Abstract
The Notch signaling pathway modulates cell fate in diverse contexts, including vascular development. Notch4 is selectively expressed in vascular endothelium and regulates vascular remodeling. The signal-dependent transcription factor activator protein 1 (AP-1) activates Notch4 transcription in endothelial cells, but other factors/signals that regulate Notch4 are largely unknown. We demonstrate that, unlike the established transrepression mechanism in which the glucocorticoid receptor (GR) antagonizes AP-1, AP-1 and GR synergistically activated Notch4 transcription in endothelial cells. Fibroblast growth factor 2 (FGF-2) and cortisol induced AP-1 and GR occupancy, respectively, at a Notch4 promoter composite response element consisting of an imperfect half-glucocorticoid response element and an AP-1 motif, which mediated signal-dependent activation. Analysis of Notch4 promoter complex assembly provided evidence that GR and AP-1 independently occupy the composite response element, but AP-1 stabilizes GR occupancy. In multipotent 10T1/2 cells, FGF-2 and cortisol induced a histone modification pattern at the Notch4 locus mimicking that present in endothelial cells and reprogrammed Notch4 from a repressed to an active state. These results establish the molecular basis for a novel AP-1/GR-Notch4 axis in vascular endothelium.
The conserved Notch signaling pathway regulates cell proliferation, differentiation, and cell fate in diverse tissues (33). Four mammalian Notch receptors (Notch1 to Notch4), which have distinct and overlapping expression patterns, share a common signaling mechanism. Binding of transmembrane Notch ligands to Notch receptors on adjacent cells induces proteolytic cleavages, liberating the Notch intracellular domain (NIC) from the plasma membrane (61). NIC translocates into the nucleus and binds the repressor CBF1/Su(H)suppressor of hairless/Lag-1 (CSL) (24, 46, 59), thus converting CSL into an activator (20, 24, 36, 74). Through this canonical pathway and CSL-independent signaling (50), Notch regulates target genes as a key step in controlling developmental processes.
Vascular development and remodeling represent important Notch-regulated biological processes (23, 57). Both Notch1 and Notch4 are expressed in vascular endothelium, but Notch4 has an almost exclusively vascular expression pattern, whereas Notch1 is expressed more broadly (71). Notch1−/− mice exhibit embryonic lethality, due to extensive developmental defects, including impaired vascular remodeling (35, 63). Notch4−/− mice do not exhibit overt phenotypes, but vascular remodeling is more severely disrupted in Notch1−/−/Notch4−/− versus Notch1−/− mice (35). Endothelial expression of constitutively active NIC4 disrupts vascular development and is embryonic lethal (70). NIC4 overexpression in adult mouse endothelium induces arteriovenous malformations and lethality (4), indicating that Notch4 might regulate determination of arterial-venous fate, consistent with zebra fish Notch5 regulating arterial-venous differentiation (38). NIC1 or NIC4 overexpression in cultured endothelial cells either inhibits (39, 45) or promotes (41, 64, 69) vascular morphogenesis.
The endothelium-specific Notch4 expression (71) and genetic evidence indicate that normal vascular development requires physiological levels of Notch4 signaling. Thus, elucidation of mechanisms regulating Notch4 expression is particularly important with regard to understanding physiological and pathological functions of Notch4. Vascular endothelium consists of functionally distinct endothelial cell subtypes (5), and Notch4 is selectively expressed at certain vascular sites (23), including mouse aorta, intersomitic vessels, arterial capillaries, pulmonary artery, pulmonary capillaries, and cardinal/subcardinal veins (35, 42, 71, 72). Notch4 transcripts are highest in the aorta and pulmonary artery in E9 and E13.5 mouse embryos, are detectable in adult pulmonary capillaries (71), and are restricted to arterial endothelium in E13.5 embryos (72). Notch4 expression in mouse heart and liver is maximal by 2 weeks and 12 weeks of postnatal development, respectively (42).
The differential Notch4 regulation at distinct vascular sites and during development might be related to unique signaling environments. Vascular endothelial growth factor 121 (VEGF121) and fibroblast growth factor 2 (FGF-2) expression in human umbilical vein endothelial cells (HUVECs) modestly increases Notch1 and Notch4 mRNA (41). The antiangiogenic factor cerivastatin downregulates Notch4 mRNA in FGF-2-treated endothelial cells (73). In arthritic, but not normal, synovial fibroblasts, tumor necrosis factor induces Notch4 mRNA (1). However, mechanisms underlying signal-dependent, endothelium-specific transcription of Notch4 are largely unknown.
Previously, we demonstrated that the Notch4 locus assembles an endothelial cell-specific histone modification pattern in HUVECs and a conserved activator protein 1 (AP-1) motif is required for endothelial cell-specific Notch4 promoter activity (78). While, in principle, AP-1 motifs can mediate signal-dependent transcriptional responses, whether signaling mechanisms target the Notch4 promoter AP-1 motif in endothelial cells is unknown. Herein, we tested whether extracellular signals regulate Notch4 transcription via AP-1 and whether signals target other Notch4 locus components. Despite multiple studies demonstrating antagonism between glucocorticoid receptor (GR) and AP-1 (28, 29, 31, 34, 47, 51, 60, 79), we demonstrate that GR and AP-1 synergistically activate Notch4 in endothelial cells and reprogram Notch4 from a repressed to an active state in a multipotent nonendothelial cell line. Molecular analyses established a novel mechanism in which AP-1 and GR function via a composite response element, consisting of an imperfect half-glucocorticoid response element (half-GRE) and an AP-1 motif, with AP-1 stabilizing GR occupancy at this element. These studies provide the molecular basis for a model in which Notch4 expression requires dual growth factor-glucocorticoid signaling. Perturbation of this multicomponent signaling mechanism in pathophysiological states would be expected to deregulate Notch4 expression in endothelial cells and/or ectopically induce Notch4 in cells that normally lack the capacity to respond to Notch4 ligands.
MATERIALS AND METHODS
Cell culture.
Mouse yolk sac endothelial cells (YSECs) were derived from a hypervascular transgenic mouse expressing the fps/fes proto-oncogene (43). YSECs were maintained in Medium 200 (Cascade Biologics) containing low-serum growth supplement (2% fetal bovine serum, 1 μg/ml cortisol, 10 ng/ml epidermal growth factor [EGF], 3 ng/ml FGF-2, and 10 μg/ml heparin [Cascade Biologics]) and 1% penicillin-streptomycin (Gibco/BRL). Mouse aortic endothelial cells (MAEs), mouse heart microvascular endothelial cells (MHECs), and mouse embryonic fibroblast 10T1/2 cells were maintained in Dulbecco's modified Eagle's medium (Biofluids) containing 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (Gibco/BRL).
Antibodies.
Anti-Notch4 antibody was from Upstate Biotechnology. Anti-NIC1 is a rabbit polyclonal antiserum directed against amino acids 1759 to 2095 of human Notch1 as described previously (26). Rabbit anti-diacetylated histone H3 (06-599), anti-tetraacetylated H4 (06-866), and anti-dimethylated H3 at K4 (07-030) antibodies were from Upstate Biotechnology. Mouse immunoglobulin G (IgG) (Upstate Biotechnology) and preimmune serum (Covance) were used as chromatin immunoprecipitation (ChIP) controls. Rabbit anti-c-Fos (K-25) was from Santa Cruz Biotechnology. Mouse monoclonal anti-GR antibody (BuGR) was described previously (2), and anti-α-tubulin was from Oncogene Research Products. The secondary antibodies goat anti-rabbit IgG conjugated with horseradish peroxidase and goat anti-mouse IgG-horseradish peroxidase were from Santa Cruz Biotechnology.
Reagents.
Cortisol, cycloheximide, and heparin were from Sigma-Aldrich. Recombinant human EGF was from Calbiochem. Recombinant human FGF-2 was from Alan Rapraeger, University of Wisconsin, Madison. Dose-response curves were generated with EGF (10, 30, or 100 ng/ml), FGF-2 (3, 10, or 30 ng/ml), and cortisol (0.03, 0.3 or 3 μM) to determine the ability of individual factors to induce Notch4 expression in YSECs (see Fig. 2A). None of the factors alone induced Notch4. In subsequent experiments, EGF (10 ng/ml), FGF-2 (10 ng/ml), and cortisol (1 μM) were used. As an FGF-2 cofactor, 10 μg/ml heparin was always included with FGF-2. All factor treatments were conducted in serum-free medium containing 0.2% bovine serum albumin. Bovine serum albumin alone had no effect on Notch4 transcription (data not shown). Cycloheximide was used at final concentration of 20 μg/ml, which quantitatively blocked supplement-induced c-Fos protein expression, while c-Fos mRNA induction was unaffected (data not shown).
FIG. 2.
