Abstract
Telomeres are key structural elements for the protection and maintenance of linear chromosomes, and they function to prevent recognition of chromosomal ends as DNA double-stranded breaks. Loss of telomere capping function brought about by telomerase deficiency and gradual erosion of telomere ends or by experimental disruption of higher-order telomere structure culminates in the fusion of defective telomeres and/or the activation of DNA damage checkpoints. Previous work has implicated the nonhomologous end-joining (NHEJ) DNA repair pathway as a critical mediator of these biological processes. Here, employing the telomerase-deficient mouse model, we tested whether the NHEJ component DNA-dependent protein kinase catalytic subunit (DNA-PKcs) was required for fusion of eroded/dysfunctional telomere ends and the telomere checkpoint responses. In late-generation mTerc−/− DNA-PKcs−/− cells and tissues, chromosomal end-to-end fusions and anaphase bridges were readily evident. Notably, nullizygosity for DNA Ligase4 (Lig4)—an additional crucial NHEJ component—was also permissive for chromosome fusions in mTerc−/− cells, indicating that, in contrast to results seen with experimental disruption of telomere structure, telomere dysfunction in the context of gradual telomere erosion can engage additional DNA repair pathways. Furthermore, we found that DNA-PKcs deficiency does not reduce apoptosis, tissue atrophy, or p53 activation in late-generation mTerc−/− tissues but rather moderately exacerbates germ cell apoptosis and testicular degeneration. Thus, our studies indicate that the NHEJ components, DNA-PKcs and Lig4, are not required for fusion of critically shortened telomeric ends and that DNA-PKcs is not required for sensing and executing the telomere checkpoint response, findings consistent with the consensus view of the limited role of DNA-PKcs in DNA damage signaling in general.
Telomeres are key structural elements for the protection and maintenance of linear chromosomes that are comprised of a series of repetitive DNA sequence elements (TTAGGG in humans) and an array of sequence-specific and -nonspecific DNA binding proteins that create a higher-order chromatin structure (21). Telomeres serve various cellular functions, including the prevention of recognition of the linear end as a DNA double-strand break (DSB) (20, 49, 85); the modulation of local chromatin structure, which influences the expression of those genes proximal to chromosome ends (5, 66, 67); and the regulation of telomere maintenance enzymes and their accessibility (21). The last function is crucial, given the end replication problem that plagues conventional DNA synthesis (65, 90). Telomere sequences can be added by telomerase, a specialized reverse transcriptase that adds telomere repeat DNA to the ends of chromosomes, thereby restoring sequences lost after RNA primer removal and end processing following DNA replication (7, 53). Human cells in culture that divide in the absence of telomerase experience telomere attrition on the order of 50 to 100 bp per cell division (43, 87). Further, mice deficient in telomerase experience approximately 5 kb of telomere erosion with every generational intercross to a point at which denuded telomeres are recognized as damaged DNA, participate in end-to-end fusions, and cause progressive degeneration of rapidly dividing tissues (8, 57).
Nonhomologous end joining (NHEJ) is a critical pathway responsible for the repair of DNA DSBs in a wide range of organisms (46). In mammals, NHEJ is mediated primarily by the DNA-dependent protein kinase (DNA-PK) complex, comprising the Ku subunits (Ku70 and Ku86), the Lig4-XRCC4 complex, Artemis, the DNA-PK catalytic subunit (DNA-PKcs), and the recently described component XRCC4-like factor (XLF)/Cernunnos (2, 13, 58). Cells and organisms deficient for any one of these proteins exhibit defective DNA DSB repair and, accordingly, are highly sensitive to DNA DSB-inducing agents, such as ionizing radiation. In addition, mammals rely on this repair pathway to generate the immune repertoire of the adaptive immune system, whereby NHEJ proteins function during V(D)J recombination to repair the RAG-mediated DNA DSBs (74). As such, mutations affecting NHEJ are associated with severe immunodeficiency in mice, humans, horses, and dogs (68, 74). The prominent role of the NHEJ pathway in DNA DSB repair also serves to maintain genome integrity, as evidenced by the presence of marked chromosomal rearrangements and the cancer predisposition of various NHEJ mutant mouse strains, particularly in the context of mutant p53 (18, 22, 30, 34, 39, 64, 75, 80, 89, 94). Lastly, deficiencies in various NHEJ components also engender accelerated senescence in cultured cells (23, 30) and segmental aging phenotypes on the whole-animal level (27, 30, 44, 89).
