6 in SNR6 transcription have led to the proposal that Nhp6 acts in this case by facilitating TFIIIC binding to the SNR6 promoter, perhaps by relieving the sterically unfavorable spacing of the SNR6 A- and B-block elements (44, 48). In another study, however, Nhp6 was also found to stimulate TFIIIC-independent transcription of SNR6 in vitro, thus suggesting a direct effect of Nhp6 on TFIIIB-DNA complex formation (47). We tend to exclude the possibility that Nhp6 stimulates tDNA transcription by facilitating TFIIIC binding because we found that tRNA gene copies having identical TFIIIC binding sites responded very differently to Nhp6. We believe, instead, that the mode of action of Nhp6 in tDNA transcription is related to its DNA binding and bending properties and to the nature of the interaction between TFIIIB and the tDNA upstream regions. TFIIIB sharply bends DNA (46), TFIIIB binding to a tDNA upstream region can be potentiated by artificially increasing DNA flexibility at particular positions (30), and computational analysis of DNA bendability has revealed that tDNA upstream sequences in the S. cerevisiae genome are characterized by sites with a high bending propensity (29). It is thus reasonable to assume that the association of TFIIIB with tDNA upstream regions is greatly favored by bent DNA conformations. By favoring the transition of tDNA upstream regions to a bent conformation, Nhp6 proteins could facilitate TFIIIB binding and tRNA gene transcription, a mechanism very similar to the one underlying Nhp6 stimulation of preinitiation complex formation in Pol II transcription (4, 5, 51). In the highly bent TFIIIB-DNA complex, TBP is thought to cause the main DNA distortion by inducing a sharp kink at the T-A base pair steps of the TATA-like elements frequently present around position −30 with respect to the TSS (45). The other two TFIIIB components, Brf1 and Bdp1, contact the DNA on both sides of the TBP-DNA subcomplex without any marked sequence specificity. The tDNA upstream region can thus be viewed as a fairly large binding surface that must provide enough flexibility points and docking sites to accommodate at least three distinct (yet interacting) proteins. We speculate that for many tRNA genes, the upstream sequence is by itself sufficient to provide both flexibility and contact sites for efficient TFIIIB assembly, while a minority of tRNA genes whose upstream sequences do not possess sufficient bendability and/or docking sites for TFIIIB components further require Nhp6 as a TFIIIB binding facilitator. The S. cerevisiae genome contains 274 mostly unclustered tRNA genes coding for 42 tRNA species with different codon specificities (31, 52). The gene copy numbers for individual tRNA species range from 1 to 16 and correlate well with both the frequency of codon occurrence and the intracellular abundance of individual tRNAs (52). Such a correlation implies that the different tRNA gene copies coding for a given tRNA are generally transcribed with similar efficiencies. Isocoding tDNAs, however, are flanked by different upstream regions that can strongly influence TFIIIB binding and transcription complex assembly. We propose that the abundant Nhp6 proteins, by facilitating TFIIIB binding to suboptimal sites, act in vivo to level the transcriptional differences that would occur within tDNA multigene families as a consequence of upstream sequence heterogeneity. Our data, however, do not exclude the possibility that Nhp6 proteins might facilitate the transcription of a subset of tDNAs as a part of the yFACT complex that has been shown to promote nucleosome rearrangements facilitating both initiation and elongation of Pol II transcription (7, 26).
Once bound to the upstream region of class III genes, the three components of TFIIIB (TBP, Brf1, and Bdp1) are sufficient for specific Pol III recruitment and transcription initiation (27). Several reports, however, suggested that additional components are required for full transcription efficiency. The yeast TFIIIE fraction greatly stimulates SUP4 and tRNALeu3 gene transcription in vitro when all-recombinant TFIIIB is used (56; G. Dieci, unpublished observations), and fully recombinant TFIIIB is considerably less effective than TFIIIB reconstituted with the crude B" fraction in supporting efficient transcription reinitiation on tRNA genes (25). The data we have presented tend to exclude the possibility that Nhp6 is a component of TFIIIE. In the presence of fully recombinant TFIIIB, TFIIIE stimulated SUP4 tRNA gene transcription without correcting the TSS selection defect. In contrast, the initiation fidelity defect was absent when the crude B" fraction was used in place of rBdp1 or when all-recombinant TFIIIB was supplemented with recombinant Nhp6 (Fig. 3 and references 1 and 39). Moreover, Nhp6 protein was detected by Western analysis in the crude B" fraction but not in the TFIIIE fraction. We suggest that crude B" contains, in addition to the key TFIIIB component Bdp1, at least two ancillary activities that contribute to class III gene transcription, i.e., Nhp6, which influences both transcription efficiency and TSS selection in a context-specific manner, and a reinitiation-stimulating activity possibly related to TFIIIE (25).