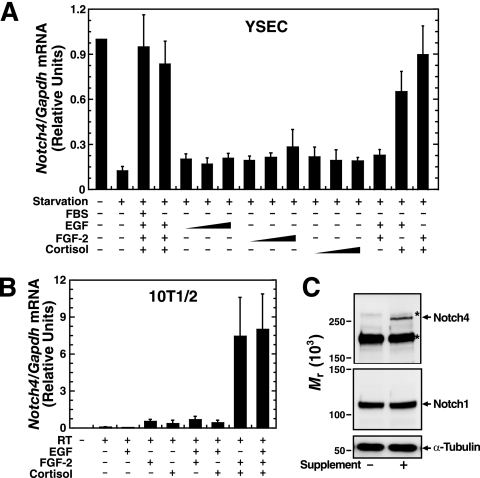
Growth factors and cortisol synergistically activate Notch4 expression. (A) YSECs were starved for 24 h and then treated with or without 2% FBS, EGF (10, 30, or 100 ng/ml), FGF-2 (3, 10, or 30 ng/ml), or cortisol (0.03, 0.3, or 3 μM) for 8 h. Notch4 mRNA was quantitated by real-time RT-PCR and normalized to Gapdh mRNA (mean ± standard error of the mean for three independent experiments). (B) 10T1/2 cells were treated with or without EGF (10 ng/ml), FGF-2 (10 ng/ml), or cortisol (1 μM) for 24 h. Notch4 mRNA was quantitated by real-time RT-PCR and normalized to Gapdh mRNA (mean ± standard error of the mean; three independent experiments). RT, reverse transcriptase. (C) 10T1/2 cells were treated with or without supplement for 24 h. Biotinylated cell surface proteins were analyzed by Western blotting, and the α-tubulin levels in the input lysates served as a loading control (representative pictures from at least three independent experiments). The asterisks denote nonspecific bands present under all conditions.
Plasmids.
For pGL3/N4pro1000, the mouse Notch4 promoter ∼1-kb fragment (−905 to +78) was amplified by PCR using the primer pair (5′→3′) AAGGCCAAGCCTCCAGACTC and CAGTCAAGCTTCAGGCAGGGACCCTC. For pGL3/N4pro500, the mouse Notch4 promoter 461-bp fragment (−383 to +78) was amplified by PCR using the primer pair (5′→3′) GGTTTCAGTTCAAGACACGTTGC and CAGTCAAGCTTCAGGCAGGGACCCTC. For pGL3/N4pro500(mGRE), the mouse Notch4 promoter with the GRE site mutated was amplified by two-step PCR using the primer pairs (5′→3′) CACAAGCGAGAGGACACCCTACTGATGG and AGGGTGTCCTCTCGCTTGTGTGACTCAGGAAACAGC and GGTTTCAGTTCAAGACACGTTGC and CAGTCAAGCTTCAGGCAGGGACCCTC. For pGL3/N4pro(mAP1), the mouse Notch4 promoter with the AP-1 motif mutated was amplified by two-step PCR using the primer pairs (5′→3′) TTGTGGCTAGACGGAAACAGCTCAGACTG and CAGTCTGAGCTGTTTCCGTCTAGCCACAA and GGTTTCAGTTCAAGACACGTTGC and CAGTCAAGCTTCAGGCAGGGACCCTC. For pGL3/N4pro(mGRE/AP1), the mouse Notch4 promoter with the imperfect half-GRE and AP-1 motif mutated was amplified by two-step PCR using the primer pairs (5′→3′) CACAAGCGAGAGGACACCCTACTGATGG and AGGGTGTCCTCTCGCTTGTGGCTAGACGGAAACAGC and GGTTTCAGTTCAAGACACGTTGC and CAGTCAAGCTTCAGGCAGGGACCCTC. The PCR products were digested with HindIII and cloned into pGL3basic.
The 4xAP1/Luc reporter containing four AP-1 motifs was from Nancy Colburn, National Cancer Institute. The GRE2/Luc reporter containing two copies of the GRE sequence from the tyrosine aminotransferase gene was from John Cidlowski, National Institute of Environmental Health sciences (44).
Quantitative real-time RT-PCR.
Total RNA was purified using Trizol (Invitrogen). cDNA was synthesized by standard procedures and quantitated by real-time reverse transcription-PCR (RT-PCR) (ABI Prism 7000). Primers were designed to amplify regions of 50 to 150 bp. Real-time RT-PCR mixtures (25 μl) contained 2 μl of cDNA, 12.5 μl of SYBR green (Applied Biosystems), and the indicated primers. Product accumulation was monitored by SYBR green fluorescence. Relative expression levels were determined from a standard curve of serial dilutions of cDNA samples. Analysis of product denaturation curves postamplification showed that primer pairs generated single products. The forward and reverse primers for real-time RT-PCR (5′→3′) were as follows: Notch4, GAGGACCTGGTTGAAGAATTGATC and TGCAGTTTTTTCCCTTTTATCCC; Notch1, TATGGCCACGAGGAAGAGCT and TAGACAATGGAGCCACGGATG; and Gapdh, TGCCCCCATGTTTGTGATG and TGTGGTCATGAGCCCTTCC.
Cell surface protein biotinylation.
YSEC and 10T1/2 cell surface proteins were biotinylated as described previously (62). Cells were washed with phosphate-buffered saline (PBS) containing Ca2+ and Mg2+ (pH 7.4) and incubated with 0.5 mg/ml EZ-link sulfo-NHS-SS-biotin (Pierce) in PBS for 30 min at 4°C. Excess biotin was quenched by incubation with 100 mM glycine in PBS for 15 min at 4°C, followed by two washes with PBS. Cells were lysed in lysis buffer (300 mM NaCl, 50 mM Tris [pH 8.0], 0.5% NP40, 0.5% deoxycholate, 1 mM CaCl2, 1 mM MgCl2, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], and 1μg/ml leupeptin) for 30 min at 4°C. Lysate was incubated overnight with streptavidin-conjugated beads (Sigma). Beads were washed three times with wash buffer (50 mM Tris [pH 8.0], 2 mM EDTA, 150 mM NaCl, 1% Triton X-100) and eluted in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris [pH 6.8], 100 mM dithiothreitol, 2% SDS, 10% glycerol, 0.1% bromophenol blue). Purified cell surface proteins were resolved by SDS-polyacrylamide gel electrophoresis on a 7.5% acrylamide gel. The proteins were transferred to an Immobilon P membrane (Millipore), detected by immunoblotting with anti-Notch4 antibody (Upstate; #07-189), and visualized with the ECL-Plus enhanced chemiluminescence system (Amersham). Antibody specificity was confirmed with expressed hemagglutinin (HA)-tagged Notch4. Anti-Notch4 and anti-HA antibodies yielded similar results in immunoblotting and immunostaining assays (data not shown).
siRNA knockdown.
Control and Notch4 small interfering RNAs (siRNAs) were from Dharmacon. RNA was transfected into YSECs via Lipofectamine (Invitrogen). YSECs were plated in six-well plates 1 day before transfection and were ∼50% confluent when transfected. RNA (200 pmol) was incubated with 8 μl Lipofectamine in 400 μl of Opti-MEM (Gibco/BRL) for 30 min at room temperature and was then added to cells that were washed with Opti-MEM. The cells were incubated with the transfection mixture for 6 h, fresh medium was added, and cells were harvested 60 h posttransfection. Total RNA was analyzed by real-time RT-PCR. Cells were biotinylated for analysis of cell surface proteins.
Quantitative ChIP analysis.
Real-time PCR-based ChIP analysis was performed as described previously (21, 78). Cells were incubated with medium containing 0.4% (for histone modifications) or 1% (for GR and AP-1) formaldehyde for 10 min at room temperature. Sonicated chromatin fragments averaged ∼300 to 500 bp. DNA was quantitated by real-time PCR. The amounts of products were determined relative to a standard curve generated from a titration of input chromatin. Measurements were made under conditions in which signals were in the linear range, and denaturation curves showed that primer pairs yielded single products. The following forward and reverse primers (5′→3′) were used for real-time PCR-based ChIP assay: N4 up, AAGGCCAAGCCTCCAGACTC and GCTTCCCGTGTTCTACACTGATACTT; N4 pro, CCATCAGTAGGGTGTCCAGGA and CGCCTCAGTCTGAGCTGTTTC; N4 pro2, GGTTTCAGTTCAAGACACGTTGC and GGTGTGACACACCCTCTTTTCAA; N4 in1, CATTGCTGGCCTTTCCTGAA and TGGGATGTGAATGGCTGAGA; N4 in1-2, TGGTCAGTGGCCTGTAAGGAG and TCCAGATGGCTTGCCCTTC; N4 ex3, GATCGATGCCAAACCCATCT and CCCCGTTGGAACAGAAAGAA; N4 in3, CAATCCACACACAAGCCTCCT and CGTCTCCCACCTGTTTTCTCA; N4 ex22, TGCCGAGATCACTTCCACAA and TTCAGCGTTATTGCAGCCTTT; RPII215 pro, GCGAATCTATAAAGGGCGTCACT and TCGGCGCTTCTGAGGAGA; β maj pro, AGTGCCAGAAGAGCCAAGGA and CAGGGTGAGGTCTAAGTGATGACA; Sgk1 up, GCATGCAAGAGCTTCTTCCCT and AATCGAAACACACGCACAGG; Sgk1 pro, GCAAGGCTCAAAATTTATGCG and TTCCAACTAATCTCCGAGAACATTC; and Sgk1 in2, TAGCAGCGAAGACTTCATGGG and GGAAAGATCTCAGCTCCAGCA.