Recent work has also described an increasingly complex relationship between NHEJ and telomeres (60). At first glance, telomeres might appear to be ideal substrates for NHEJ activities by nature of their similarity to internal chromosomal DNA DSBs. However, the unique physical structure of the telomere, designated the t-loop (38), appears to prevent the native end of chromosomes from being recognized as aberrant DNA damage (20, 21, 49, 85). Indeed, experiments using mechanisms that disrupt the chromatin structure of telomeres (for example, via expression of dominant negative TRF2) (86) produce telomeres that are substrates for the NHEJ machinery, which subsequently creates interchromosomal fusions that can retain substantial telomere DNA repeats (81). These results corroborate the finding that LIG4-mediated NHEJ creates telomere fusions in Tel1- and Mec1-deficient strains of Saccharomyces cerevisiae (61), as well as in Arabidopsis thaliana, where Ku70 plays at least some role in fusing critically shortened telomeres (45). Further, experiments conducted with NHEJ mutant mice implicate this pathway as a critical mediator of the telomere response when telomeres are eroded beyond their minimum functional length (24, 25). Specifically, in the mTerc−/− mouse model, DNA-PKcs and Ku86 were reported to be required for the creation of the hallmark end-to-end fusions that are devoid of telomere sequences at their junction as a result of telomere attrition (24, 25). Additionally, in the context of these studies, these proteins have been reported to facilitate the induction of the p53-dependent apoptotic response in telomere-eroded tissues, such as the intestine and the gonads (24, 25). Telomere attrition and the presence of telomere signal-free ends is increased in the combined absence of NHEJ and telomerase in mice (24, 25), although it remains unclear whether the accelerated telomere attrition seen in these contexts is a direct result of capping function or is from the increased cellular turnover required to maintain tissue cellularity and function in these genetic backgrounds.
In counterpoint to these studies, much work has also conveyed that, rather than functioning solely in response to telomere dysfunction, NHEJ proteins play an important role in normal telomere maintenance (29, 60). In the yeast S. cerevisiae, the Ku subunit binds to the telomerase RNA, perhaps to facilitate normal and de novo telomere access to telomerase (37, 83). Ku mutants in both S. cerevisiae and Schizosaccharomyces pombe have shorter but stable telomeres (4, 12, 69), further implicating the Ku complex in normal telomere maintenance. Mammalian cells also appear to depend on activities of the NHEJ complex for normal telomere maintenance. For instance, cells derived from Ku70, Ku86, Artemis, and DNA-PKcs knockout mice and from DNA-PKcs point mutant (SCID) mice harbor chromosome end-to-end fusions that retain telomere sequences and show increased genomic instability, implicating NHEJ proteins in telomere capping (3, 35, 36, 52, 73, 78). Ku proteins can bind to telomere repeats in vitro (6) and can be localized to telomeric DNA in cells via chromatin immunoprecipitation, and Ku deficiency results in shortened telomeres (19, 51); further, Ku has been reported to bind to telomere repeat binding proteins TRF1 and TRF2 (52, 82). On the other hand, Ku70 deficiency in other species, including chickens and plant species, had either no effect on telomere length (91) or resulted in dramatic telomere lengthening (14, 72). Thus, the impact of NHEJ deficiency on telomere dynamics appears to vary in a manner dependent upon the specific NHEJ mutation, species, and experimental context, and the precise roles of these factors in telomere length regulation continue to be areas of active investigation (19, 24, 35, 36, 42, 78).