The absence of Nhp6 proteins negatively affected both transcription and barrier activity of the HMR tDNAThr. Such parallel effects suggest that the presence of stable Pol III transcription complexes on tDNAs is required for their ability to block the spread of silent heterochromatin. This is consistent with previous results that demonstrated that weakened Pol III complex formation, by either box A or box B mutations or conditional mutations in TFIIIB or TFIIIC subunits, weakened HMR-tDNA barrier function (22). How Nhp6 proteins might affect chromatin structure and complex assembly at Pol III promoters has not been investigated in detail, but studies on the effects of nhp6 null mutations and Nhp6p overexpression on Pol II transcription provide some insight. Several recent studies have revealed genetic interactions between the Nhp6 proteins and TBP assembly at Pol II promoters (6, 24, 62). Overexpression of TBP partially restored HO gene transcription in nhp6ab mutants (62), and overexpression of Nhp6p suppressed TFIIA mutations and directly stimulated in vitro TFIIA-TBP-DNA complex formation (5). In vitro binding of purified Nhp6p to reconstituted nucleosomes resulted in an altered DNase I digestion pattern and changes in restriction enzyme accessibility, suggesting a reorganization of histone-DNA interactions upon Nhp6p binding (53, 55). In the presence of TFIIA, direct binding of TBP to a TATA box positioned at a nucleosomal dyad was stimulated by the yeast yFACT complex, which contains Nhp6p (7, 26). Genetic screens for TBP mutants that are lethal in the nhp6ΔΔ background yielded mutations on the TBP surfaces known to interact with Spt3p, TFIIA, and the TFIIIB subunit Brf1p (24). The same study demonstrated reduced TBP binding to the Pol III-transcribed SNR6 gene in nhp6ab strains. Taken together, these studies suggest a general role for Nhp6 proteins in assisting the assembly of TBP-containing complexes onto both Pol II and Pol III promoters within chromatin, perhaps by localized reorganization of nucleosome-DNA interactions. Inefficient TFIIIB assembly at tDNAs in the absence of Nhp6 proteins would explain both the reduced transcription efficiency and weakened heterochromatin barrier activity of selected tDNAs with less-than-optimal 5′-flanking sequence motifs.
Acknowledgments
We thank Reid Johnson (UCLA) for anti-Nhp6 antiserum; Michael Snyder (Yale University), Marie-Claude Marsolier (CEA-Saclay), and Tiziana Lodi (University of Parma) for strains and plasmids; Joël Acker (CEA-Saclay) for pure, recombinant Bdp1 protein; and Mircko Alzapiedi, Milena Preti, Ilaria Lamberto, and Gloria Fiorino (University of Parma) for help and discussions. Special thanks to Nick Proudfoot (University of Oxford) for allowing some experiments to be carried out in his laboratory.
This work was supported by the Human Frontier Science Program Organization (grant RGY0011/2002-C to G.D. and D.D.); by the Italian Ministry of Education, University and Research (MIUR, 2005 PRIN and FIRB Programs); and by the National Science Foundation (grant MCB-0342113 to D.D.).