Nuclear extract preparation.
YSEC or 10T1/2 cells were harvested by scraping and collected by centrifugation at 400 × g for 8 min. Cells were washed once with ice-cold PBS and resuspended in 1.5 volumes of nuclei lysis buffer (10 mM Tris-HCl [pH 7.5], 10 mM NaCl, 3 mM MgCl2, and 0.2% Nonidet P-40) on ice for 5 min. Nuclei were collected by centrifugation for 5 min at 400 × g. Nuclei were washed by gentle resuspension in 1.5 volumes of nuclei wash buffer (10 mM Tris-HCl [pH 7.5], 10 mM NaCl, and 3 mM MgCl2) and collected by centrifugation for 4 min at 400 × g. Nuclei were immediately resuspended in an equal volume of low-KCl extract buffer (20 mM HEPES [pH 7.5], 20 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 25% glycerol), and 1.33 volumes of the same buffer containing 1.2 M KCl was added dropwise. Nuclei were extracted for 45 min at 4°C with constant rotating. The suspension was centrifuged for 30 min at 150,000 × g. The supernatant was dialyzed against 500 volumes of a mixture of 20 mM HEPES (pH 7.4), 20% glycerol, 60 mM KCl, and 0.2 mM EDTA overnight at 4°C and centrifuged for 30 min at 150,000 × g. Aliquots of the supernatant were frozen on dry ice and stored at −80°C. The protein concentration was measured by the Bradford assay with gamma globulin as a standard. Dithiothreitol (10 mM), phenylmethylsulfonyl fluoride (0.5 mM), leupeptin (20 μg/ml), and β-glycerophosphate (1 mM) were included in all buffers.
In vitro promoter complex assembly assay.
Biotinylated double-stranded oligonucleotides (Operon) were immobilized on streptavidin-conjugated Dynabeads M-280 (Dynal). Nuclear extract (300 μg total protein) was incubated with DNA-bound Dynabeads (0.2 mg) in a mixture of 10 mM HEPES (pH 7.5), 60 mM KCl, 10% glycerol, 1 mM MgCl2, and 1 μg/μl poly(dI-dC) for 45 min at 4°C with rotation. After magnetic separation, beads were washed three times with 10 mM HEPES (pH 7.5), 60 or 150 mM KCl, 10% glycerol, and 1 mM MgCl2. Absorbed proteins were eluted in SDS-sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis on an 8% acrylamide gel. Proteins were transferred to Immobilon P membrane, detected by immunoblotting with anti-GR or anti-Fos antibody, and visualized with ECL-Plus.
Transient transfection assay.
10T1/2 cells were plated in 24-well plates 1 day before transfection and were ∼90% confluent when transfected. Samples of plasmid DNA (0.4 μg) were added to 50 μl of Opti-MEM (Gibco/BRL), incubated with Lipofectamine 2000 reagent (2.5 μl/1 μg DNA; Invitrogen) for 30 min at room temperature, and then added to cells. Cells were incubated with the mixture for 6 h and then treated with cortisol and/or FGF-2 for 16 h. Cells were harvested, and lysates were assayed for luciferase activity (Promega). The luciferase activity was normalized by the protein content of the lysates, as determined by Bradford assay (Bio-Rad) using gamma globulin as a standard.
RESULTS AND DISCUSSION
Dual growth factor-glucocorticoid signaling requirement for Notch4 transcription in endothelial cells.
Despite the strict temporospatial Notch4 expression, Notch4 transcriptional regulation is poorly understood. Growth factors and hormones are crucial for maintaining cultured endothelial cell phenotypes. Notch4-expressing mouse YSECs are cultured in medium with 2% FBS and endothelial cell supplement containing cortisol, EGF, FGF-2, and heparin, an FGF-2 cofactor. We asked whether Notch4 expression requires FBS and the supplement. Starvation of YSECs for 24 h without FBS and the supplement strongly decreased Notch4 mRNA (∼10-fold) (Fig. 1A, left). To assess whether downregulation is reversible, FBS and the supplement were added back to starved cells. Whereas FBS did not rescue expression, FBS/supplement reestablished normal Notch4 mRNA levels (Fig. 1A, left). FBS/supplement did not regulate Notch1 expression (Fig. 1B, left). Notch4, but not Notch1, expression was also signal dependent in MAEs and MHECs (Fig. 1A and B, middle and right). Thus, the signaling mechanism that regulates Notch4 expression operates in diverse endothelial cell subtypes.
FIG. 1.
Signal-dependent Notch4 expression in endothelial cells. YSECs, MAEs, or MHECs were starved for 24 h and treated with or without FBS and/or endothelial supplement for 24 h. Notch4 (A) and Notch1 (B) mRNA levels were quantitated by real-time RT-PCR and normalized to Gapdh mRNA (mean ± standard error of the mean for at least three independent experiments). (C) YSECs were transfected with control siRNA or Notch4 siRNA. Total RNA was isolated 60 h posttransfection. Notch4 and Notch1 mRNA levels were quantitated by real-time RT-PCR and normalized to Gapdh mRNA (left; two independent experiments). The Gapdh mRNA level was unaffected by Notch4 siRNA. Cell surface proteins were biotinylated, adsorbed to streptavidin-conjugated beads, and analyzed by Western blotting with anti-Notch4 and anti-Notch1 antibodies. The α-tubulin levels in the input lysates served as a loading control (middle; representative pictures from two independent experiments). The density of the specific bands was quantitated and plotted with the values obtained from cells transfected with control siRNA designated 1.0 (right; two independent experiments). (D) YSECs were starved for 24 h and treated with or without supplement for 24 h. Biotinylated cell surface proteins were analyzed by Western blotting, and the α-tubulin levels in the input lysates served as a loading control (left; representative pictures from at least three independent experiments). The density of the specific bands was quantitated and plotted with control cells designated 1.0 (right; mean ± standard error of the mean for three independent experiments). The asterisks denote nonspecific bands present under all conditions. RT, reverse transcriptase.
To determine if signal-dependent changes in Notch4 mRNA are accompanied by altered endogenous Notch4 protein levels, Notch4 was measured by Western blotting. Due to the low abundance of Notch4, cell surface proteins were biotinylated and enriched via adsorption to streptavidin-conjugated beads (62). Due to the reactivity of available anti-Notch4 antibodies with both Notch4 and cross-reactive proteins, Notch4 was knocked down in YSECs to identify the specific Notch4 band on the blot. siRNA-mediated knockdown of Notch4 reduced Notch4 mRNA by ∼90% (Fig. 1C, left) and downregulated a 245- ± 6-kDa band (Fig. 1C, middle and right), consistent with the size of full-length Notch4. An apparently identical ∼250-kDa Notch4 band was reported previously as a biotinylated cell surface protein representing the unprocessed form of Notch4 (62). Consistent with this, it was reported that unprocessed form of Notch receptors is functionally expressed on the cell surface (3, 32). Neither Notch1 mRNA (Fig. 1C, left) nor protein (Fig. 1C, middle and right) was significantly affected by Notch4 siRNA (Fig. 1C). Notch1 was detected with an antibody generated against the Notch1 C terminus, which recognizes an ∼110-kDa band, representing the Notch1 intracellular domain (6, 26). As a control, α-tubulin expression was unaffected. Consistent with the mRNA analysis of Fig. 1A and B, starvation downregulated the ∼250-kDa Notch4 band, and the supplement rescued Notch4 expression (Fig. 1D); levels of Notch1 and α-tubulin expression were constant (Fig. 1D). The syntheses of both Notch4 mRNA and protein are therefore regulated via cell signaling in YSECs.
The endothelial supplement contains cortisol, EGF, FGF-2, and heparin. We tested whether these components are required for Notch4 transcription. After 24 h of starvation, YSECs were treated with different concentrations of EGF, FGF-2, or cortisol. While the combined components activated Notch4 expression to a level equivalent to that in the supplement, the individual components did not rescue expression, even at the maximal concentrations (Fig. 2A). However, cortisol combined with EGF or FGF-2 strongly induced Notch4 expression (Fig. 2A), indicating that expression requires synergism between a growth factor, either EGF or FGF-2, and cortisol. Furthermore, identical to cortisol, the highly specific synthetic glucocorticoid agonist dexamethasone (30 nM and 300 nM) synergized with FGF-2 to induce Notch4 expression in YSECs (data not shown).
Growth factor-glucocorticoid synergism reprograms Notch4 from a repressed to an active state in a multipotent cell line.
Notch4 was identified as the int3 oncogene in mouse mammary tumors (12, 14). Thereafter, Notch4 expression was described in certain nonendothelial cells (1, 22). Overexpression of constitutively active Notch4/Int3, in which the extracellular region of Notch4 is deleted, is oncogenic (13, 27, 54, 56). Based on these results, and restricted Notch4 expression by endothelial cells, we tested whether Notch4 expression is ectopically activated in nonendothelial cells and whether ectopic expression involves a mechanism similar to that of endothelial cells.
Multipotent mouse embryonic 10T1/2 cells (55, 66) can be induced to express neuronal, adipocyte, smooth muscle cell, myoblast, and osteoblast markers (7, 10, 15, 19, 37, 65). In 10T1/2 cells treated for 24 h with EGF, FGF-2, or cortisol, individually or in combinations, FGF-2/cortisol strongly activated Notch4 expression (Fig. 2B). Unlike YSECs, EGF/cortisol did not induce Notch4 expression. Unlike YSECs, which were starved prior to supplement addition, 10T1/2 cells were maintained in medium containing 10% fetal bovine serum and stimulated by replacing this medium with medium containing the indicated factors. The distinct culture conditions, or fundamental differences in signaling circuitry, might underlie the differential EGF responsiveness. Previously, we demonstrated that the supplement induces Notch4 mRNA in HeLa cells to a low level (78). The level induced in 10T1/2 cells was comparable to that of Notch4 expression in YSECs (Fig. 2B), which was considerably higher than that of HeLa cells. The supplement also strongly induced Notch4, but not Notch1, protein in 10T1/2 cells (Fig. 2C). These results indicate that ectopic FGF-2/cortisol signaling synergistically reprograms the Notch4 locus from a repressed to an active state in a multipotent progenitor cell.
Signal-dependent establishment of an endothelial cell-like histone modification pattern at the endogenous Notch4 locus in a nonendothelial cell.
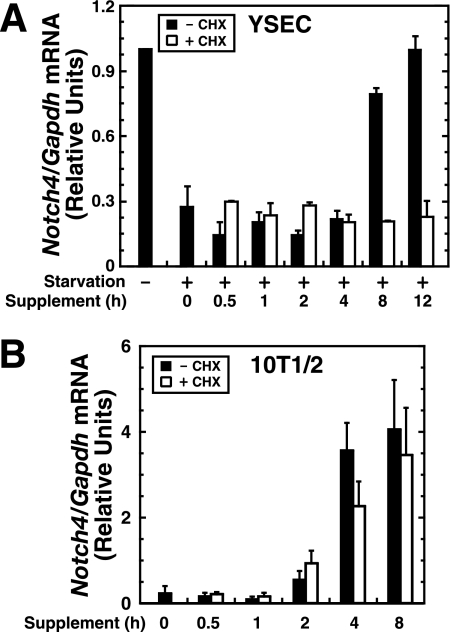
In contrast to the established mechanism in which glucocorticoids antagonize growth factor signaling, cortisol and FGF-2 synergistically activate Notch4 expression. To define the underlying mechanisms, we tested whether cortisol and growth factor signaling directly targets Notch4 or if one or both factors indirectly regulate Notch4 via induction of an intermediate protein. Such an indirect mechanism should be sensitive to protein synthesis inhibition. In YSECs starved for 24 h, supplement-dependent Notch4 transcription was maximal by 8 h. Cycloheximide abrogated Notch4 induction (Fig. 3A), indicating that protein synthesis is required. In 10T1/2 cells, a 4-h supplement treatment maximally induced Notch4 mRNA, which was cycloheximide insensitive (Fig. 3B). Thus, cortisol- and FGF-2-mediated Notch4 activation in 10T1/2 cells does not require new protein synthesis. As noted above, 10T1/2 cells were not starved prior to the supplement/2% serum addition. Thus, the selective protein synthesis requirement in YSECs could be related to the need to restore the levels of supplement/serum-sensitive factors.
FIG. 3.
Cell-type-specific modes of Notch4 transcriptional activation. (A) YSECs were starved for 24 h and treated with the supplement with or without the protein synthesis inhibitor cycloheximide (CHX) for the indicated times. Notch4 mRNA was quantitated by real-time RT-PCR and normalized to Gapdh mRNA (mean ± standard deviation for two independent experiments). (B) 10T1/2 cells were treated with the supplement with or without CHX for the indicated times. Notch4 mRNA was quantitated by real-time RT-PCR and normalized to Gapdh mRNA (mean ± standard error of the mean for three independent experiments).
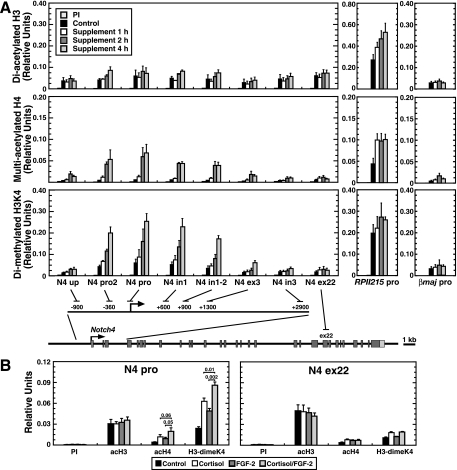
The endogenous Notch4 locus in HUVECs has a characteristic histone modification pattern, which is lacking in nonendothelial HeLa cells (78). Since cortisol/FGF-2 directly activates Notch4 in 10T1/2 cells (Fig. 3B), we tested whether cortisol and/or FGF-2 establishes a histone modification pattern resembling the HUVEC pattern. Quantitative ChIP analysis was used to measure diacetylated histone H3 (acH3), multiacetylated histone H4 (acH4), and histone H3 dimethylated at lysine 4 (H3-dimeK4) at Notch4 in 10T1/2 cells (Fig. 4). Cells were treated with the supplement for 1, 2, or 4 h, under conditions in which Notch4 transcription is maximally activated (Fig. 3B). Whereas the supplement did not affect acH3, acH4 and H3-dimeK4 were strongly induced at the promoter (N4 pro). Similar increases occurred ∼400 bp upstream (N4 pro2) and within intron 1 (N4 in1; N4 in1-2), whereas little to no changes occurred ∼900 bp upstream (N4 up) at exon 3 (N4 ex3), intron 3 (N4 in3), and exon 22 (N4 ex22) (Fig. 4A, left). In untreated 10T1/2 cells, the acH3 level was comparable to that detected previously in HUVECs (∼0.05; Fig. 4A, upper), indicating that acH3 is preestablished at the repressed Notch4 locus in 10T1/2 cells. The induced acH4 and H3-dimeK4 levels establish a pattern resembling the HUVEC pattern. The supplement did not affect epigenetic marks at the active RPII215 promoter and the repressed β-globin promoter (Fig. 4A, right).
FIG. 4.
Signal-dependent Notch4 chromatin domain activation. (A) Quantitative ChIP analysis of diacetylated histone H3 (acH3), multiacetylated histone H4 (acH4), and dimethylated histone H3 at lysine 4 (H3-dimeK4) at the Notch4 locus in 10T1/2 cells. Cells were treated with the supplement for 0, 1, 2, or 4 h. Histone modifications were analyzed at the constitutively active RPII215 promoter and erythroid cell-specific β major promoter as controls (mean ± standard error of the mean for three independent experiments). The positions of the amplicons analyzed by ChIP assay at the Notch4 locus are indicated at the bottom of the figure (dark gray boxes, exons; light gray boxes, untranslated regions). (B) ChIP analysis of histone modifications at the Notch4 promoter and exon 22 in 10T1/2 cells. Cells were treated with or without cortisol and/or FGF-2 for 4 h (mean ± standard error of the mean for three independent experiments). PI, preimmune sera; up, upstream; pro, promoter; in, intron; ex, exon.
We tested whether cortisol and FGF-2 are both required or if a single component suffices to induce acH4 and H3-dimeK4 at Notch4. 10T1/2 cells were treated with cortisol, FGF-2, or cortisol/FGF-2 (Fig. 4B). Although cortisol and FGF-2 individually elevated acH4 and H3-dimeK4 at the promoter, cortisol/FGF-2 maximally increased acH4 and H3-dimeK4 without affecting acH3 (Fig. 4B, left). AcH4 and H3-dimeK4 were constant at exon 22 (Fig. 4B, right). These results indicate that establishment of the endothelial cell-like histone modification pattern requires cortisol and FGF-2.
Signal-dependent GR/AP-1 complex assembly at the endogenous Notch4 locus.
Cortisol activates the GR, but can activate the mineralocorticoid receptor in certain contexts (11). Similar to cortisol, the highly specific GR agonist dexamethasone, which does not activate the mineralocorticoid receptor at the concentrations used, synergized with FGF-2 to activate Notch4 expression (data not shown), strongly implicating the GR. FGF-2 signals through plasma membrane FGF receptor tyrosine kinases, which activate signaling components, such as the mitogen-activated protein kinase pathway (75). A conserved AP-1 motif at the Notch4 promoter is required for endothelial cell-specific transcription (78). AP-1 subunits are induced and activated by receptor tyrosine kinase signaling (9), and FGF-2 induces and activates AP-1 subunits (48, 49). Under conditions in which supplement starvation of YSECs downregulates Notch4 expression, c-Fos, Fra-1, and JunB were also downregulated (data not shown). The cycloheximide sensitivity of Notch4 expression in YSECs (Fig. 3A) is consistent with a mechanism in which growth factor signaling is required to establish normal levels of AP-1 subunit(s) prior to signal-dependent AP-1 activation (see Fig. 8).