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Andrau, J. C., and M. Werner. 2001. B"-associated factor(s) involved in RNA polymerase III preinitiation complex formation and start-site selection. Eur. J. Biochem. 268:5167-5175. [DOI] [PubMed] [Google Scholar]
- 2.Bachman, N., M. E. Gelbart, T. Tsukiyama, and J. D. Boeke. 2005. TFIIIB subunit Bdp1p is required for periodic integration of the Ty1 retrotransposon and targeting of Isw2p to S. cerevisiae tDNAs. Genes Dev. 19:955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, R. E., S. Camier, A. Sentenac, and B. D. Hall. 1987. Gene size differentially affects the binding of yeast transcription factor τ to two intragenic regions. Proc. Natl. Acad. Sci. USA 84:8768-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi, M. E., and A. Agresti. 2005. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15:496-506. [DOI] [PubMed] [Google Scholar]
- 5.Biswas, D., A. N. Imbalzano, P. Eriksson, Y. Yu, and D. J. Stillman. 2004. Role for Nhp6, Gcn5, and the Swi/Snf complex in stimulating formation of the TATA-binding protein-TFIIA-DNA complex. Mol. Cell. Biol. 24:8312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas, D., Y. Yu, D. Mitra, and D. J. Stillman. 2006. Genetic interactions between Nhp6 and Gcn5 with Mot1 and the Ccr4-Not complex that regulate binding of TATA-binding protein in Saccharomyces cerevisiae. Genetics 172:837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, D., Y. Yu, M. Prall, T. Formosa, and D. J. Stillman. 2005. The yeast FACT complex has a role in transcriptional initiation. Mol. Cell. Biol. 25:5812-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolchi, A., S. Ottonello, and S. Petrucco. 2005. A general one-step method for the cloning of PCR products. Biotechnol. Appl. Biochem. 42:205-209. [DOI] [PubMed] [Google Scholar]
- 9.Bolton, E. C., and J. D. Boeke. 2003. Transcriptional interactions between yeast tRNA genes, flanking genes and Ty elements: a genomic point of view. Genome Res. 13:254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonneaud, N., O. Ozier-Kalogeropoulos, G. Y. Li, M. Labouesse, L. Minvielle-Sebastia, and F. Lacroute. 1991. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7:609-615. [DOI] [PubMed] [Google Scholar]
- 11.Braglia, P., R. Percudani, and G. Dieci. 2005. Sequence context effects on oligo(dT) termination signal recognition by Saccharomyces cerevisiae RNA polymerase III. J. Biol. Chem. 280:19551-19562. [DOI] [PubMed] [Google Scholar]
- 12.Burnol, A. F., F. Margottin, P. Schultz, M.-C. Marsolier, P. Oudet, and A. Sentenac. 1993. Basal promoter and enhancer element of yeast U6 snRNA gene. J. Mol. Biol. 233:644-658. [DOI] [PubMed] [Google Scholar]
- 13.Bustin, M., and R. Reeves. 1996. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 54:35-100. [DOI] [PubMed] [Google Scholar]
- 14.Chédin, S., M. L. Ferri, G. Peyroche, J. C. Andrau, S. Jourdain, O. Lefebvre, M. Werner, C. Carles, and A. Sentenac. 1998. The yeast RNA polymerase III transcription machinery: a paradigm for eukaryotic gene activation. Cold Spring Harbor Symp. Quant. Biol. 63:381-389. [DOI] [PubMed] [Google Scholar]
- 15.Costigan, C., D. Kolodrubetz, and M. Snyder. 1994. NHP6A and NHP6B, which encode HMG1-like proteins, are candidates for downstream components of the yeast SLT2 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 14:2391-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devine, S. E., and J. D. Boeke. 1996. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 10:620-633. [DOI] [PubMed] [Google Scholar]
- 17.Dieci, G., L. Duimio, F. Coda-Zabetta, K. U. Sprague, and S. Ottonello. 1993. A novel RNA polymerase III transcription factor fraction that is not required for template commitment. J. Biol. Chem. 268:11199-11207. [PubMed] [Google Scholar]
- 18.Dieci, G., R. Percudani, S. Giuliodori, L. Bottarelli, and S. Ottonello. 2000. TFIIIC-independent in vitro transcription of yeast tRNA genes. J. Mol. Biol. 299:601-613. [DOI] [PubMed] [Google Scholar]
- 19.Dieci, G., and A. Sentenac. 2003. Detours and shortcuts to transcription reinitiation. Trends Biochem. Sci. 28:202-209. [DOI] [PubMed] [Google Scholar]
- 20.Dieci, G., and A. Sentenac. 1996. Facilitated recycling pathway for RNA polymerase III. Cell 84:245-252. [DOI] [PubMed] [Google Scholar]