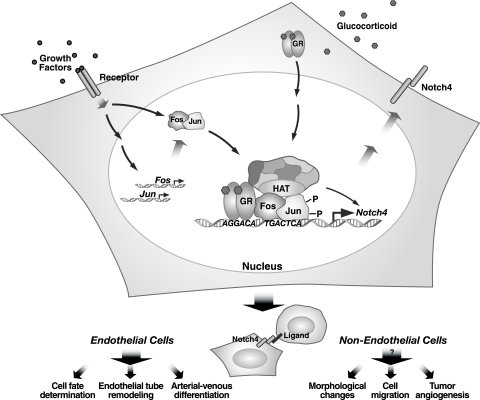
FIG. 8.
Growth factor-glucocorticoid/Notch4 signaling axis. The model depicts growth factor- and glucocorticoid-mediated synergistic activation of Notch4 transcription. The angiogenic factor FGF-2 induces genes encoding AP-1 subunits and activates AP-1 via posttranslational modifications, thereby increasing AP-1-mediated transactivation. Activated AP-1 occupies the Notch4 promoter composite response element and enhances cortisol-bound GR occupancy at the imperfect half-GRE. The AP-1/GR complex at the composite response element recruits coactivators that increase acH4 and H3-dimeK4 at restricted regions of the locus and synergistically activate Notch4 transcription. Notch4 is engaged by ligands on the surface of neighboring cells to stimulate angiogenic vascular remodeling. The integrity of this network is predicted to be crucial for establishment of physiological levels of Notch4 signaling in vascular endothelium. Since synergistic signaling reprograms the repressed Notch4 locus to an active locus in a nonendothelial cell, we hypothesize that the growth factor-glucocorticoid/Notch4 axis can be engaged in pathophysiological scenarios (e.g., in malignancies in which AP-1 and GR are deregulated, thereby ectopically activating Notch4). As deregulated Notch signaling is oncogenic, derailing the growth factor-glucocorticoid/Notch4 axis would likely contribute to tumor progression.
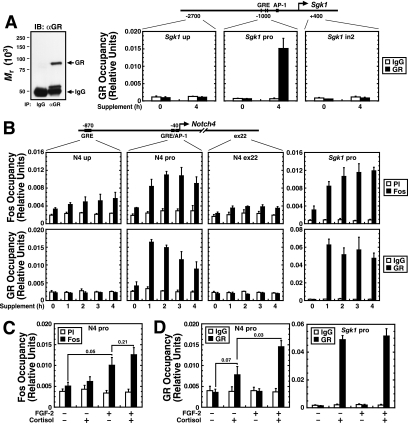
To determine whether GR and AP-1 synergistically induce Notch4 expression and to dissect the underlying mechanisms, we examined the Notch4 promoter for GRE and AP-1 motifs. The promoter region contains an AP-1 motif (78) and a consensus half-GRE, ∼40 bp and 900 bp upstream of the start site, respectively (Fig. 5B). Quantitative ChIP analysis was conducted to determine whether endogenous GR and AP-1 occupy the Notch4 locus in 10T1/2 cells. Antibodies against Fos, but not Jun, subunits, were sufficiently specific and efficacious for ChIP (data not shown) (78), and the anti-GR antibody specifically immunoprecipitated only the GR (Fig. 5A, left). The serum- and glucocorticoid-inducible kinase 1 (Sgk1) promoter, which contains three consensus GREs and an AP-1 motif and is activated by both glucocorticoid and growth factors (77), was a positive control (Fig. 5A). The supplement induced rapid Fos and GR occupancy at the Sgk1 promoter (Fig. 5A and B). At the Notch4 locus, the supplement induced Fos occupancy at the promoter by 1 h, which persisted until 4 h (Fig. 5B, upper). Little to no Fos occupancy was detected at exon 22. Similarly, only small enrichments were detected at the upstream GRE (P = 0.08; 0.12, 0.17, and 0.09 for 1-, 2-, 3-, and 4-h supplement treatments, respectively, relative to time zero), and this region lacks AP-1 motifs. No GR occupancy was detected at the upstream GRE and exon 22 with or without the supplement. However, the GR occupied the promoter region containing the AP-1 motif (Fig. 5B, lower). GR occupancy was maximal by 1 h and subsequently declined slightly. No GR occupancy was detected at other consensus GREs (at −6316, −4360, −3691, +2834, and +4138; data not shown) at the locus. Five base pairs away from the AP-1 motif resides an imperfect half-GRE (AGGACA), differing by one nucleotide from the consensus half-GRE (AGAACA) (Fig. 6A, wild-type oligonucleotide [WT oligo]). Thus, the GR might bind the imperfect half-GRE, or might be tethered to the Notch4 promoter via binding AP-1, since GR and AP-1 physically interact (28, 79).
FIG. 5.
Signal-dependent AP-1 and GR complex assembly at the Notch4 promoter. (A) The Western blot on the left demonstrates the specific immunoprecipitation (IP) of endogenous GR from 10T1/2 whole-cell lysates. Shown are the results of ChIP analysis of GR occupancy at the Sgk1 locus in 10T1/2 cells. Cells were treated with supplement for 4 h. GR was specifically recruited to the Sgk1 promoter GREs. IB, immunoblot; αGR, anti-GR antibody. (B) ChIP analysis of Fos subunits (upper) and GR (lower) occupancy at the Notch4 locus in 10T1/2 cells. Cells were treated with supplement for 0, 1, 2, 3, or 4 h. Fos or GR occupancy at the Sgk1 promoter was used as a positive control (mean ± standard error of the mean for three independent experiments). (C) ChIP analysis of Fos occupancy at the Notch4 promoter. Cells were treated with or without cortisol and/or FGF-2 for 4 h (mean ± standard error of the mean for three independent experiments). (D) ChIP analysis of GR occupancy at the Notch4 promoter (left) and Sgk1 promoter (right). Cells were treated with or without cortisol and/or FGF-2 for 1 h (mean ± standard error of the mean for three independent experiments). PI, preimmune sera; up, upstream; pro, promoter; ex, exon.
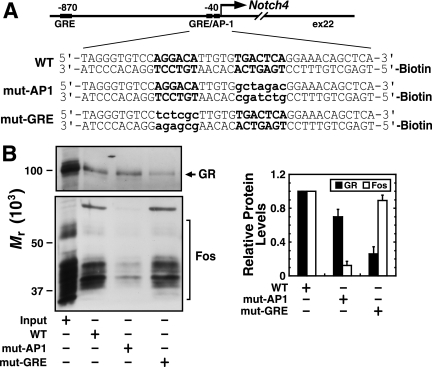
FIG. 6.
GR and AP-1 independently occupy a composite response element, consisting of an imperfect half-GRE and a neighboring AP-1 motif. (A) Sequences of 5′-biotinylated double-strand oligonucleotides used in the in vitro promoter complex assembly assay: WT, 40-bp Notch4 promoter oligonucleotides with wild-type GRE and AP-1 motifs; mut-AP1, 40-bp Notch4 promoter oligonucleotides with mutated AP-1 motif; mut-GRE, 40-bp Notch4 promoter oligonucleotides with mutated GRE site. The GRE and AP-1 motifs are indicated in uppercase boldface type. The mutated nucleotides are indicated in lowercase boldface type. (B) Western blotting analysis of proteins adsorbed to biotinylated oligonucleotides (representative pictures from at least three independent experiments). Monoclonal anti-GR antibody and polyclonal antibody against all Fos subunits were used for immunoblotting as indicated. The density of the specific bands was quantitated, and the value obtained for the WT condition was designated 1.0 (mean ± standard error of the mean for three independent experiments).
To investigate how the GR is recruited to the promoter, we asked whether AP-1 and GR occupy the promoter independently. 10T1/2 cells were treated with cortisol, FGF-2, FGF-2/cortisol. The levels of AP-1 occupancy in FGF-2- and cortisol- and FGF-2-treated cells were similar (Fig. 5C) (P = 0.21). Cortisol-mediated GR activation therefore does not affect AP-1 occupancy. Cortisol induced a twofold increase of GR occupancy, which was significantly (P = 0.03) less than that achieved with cortisol/FGF-2 (Fig. 5D, left). Thus, FGF-2 facilitates cortisol-mediated GR occupancy. Since cortisol alone induced GR occupancy at the promoter without AP-1 occupancy (Fig. 5D), this suggests that GR directly binds the promoter, inconsistent with a model in which GR is solely tethered to AP-1. However, AP-1-mediated enhancement of GR occupancy supports the notion that AP-1 stabilizes GR occupancy at the imperfect half-GRE. Importantly, AP-1 activation did not affect GR occupancy at the Sgk1 promoter containing consensus GREs (Fig. 5D, right).
We further tested whether the GR occupies the imperfect half-GRE by analyzing whether GR and AP-1 assemble on the Notch4 promoter in vitro. Double-stranded oligonucleotides containing 40 bp of the promoter (wild type [WT]), the AP-1 motif-mutated promoter (mut-AP1), and the imperfect half-GRE site-mutated promoter (mut-GRE) were biotin labeled (Fig. 6A), immobilized on streptavidin-conjugated beads, and incubated with nuclear extract from supplement-treated 10T1/2 cells. Western blotting indicated that AP-1 and GR absorbed to the WT oligonucleotide (Fig. 6B). Mutation of the AP-1 motif significantly reduced AP-1 binding (88% decrease; P = 0.002), while GR binding persisted, and mutation of the GRE significantly decreased GR binding (74% decrease; P = 0.008), while AP-1 binding persisted (Fig. 6B). These results provide further evidence that AP-1 and GR directly occupy the Notch4 promoter, and the AP-1 motif and imperfect half-GRE mediate AP-1 occupancy and GR occupancy, respectively.
A conserved composite response element, consisting of an imperfect half-GRE and an AP-1 motif, mediates synergistic activation.
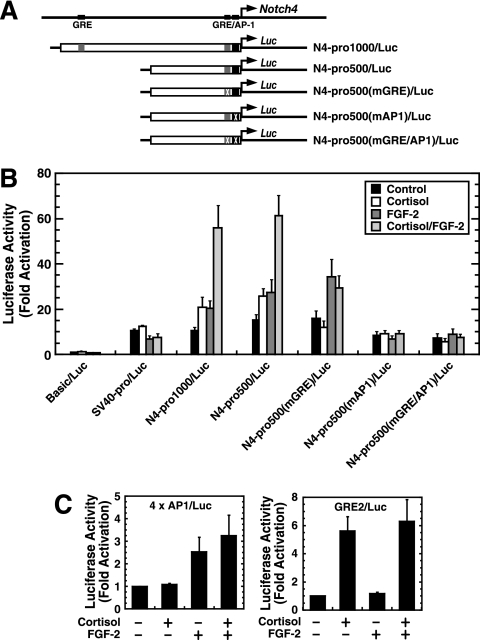
Cortisol and FGF-2 synergistically activate Notch4 transcription, and signals recruit GR and AP-1 to the Notch4 promoter imperfect half-GRE and AP-1 motif, respectively. As the Notch4 promoter has endothelial cell-specific activity that requires the AP-1 motif (78), the Notch4 reporter might have signal-dependent activity which requires these motifs. Notch4 promoter-luciferase constructs (Fig. 7A) were transiently transfected into 10T1/2 cells, and cells were treated with cortisol, FGF-2, or FGF-2/cortisol. Cortisol and/or FGF-2 did not affect activity of the promoterless reporter (Basic/Luc) nor that of the simian virus 40 (SV40) promoter reporter (SV40pro/Luc). Cortisol or FGF-2 alone activated the ∼1-kb Notch4 reporter (N4-pro1000/Luc) less than twofold (P = 0.02 and 0.002, respectively). In contrast, cortisol and FGF-2 activated N4-pro1000/Luc more than fivefold (P = 0.0015) (Fig. 7B), indicating that cortisol and FGF-2 synergistically activate the promoter. This synergism is specific to the Notch4 promoter, since cortisol and FGF-2 did not synergistically activate the AP-1 reporter (4xAP1/Luc) containing four AP-1 motifs nor the GRE reporter (GRE2/Luc) containing two GREs (Fig. 7C).
FIG. 7.
The composite response element mediates synergistic transcriptional activation. (A) Notch4 promoter luciferase reporter constructs. (B) 10T1/2 cells were transiently transfected with the following luciferase reporter constructs, respectively: pGL3/Basic vector (Basic/Luc), SV40-pro/Luc, the ∼1-kb Notch4 promoter N4-pro1000/Luc), the ∼500-bp Notch4 promoter (N4-pro500/Luc), the imperfect half-GRE-mutated Notch4 promoter [N4-pro500(mGRE)/Luc], the AP-1 motif-mutated promoter [N4-pro500(mAP1)/Luc], and the imperfect half-GRE and AP-1 motif-mutated promoter [N4-pro500(mGRE/AP1)/Luc]. Transfected cells were treated with or without cortisol and/or FGF-2 for 16 h. Luciferase activities are shown, with pGL3/Basic activity designated 1.0 (mean ± standard error of the mean for at least three independent experiments). (C) 10T1/2 cells were transiently transfected with the AP-1 reporter (4xAP1/Luc) containing four synthetic AP-1 motifs (left) or the GRE reporter (GRE2/Luc) containing two copies of the GRE sequence from the tyrosine aminotransferase gene (right). Luciferase activities are shown, with the untreated condition designated 1.0 (mean ± standard error of the mean for at least three independent experiments).
N4-pro500/Luc differed from N4-pro1000/Luc in that the upstream ∼500 bp containing the consensus GRE was deleted (Fig. 7A). Both constructs responded similarly (Fig. 7B), indicating that the consensus GRE is not required and consistent with the ChIP analysis in which GR did not occupy the consensus GRE. Mutation of the imperfect half-GRE of N4-pro500/Luc [N4-pro500(mGRE)/Luc] abolished its cortisol responsiveness, while FGF-2 responsiveness persisted (P = 0.036). FGF-2/cortisol had a similar activity to FGF-2 alone (P = 0.212) (Fig. 7B). Mutation of the AP-1 motif of N4-pro500/Luc [N4-pro500(mAP1)/Luc] abrogated its cortisol and FGF-2 responsiveness. Mutation of both the imperfect half-GRE and AP-1 motif [N4-pro500(mGRE/AP1)/Luc] yielded activity equivalent to that of N4-pro500(mAP1)/Luc (Fig. 7B).
The results of Fig. 7 indicate that cortisol and FGF-2 synergistically activate the Notch4 promoter. The imperfect half-GRE mediates the cortisol response, while the AP-1 motif mediates cortisol and FGF-2 responses. Similarly, FGF-2 alone stimulates AP-1 occupancy at the promoter, while FGF-2 significantly enhanced cortisol-mediated GR occupancy (Fig. 5C and D). Thus, mutation of the AP-1 motif abolished Notch4 promoter responsiveness to both FGF-2 and cortisol, even though the imperfect half-GRE remained intact. Conversion of the imperfect half-GRE to a perfect half-GRE yielded an activity and signal responsiveness identical to that of the wild-type construct (data not shown).
Notch4 regulation via synergistic growth factor-glucocorticoid signaling: mechanistic insights.
We describe herein growth factor and glucocorticoid synergism to establish an active Notch4 locus in endothelial and nonendothelial cells. Although Notch signaling controls numerous biological processes, mechanisms regulating Notch receptor expression are poorly understood. Previously, we implicated AP-1 in mediating endothelial cell-specific Notch4 expression, but other important factors/signals were not determined (78). As FGF-2, which activates AP-1, is a crucial proangiogenic factor that regulates vascular remodeling and tumor angiogenesis (25, 30, 76), an FGF-2-AP-1-Notch4 pathway is likely to be important in both physiological and pathophysiological contexts. Endothelial cells express FGFR1 and/or FGFR2, and FGF-2 signaling modulates diverse endothelial cell functions, including proliferation, migration, morphology, and transcription (25). Glucocorticoids exert extensive physiological and pathological activities, including the direct regulation of endothelial cell functions (16, 17, 40, 53, 68). It was reported that cortisol induced Notch1 and Notch2, but not Notch4, mRNA in osteoblast MC3T3 cells (52). However, mechanisms of cortisol-induced Notch1 and Notch2 expression are unknown. Furthermore, during dexamethasone-induced osteoblast differentiation, Notch1 and Notch2 mRNAs are downregulated in differentiating cells, whereas Notch4 mRNA was slightly upregulated (58). It is unclear whether the apparent regulation of Notch receptor expression in these systems represents a direct action of glucocorticoids or is secondary to osteoblast differentiation. Cortisol did not induce Notch1 expression in the endothelial cells that we have studied (Fig. 1B), and cortisol alone is insufficient to induce Notch4 expression (Fig. 2A).
Almost all reports of GR-AP-1 interactions involve GR antagonism of AP-1-mediated transactivation (28, 60, 67, 79), and this mechanism is important for glucocorticoid anti-inflammatory activity. The GR binds AP-1, preventing AP-1 DNA binding, or the GR-AP-1 complex recruits corepressors at a target site. In a transient transfection assay with synthetic reporter constructs, the GR potentiates AP-1-mediated transcription, dependent upon AP-1 subunit composition and the distance between response elements (8, 18, 47, 51). GR positively regulates AP-1-mediated transactivation on a 25-bp composite response element, consisting of a GRE and an AP-1 motif, but only when the Jun level exceeds that of Fos (8). When the center-to-center distance between the GRE and the AP-1 motif is >26 bp, the GR positively regulates both Fos- and Jun-mediated transcription (51). At the Notch4 promoter, the composite response element that mediates synergism consists of an imperfect half-GRE and an AP-1 motif with a 5-bp spacer, which is the first example that GR and AP-1 synergistically activate an endogenous locus.