- 21.Donze, D., C. R. Adams, J. Rine, and R. T. Kamakaka. 1999. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 13:698-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donze, D., and R. T. Kamakaka. 2001. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 20:520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumay-Odelot, H., J. Acker, R. Arrebola, A. Sentenac, and C. Marck. 2002. Multiple roles of the τ131 subunit of yeast transcription factor IIIC (TFIIIC) in TFIIIB assembly. Mol. Cell. Biol. 22:298-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson, P., D. Biswas, Y. Yu, J. M. Stewart, and D. J. Stillman. 2004. TATA-binding protein mutants that are lethal in the absence of the Nhp6 high-mobility-group protein. Mol. Cell. Biol. 24:6419-6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari, R., C. Rivetti, J. Acker, and G. Dieci. 2004. Distinct roles of transcription factors TFIIIB and TFIIIC in RNA polymerase III transcription reinitiation. Proc. Natl. Acad. Sci. USA 101:13442-13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formosa, T., P. Eriksson, J. Wittmeyer, J. Ginn, Y. Yu, and D. J. Stillman. 2001. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 20:3506-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiduschek, E. P., and G. A. Kassavetis. 2001. The RNA polymerase III transcription apparatus. J. Mol. Biol. 310:1-26. [DOI] [PubMed] [Google Scholar]
- 28.Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle, N. Dephoure, E. K. O'Shea, and J. S. Weissman. 2003. Global analysis of protein expression in yeast. Nature 425:737-741. [DOI] [PubMed] [Google Scholar]
- 29.Giuliodori, S., R. Percudani, P. Braglia, R. Ferrari, E. Guffanti, S. Ottonello, and G. Dieci. 2003. A composite upstream sequence motif potentiates tRNA gene transcription in yeast. J. Mol. Biol. 333:1-20. [DOI] [PubMed] [Google Scholar]
- 30.Grove, A., G. A. Kassavetis, T. E. Johnson, and E. P. Geiduschek. 1999. The RNA polymerase III-recruiting factor TFIIIB induces a DNA bend between the TATA box and the transcriptional start site. J. Mol. Biol. 285:1429-1440. [DOI] [PubMed] [Google Scholar]
- 31.Hani, J., and H. Feldmann. 1998. tRNA genes and retroelements in the yeast genome. Nucleic Acids Res. 26:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harismendy, O., C. G. Gendrel, P. Soularue, X. Gidrol, A. Sentenac, M. Werner, and O. Lefebvre. 2003. Genome-wide location of yeast RNA polymerase III transcription machinery. EMBO J. 22:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huet, J., N. Manaud, G. Dieci, G. Peyroche, C. Conesa, O. Lefebvre, A. Ruet, M. Riva, and A. Sentenac. 1996. RNA polymerase III and class III transcription factors from Saccharomyces cerevisiae. Methods Enzymol. 273:249-267. [DOI] [PubMed] [Google Scholar]
- 34.Hull, M. W., J. Erickson, M. Johnston, and D. R. Engelke. 1994. tRNA genes as transcriptional repressor elements. Mol. Cell. Biol. 14:1266-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jambunathan, N., A. W. Martinez, E. C. Robert, N. B. Agochukwu, M. E. Ibos, S. L. Dugas, and D. Donze. 2005. Multiple bromodomain genes are involved in restricting the spread of heterochromatic silencing at the Saccharomyces cerevisiae HMR-tRNA boundary. Genetics 171:913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassavetis, G. A., B. R. Braun, L. H. Nguyen, and P. E. Geiduschek. 1990. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell 60:235-245. [DOI] [PubMed] [Google Scholar]
- 37.Kassavetis, G. A., C. A. P. Joazeiro, M. Pisano, E. P. Geiduschek, T. Colbert, S. Hahn, and J. A. Blanco. 1992. The role of the TATA binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor TFIIIB. Cell 71:1055-1064. [DOI] [PubMed] [Google Scholar]
- 38.Kassavetis, G. A., E. Soragni, R. Driscoll, and E. P. Geiduschek. 2005. Reconfiguring the connectivity of a multiprotein complex: fusions of yeast TATA-binding protein with Brf1, and the function of transcription factor IIIB. Proc. Natl. Acad. Sci. USA 102:15406-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassavetis, G. A., and D. F. Steiner. 2006. NHP6 is a transcriptional initiation fidelity factor for RNA polymerase III transcription in vitro and in vivo. J. Biol. Chem. 281:7445-7451. [DOI] [PubMed] [Google Scholar]
- 40.Kirchner, J., C. M. Connolly, and S. B. Sandmeyer. 1995. Requirement of RNA polymerase III transcription factors for in vitro position-specific integration of a retroviruslike element. Science 267:1488-1491. [DOI] [PubMed] [Google Scholar]
- 41.Kolodrubetz, D., M. Kruppa, and A. Burgum. 2001. Gene dosage affects the expression of the duplicated NHP6 genes of Saccharomyces cerevisiae. Gene 272:93-101. [DOI] [PubMed] [Google Scholar]