Our results indicate that growth factor/glucocorticoid signaling converges upon AP-1 and GR to synergistically activate Notch4 transcription. Activation is mediated by a promoter-localized imperfect half-GRE and a neighboring AP-1 motif. The imperfect half-GRE is insufficient for maximal GR occupancy and function when AP-1 is not promoter bound, and AP-1 enhances GR occupancy (Fig. 5D) and transcriptional activation (Fig. 7B). In aggregate (Fig. 8), the results support a model in which AP-1 stabilizes GR at the imperfect half-GRE, which constitutes a novel mode of GR function. It will be of considerable interest to determine whether perturbations of the growth factor-glucocorticoid axis deregulate Notch4 expression in pathological states, such as tumor angiogenesis, and ectopically activate Notch4 in cells that normally lack the capacity to respond to Notch4 ligand-expressing cells in the microenvironment.
Acknowledgments
We acknowledge support from NIH grant DK50107 (E.H.B.) and an American Heart Association Predoctoral Fellowship (J.W.).
We thank Soumen Paul, Kirby Johnson, and Shin-Il Kim for critical comments. We thank Bob Auerbach for YSECs and Alan Rapraeger for FGF-2.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Ando, K., S. Kanazawa, T. Tetsuka, S. Ohta, X. Jiang, T. Tada, M. Kobayashi, N. Matsui, and T. Okamoto. 2003. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene 22:7796-7803. [DOI] [PubMed] [Google Scholar]
- 2.Bresnick, E. H., F. C. Dalman, E. R. Sanchez, and W. B. Pratt. 1989. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem. 264:4992-4997. [PubMed] [Google Scholar]
- 3.Bush, G., G. diSibio, A. Miyamoto, J. B. Denault, R. Leduc, and G. Weinmaster. 2001. Ligand-induced signaling in the absence of furin processing of Notch1. Dev. Biol. 229:494-502. [DOI] [PubMed] [Google Scholar]
- 4.Carlson, T. R., Y. Yan, X. Wu, M. T. Lam, G. L. Tang, L. J. Beverly, L. M. Messina, A. J. Capobianco, Z. Werb, and R. Wang. 2005. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc. Natl. Acad. Sci. USA 102:9884-9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi, J. T., H. Y. Chang, G. Haraldsen, F. L. Jahnsen, O. G. Troyanskaya, D. S. Chang, Z. Wang, S. G. Rockson, M. van de Rijn, D. Botstein, and P. O. Brown. 2003. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 100:10623-10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu, J., and E. H. Bresnick. 2004. Evidence that C promoter-binding factor 1 binding is required for Notch-1-mediated repression of activator protein-1. J. Biol. Chem. 279:12337-12345. [DOI] [PubMed] [Google Scholar]
- 7.Davis, R. L., H. Weintraub, and A. B. Lassar. 1987. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51:987-1000. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, M. I., J. N. Miner, S. K. Yoshinaga, and K. R. Yamamoto. 1990. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249:1266-1272. [DOI] [PubMed] [Google Scholar]
- 9.Eferl, R., and E. F. Wagner. 2003. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3:859-868. [DOI] [PubMed] [Google Scholar]
- 10.Farah, M. H., J. M. Olson, H. B. Sucic, R. I. Hume, S. J. Tapscott, and D. L. Turner. 2000. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127:693-702. [DOI] [PubMed] [Google Scholar]
- 11.Funder, J. W., P. T. Pearce, R. Smith, and A. I. Smith. 1988. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242:583-585. [DOI] [PubMed] [Google Scholar]
- 12.Gallahan, D., and R. Callahan. 1997. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4). Oncogene 14:1883-1890. [DOI] [PubMed] [Google Scholar]
- 13.Gallahan, D., C. Jhappan, G. Robinson, L. Hennighausen, R. Sharp, E. Kordon, R. Callahan, G. Merlino, and G. H. Smith. 1996. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res. 56:1775-1785. [PubMed] [Google Scholar]
- 14.Gallahan, D., C. Kozak, and R. Callahan. 1987. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J. Virol. 61:218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazit, D., R. Ebner, A. J. Kahn, and R. Derynck. 1993. Modulation of expression and cell surface binding of members of the transforming growth factor-beta superfamily during retinoic acid-induced osteoblastic differentiation of multipotential mesenchymal cells. Mol. Endocrinol. 7:189-198. [DOI] [PubMed] [Google Scholar]
- 16.Gloddek, J., U. Pagotto, M. Paez Pereda, E. Arzt, G. K. Stalla, and U. Renner. 1999. Pituitary adenylate cyclase-activating polypeptide, interleukin-6 and glucocorticoids regulate the release of vascular endothelial growth factor in pituitary folliculostellate cells. J. Endocrinol. 160:483-490. [DOI] [PubMed] [Google Scholar]
- 17.Hafezi-Moghadam, A., T. Simoncini, Z. Yang, F. P. Limbourg, J. C. Plumier, M. C. Rebsamen, C. M. Hsieh, D. S. Chui, K. L. Thomas, A. J. Prorock, V. E. Laubach, M. A. Moskowitz, B. A. French, K. Ley, and J. K. Liao. 2002. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 8:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison, R. J., G. P. McNeil, and P. R. Dobner. 1995. Synergistic activation of neurotensin/neuromedin N gene expression by c-Jun and glucocorticoids: novel effects of Fos family proteins. Mol. Endocrinol. 9:981-993. [DOI] [PubMed] [Google Scholar]
- 19.Hirschi, K. K., S. A. Rohovsky, and P. A. D'Amore. 1998. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 141:805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh, J. J.-D., T. Henkel, P. Salmon, E. Robey, M. G. Peterson, and S. D. Hayward. 1996. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol. Cell. Biol. 16:952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Im, H., J. A. Grass, K. D. Johnson, M. E. Boyer, J. Wu, and E. H. Bresnick. 2004. Measurement of protein-DNA interactions in vivo by chromatin immunoprecipitation. Methods Mol. Biol. 284:129-146. [DOI] [PubMed] [Google Scholar]
- 22.Imatani, A., and R. Callahan. 2000. Identification of a novel NOTCH-4/INT-3 RNA species encoding an activated gene product in certain human tumor cell lines. Oncogene 19:223-231. [DOI] [PubMed] [Google Scholar]
- 23.Iso, T., Y. Hamamori, and L. Kedes. 2003. Notch signaling in vascular development. Arterioscler. Thromb. Vasc. Biol. 23:543-553. [DOI] [PubMed] [Google Scholar]
- 24.Jarriault, S., C. Brou, F. Logeat, E. H. Schroeter, R. Kopan, and A. Israel. 1995. Signalling downstream of activated mammalian Notch. Nature 377:355-358. [DOI] [PubMed] [Google Scholar]
- 25.Javerzat, S., P. Auguste, and A. Bikfalvi. 2002. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 8:483-489. [DOI] [PubMed] [Google Scholar]
- 26.Jeffries, S., and A. J. Capobianco. 2000. Neoplastic transformation by Notch requires nuclear localization. Mol. Cell. Biol. 20:3928-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhappan, C., D. Gallahan, C. Stahle, E. Chu, G. H. Smith, G. Merlino, and R. Callahan. 1992. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 6:345-355. [DOI] [PubMed] [Google Scholar]
- 28.Jonat, C., H. J. Rahmsdorf, K. K. Park, A. C. Cato, S. Gebel, H. Ponta, and P. Herrlich. 1990. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62:1189-1204. [DOI] [PubMed] [Google Scholar]
- 29.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 30.Kandel, J., E. Bossy-Wetzel, F. Radvanyi, M. Klagsbrun, J. Folkman, and D. Hanahan. 1991. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell 66:1095-1104. [DOI] [PubMed] [Google Scholar]
- 31.Karin, M., and L. Chang. 2001. AP-1-glucocorticoid receptor crosstalk taken to a higher level. J. Endocrinol. 169:447-451. [DOI] [PubMed] [Google Scholar]
- 32.Kidd, S., and T. Lieber. 2002. Furin cleavage is not a requirement for Drosophila Notch function. Mech. Dev. 115:41-51. [DOI] [PubMed] [Google Scholar]
- 33.Kimble, J., and P. Simpson. 1997. The LIN-12/Notch signaling pathway and its regulation. Annu. Rev. Cell Dev. Biol. 13:333-361. [DOI] [PubMed] [Google Scholar]
- 34.Konig, H., H. Ponta, H. J. Rahmsdorf, and P. Herrlich. 1992. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 11:2241-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebs, L. T., Y. Xue, C. R. Norton, J. R. Shutter, M. Maguire, J. P. Sundberg, D. Gallahan, V. Closson, J. Kitajewski, R. Callahan, G. H. Smith, K. L. Stark, and T. Gridley. 2000. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 14:1343-1352. [PMC free article] [PubMed] [Google Scholar]
- 36.Kurooka, H., and T. Honjo. 2000. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 275:17211-17220. [DOI] [PubMed] [Google Scholar]
- 37.Lassar, A. B., B. M. Paterson, and H. Weintraub. 1986. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell 47:649-656. [DOI] [PubMed] [Google Scholar]