- 42.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieg, R., R. Stucka, S. Clark, and H. Feldmann. 1991. The use of a synthetic tRNA gene as a novel approach to study in vivo transcription and chromatin structure in yeast. Nucleic Acids Res. 19:3849-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruppa, M., R. D. Moir, D. Kolodrubetz, and I. M. Willis. 2001. Nhp6, an HMG1 protein, functions in SNR6 transcription by RNA polymerase III in S. cerevisiae. Mol. Cell 7:309-318. [DOI] [PubMed] [Google Scholar]
- 45.Kumar, A., A. Grove, G. A. Kassavetis, and E. P. Geiduschek. 1998. Transcription factor IIIB: the architecture of its DNA complex, and its roles in initiation of transcription by RNA polymerase III. Cold Spring Harbor Symp. Quant. Biol. 63:121-129. [DOI] [PubMed] [Google Scholar]
- 46.Léveillard, T., G. A. Kassavetis, and E. P. Geiduschek. 1991. Saccharomyces cerevisiae transcription factors IIIB and IIIC bend the DNA of a tRNA(Gln) gene. J. Biol. Chem. 266:5162-5168. [PubMed] [Google Scholar]
- 47.Lopez, S., M. Livingstone-Zatchej, S. Jourdain, F. Thoma, A. Sentenac, and M.-C. Marsolier. 2001. High-mobility-group proteins NHP6A and NHP6B participate in activation of the RNA polymerase III SNR6 gene. Mol. Cell. Biol. 21:3096-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin, M. P., V. L. Gerlach, and D. A. Brow. 2001. A novel upstream RNA polymerase III promoter element becomes essential when the chromatin structure of the yeast U6 RNA gene is altered. Mol. Cell. Biol. 21:6429-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moqtaderi, Z., and K. Struhl. 2004. Genome-wide occupancy profile of the RNA polymerase III machinery in Saccharomyces cerevisiae reveals loci with incomplete transcription complexes. Mol. Cell. Biol. 24:4118-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira, J. M., and S. Holmberg. 2000. Chromatin-mediated transcriptional regulation by the yeast architectural factors NHP6A and NHP6B. EMBO J. 19:6804-6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paull, T. T., M. Carey, and R. C. Johnson. 1996. Yeast HMG proteins NHP6A/B potentiate promoter-specific transcriptional activation in vivo and assembly of preinitiation complexes in vitro. Genes Dev. 10:2769-2781. [DOI] [PubMed] [Google Scholar]
- 52.Percudani, R., A. Pavesi, and S. Ottonello. 1997. Transfer RNA gene redundancy and translational selection in Saccharomyces cerevisiae. J. Mol. Biol. 268:322-330. [DOI] [PubMed] [Google Scholar]
- 53.Rhoades, A. R., S. Ruone, and T. Formosa. 2004. Structural features of nucleosomes reorganized by yeast FACT and its HMG box component, Nhp6. Mol. Cell. Biol. 24:3907-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts, D. N., A. J. Stewart, J. T. Huff, and B. R. Cairns. 2003. The RNA polymerase III transcriptome revealed by genome-wide localization and activity-occupancy relationships. Proc. Natl. Acad. Sci. USA 100:14695-14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruone, S., A. R. Rhoades, and T. Formosa. 2003. Multiple Nhp6 molecules are required to recruit Spt16-Pob3 to form yFACT complexes and to reorganize nucleosomes. J. Biol. Chem. 278:45288-45295. [DOI] [PubMed] [Google Scholar]
- 56.Rüth, J., C. Conesa, G. Dieci, O. Lefebvre, A. Düsterhoft, S. Ottonello, and A. Sentenac. 1996. A suppressor of mutations in the class III transcription system encodes a component of yeast TFIIIB. EMBO J. 15:1941-1949. [PMC free article] [PubMed] [Google Scholar]
- 57.Sethy-Coraci, I., R. D. Moir, A. Lopez-de-Leon, and I. M. Willis. 1998. A differential response of wild type and mutant promoters to TFIIIB70 overexpression in vivo and in vitro. Nucleic Acids Res. 26:2344-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simms, T. A., E. C. Miller, N. P. Buisson, N. Jambunathan, and D. Donze. 2004. The Saccharomyces cerevisiae TRT2 tRNAThr gene upstream of STE6 is a barrier to repression in MATα cells and exerts a potential tRNA position effect in MATa cells. Nucleic Acids Res. 32:5206-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, J. O., and A. A. Travers. 2001. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem. Sci. 26:167-174. [DOI] [PubMed] [Google Scholar]
- 61.White, R. J. 2002. RNA polymerase III transcription, 3rd edition. Landes Bioscience, Austin, TX.
- 62.Yu, Y., P. Eriksson, L. T. Bhoite, and D. J. Stillman. 2003. Regulation of TATA-binding protein binding by the SAGA complex and the Nhp6 high-mobility group protein. Mol. Cell. Biol. 23:1910-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]