- 38.Lawson, N. D., N. Scheer, V. N. Pham, C. H. Kim, A. B. Chitnis, J. A. Campos-Ortega, and B. M. Weinstein. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128:3675-3683. [DOI] [PubMed] [Google Scholar]
- 39.Leong, K. G., X. Hu, L. Li, M. Noseda, B. Larrivée, C. Hull, L. Hood, F. Wong, and A. Karsan. 2002. Activated Notch4 inhibits angiogenesis: role of β1-integrin activation. Mol. Cell. Biol. 22:2830-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limbourg, F. P., Z. Huang, J. C. Plumier, T. Simoncini, M. Fujioka, J. Tuckermann, G. Schutz, M. A. Moskowitz, and J. K. Liao. 2002. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J. Clin. Investig. 110:1729-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, Z.-J., T. Shirakawa, Y. Li, A. Soma, M. Oka, G. P. Dotto, R. M. Fairman, O. C. Velazquez, and M. Herlyn. 2003. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell. Biol. 23:14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loomes, K. M., D. B. Taichman, C. L. Glover, P. T. Williams, J. E. Markowitz, D. A. Piccoli, H. S. Baldwin, and R. J. Oakey. 2002. Characterization of Notch receptor expression in the developing mammalian heart and liver. Am. J. Med. Genet. 112:181-189. [DOI] [PubMed] [Google Scholar]
- 43.Lu, L. S., S. J. Wang, and R. Auerbach. 1996. In vitro and in vivo differentiation into B cells, T cells, and myeloid cells of primitive yolk sac hematopoietic precursor cells expanded >100-fold by coculture with a clonal yolk sac endothelial cell line. Proc. Natl. Acad. Sci. USA 93:14782-14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, N. Z., and J. A. Cidlowski. 2005. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 18:331-342. [DOI] [PubMed] [Google Scholar]
- 45.MacKenzie, F., P. Duriez, B. Larrivee, L. Chang, I. Pollet, F. Wong, C. Yip, and A. Karsan. 2004. Notch4-induced inhibition of endothelial sprouting requires the ankyrin repeats and involves signaling through RBP-Jkappa. Blood 104:1760-1768. [DOI] [PubMed] [Google Scholar]
- 46.Matsunami, N., Y. Hamaguchi, Y. Yamamoto, K. Kuze, K. Kangawa, H. Matsuo, M. Kawaichi, and T. Honjo. 1989. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature 342:934-937. [DOI] [PubMed] [Google Scholar]
- 47.Miner, J. N., and K. R. Yamamoto. 1992. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 6:2491-2501. [DOI] [PubMed] [Google Scholar]
- 48.Mohan, R., J. Sivak, P. Ashton, L. A. Russo, B. Q. Pham, N. Kasahara, M. B. Raizman, and M. E. Fini. 2000. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J. Biol. Chem. 275:10405-10412. [DOI] [PubMed] [Google Scholar]
- 49.Newberry, E. P., D. Willis, T. Latifi, J. M. Boudreaux, and D. A. Towler. 1997. Fibroblast growth factor receptor signaling activates the human interstitial collagenase promoter via the bipartite Ets-AP1 element. Mol. Endocrinol. 11:1129-1144. [DOI] [PubMed] [Google Scholar]
- 50.Nofziger, D., A. Miyamoto, K. M. Lyons, and G. Weinmaster. 1999. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126:1689-1702. [DOI] [PubMed] [Google Scholar]
- 51.Pearce, D., W. Matsui, J. N. Miner, and K. R. Yamamoto. 1998. Glucocorticoid receptor transcriptional activity determined by spacing of receptor and nonreceptor DNA sites. J. Biol. Chem. 273:30081-30085. [DOI] [PubMed] [Google Scholar]
- 52.Pereira, R. M., A. M. Delany, D. Durant, and E. Canalis. 2002. Cortisol regulates the expression of Notch in osteoblasts. J. Cell. Biochem. 85:252-258. [DOI] [PubMed] [Google Scholar]
- 53.Pitzalis, C., N. Pipitone, and M. Perretti. 2002. Regulation of leukocyte-endothelial interactions by glucocorticoids. Ann. N. Y. Acad. Sci. 966:108-118. [DOI] [PubMed] [Google Scholar]
- 54.Raafat, A., S. Bargo, M. R. Anver, and R. Callahan. 2004. Mammary development and tumorigenesis in mice expressing a truncated human Notch4/Int3 intracellular domain (h-Int3sh). Oncogene 23:9401-9407. [DOI] [PubMed] [Google Scholar]
- 55.Reznikoff, C. A., J. S. Bertram, D. W. Brankow, and C. Heidelberger. 1973. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 33:3239-3249. [PubMed] [Google Scholar]
- 56.Robbins, J., B. J. Blondel, D. Gallahan, and R. Callahan. 1992. Mouse mammary tumor gene int-3: a member of the notch gene family transforms mammary epithelial cells. J. Virol. 66:2594-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossant, J., and L. Howard. 2002. Signaling pathways in vascular development. Annu. Rev. Cell Dev. Biol. 18:541-573. [DOI] [PubMed] [Google Scholar]
- 58.Schnabel, M., I. Fichtel, L. Gotzen, and J. Schlegel. 2002. Differential expression of Notch genes in human osteoblastic cells. Int. J. Mol. Med. 9:229-232. [PubMed] [Google Scholar]
- 59.Schroeter, E. H., J. A. Kisslinger, and R. Kopan. 1998. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393:382-386. [DOI] [PubMed] [Google Scholar]
- 60.Schule, R., P. Rangarajan, S. Kliewer, L. J. Ransone, J. Bolado, N. Yang, I. M. Verma, and R. M. Evans. 1990. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 62:1217-1226. [DOI] [PubMed] [Google Scholar]
- 61.Selkoe, D., and R. Kopan. 2003. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 26:565-597. [DOI] [PubMed] [Google Scholar]
- 62.Shawber, C. J., I. Das, E. Francisco, and J. Kitajewski. 2003. Notch signaling in primary endothelial cells. Ann. N. Y. Acad. Sci. 995:162-170. [DOI] [PubMed] [Google Scholar]
- 63.Swiatek, P. J., C. E. Lindsell, F. F. del Amo, G. Weinmaster, and T. Gridley. 1994. Notch1 is essential for postimplantation development in mice. Genes Dev. 8:707-719. [DOI] [PubMed] [Google Scholar]
- 64.Taylor, K. L., A. M. Henderson, and C. C. Hughes. 2002. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc. Res. 64:372-383. [DOI] [PubMed] [Google Scholar]
- 65.Taylor, S. M., and P. A. Jones. 1979. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17:771-779. [DOI] [PubMed] [Google Scholar]
- 66.Terzaghi, M., and J. B. Little. 1975. Repair of potentially lethal radiation damage in mammalian cells is associated with enhancement of malignant transformation. Nature 253:548-549. [DOI] [PubMed] [Google Scholar]
- 67.Tuckermann, J. P., H. M. Reichardt, R. Arribas, K. H. Richter, G. Schutz, and P. Angel. 1999. The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J. Cell Biol. 147:1365-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ullian, M. E. 1999. The role of corticosteroids in the regulation of vascular tone. Cardiovasc. Res. 41:55-64. [DOI] [PubMed] [Google Scholar]
- 69.Uyttendaele, H., V. Closson, G. Wu, F. Roux, G. Weinmaster, and J. Kitajewski. 2000. Notch4 and Jagged-1 induce microvessel differentiation of rat brain endothelial cells. Microvasc. Res. 60:91-103. [DOI] [PubMed] [Google Scholar]
- 70.Uyttendaele, H., J. Ho, J. Rossant, and J. Kitajewski. 2001. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc. Natl. Acad. Sci. USA 98:5643-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uyttendaele, H., G. Marazzi, G. Wu, Q. Yan, D. Sassoon, and J. Kitajewski. 1996. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122:2251-2259. [DOI] [PubMed] [Google Scholar]
- 72.Villa, N., L. Walker, C. E. Lindsell, J. Gasson, M. L. Iruela-Arispe, and G. Weinmaster. 2001. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 108:161-164. [DOI] [PubMed] [Google Scholar]
- 73.Vincent, L., P. Albanese, H. Bompais, G. Uzan, J. P. Vannier, P. G. Steg, J. Soria, and C. Soria. 2003. Insights in the molecular mechanisms of the anti-angiogenic effect of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Thromb. Haemost. 89:530-537. [PubMed] [Google Scholar]
- 74.Wallberg, A. E., K. Pedersen, U. Lendahl, and R. G. Roeder. 2002. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by Notch intracellular domains in vitro. Mol. Cell. Biol. 22:7812-7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, J.-K., G. Gao, and M. Goldfarb. 1994. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol. Cell. Biol. 14:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, Y., and D. Becker. 1997. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat. Med. 3:887-893. [DOI] [PubMed] [Google Scholar]
- 77.Webster, M. K., L. Goya, Y. Ge, A. C. Maiyar, and G. L. Firestone. 1993. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 13:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu, J., F. Iwata, J. A. Grass, C. S. Osborne, L. Elnitski, P. Fraser, O. Ohneda, M. Yamamoto, and E. H. Bresnick. 2005. Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol. Cell. Biol. 25:1458-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang-Yen, H. F., J. C. Chambard, Y. L. Sun, T. Smeal, T. J. Schmidt, J. Drouin, and M. Karin. 1990. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62:1205-1215. [DOI] [PubMed] [Google Scholar]