Given the range of functions and complex phenotypes associated with the core NHEJ components in telomere biology, we sought to understand the genetic interactions of NHEJ and telomeres in the response to physiological telomere attrition in vivo by generating and characterizing mTerc−/− mice and cells that are also null for DNA-PKcs or Lig4. Our results indicate that classical NHEJ is dispensable for the telomere erosion-dependent chromosome fusions and, further, that DNA-PKcs has no impact on the apoptosis response induced by telomere erosion in multiple organ compartments. The results of this study stand in sharp contrast to the proposed roles attributed to the NHEJ complex, including DNA-PKcs, in the telomerase-deficient mouse (26). Furthermore, the strict requirement of NHEJ in the repair of dysfunctional telomeres generated by acute experimental disruption of telomere binding proteins (80) versus the dispensability of NHEJ in the fusion of dysfunctional telomeres arising in the setting of telomerase deficiency and telomere erosion indicates that physiological telomere attrition can engage non-NHEJ pathways to resolve dysfunctional telomeres.
MATERIALS AND METHODS
Mice.
mTerc, DNA-PKcs, and Lig4 mutant mouse strains have been described in detailed elsewhere and represent null alleles in each case (8, 31, 33). The mTerc- and Lig4-deficient mouse cross was also maintained with p53 heterozygosity, as has been described previously, in order to provide for enhanced survival of Lig4−/− cells (30). Mice heterozygous for each null allele (mTerc+/− or DNA-PKcs+/− or Lig4+/− mice, designated G0) were intercrossed to produce first-generation (G1) mTerc-null mice that were the wild type or a heterozygous mutant for either DNA-PKcs or Lig4. These mice were intercrossed to produce second-generation telomerase-deficient mice (G2) and so on as described previously. Organs from harvested mice were fixed in 10% neutral-buffered formalin and embedded in paraffin. Apoptotic and anaphase bridging indices, correlates of the degree of telomere dysfunction as defined previously (77), were determined from 5-μm-thick sections of fixed tissues stained with hematoxylin and eosin (H&E).
Cell culture and cytogenetic and phenotypic analysis.
Day 13.5 embryos were harvested, and mouse embryonic fibroblasts (MEFs) were isolated by standard methods. Ear fibroblasts were isolated as described previously (79). Whole bone marrow cells were flushed from tibias and femurs with a 25-gauge needle and grown in RPMI-10% fetal calf serum-50 μM beta-mercaptoethanol with the addition of a 1/10 volume of MethoCult M3434 (Stem Cell Technologies, Vancouver, Canada). Cultures were grown for 2 days prior to harvesting for metaphase analyses. MEFs and ear fibroblasts were cultured in the presence of KaryoMAX Colcemid solution for 5 h prior to harvesting, and bone marrow cells were incubated for 2 to 3 h. For quantification of chromatin bridges in ear fibroblast cultures, a chromatin bridge was defined as an observable 4′,6′-diamidino-2-phenylindole (DAPI) staining continuity between two distinct (typically postmitotic) nuclei. Telomere fluorescence in situ hybridization (FISH) was performed as described previously (8). At least twenty metaphases from harvested cell cultures were analyzed for telomere integrity by telomere FISH. For apoptosis assays, sections from paraffin-embedded testes were deparaffinized and processed for apoptotic staining (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling [TUNEL]) according to the manufacturer's instructions (Chemicon, Temucula, CA). p53 immunostaining was performed following antigen retrieval in citrate buffer with CM5 antibody (Vector, Burlingame, CA). For immunofluorescence studies, early passage MEFs were fixed in 3.5% paraformaldehyde in phosphate-buffered saline for 10 min, permeabilized (50 mM NaCl, 3 mM MgCl2, 200 mM sucrose, 10 mM HEPES [pH 7.9], 0.5% TX-100) for 5 min, and then stained with primary antibodies against 53BP1 (Bethyl, Montgomery, TX) and phospho-serine-1981 Atm (Rockland, Gilbertsville, PA) and fluorescence isothiocyanate anti-rabbit and rhodamine anti-mouse secondary antibodies (Rockland). Cells were counterstained with DAPI before being mounted in antifade solution (90% glycerol, 0.1 M Tris-HCl [pH 8.0], and 2.3% 1,4-diazabicyclo[2.2.2]octane [DABCO]). Foci were scored by eye from a minimum of 120 randomly chosen nuclei by using a 60× objective, and scoring was performed in a blinded manner with respect to genotype. Immunofluorescence images were captured in grayscale for each fluorophore and were merged by compilation in respective red-green-blue (RGB) channels using Adobe Photoshop CS 8.0. Student's t test and Fisher's exact test were used to determine significance.
RESULTS
Increased genomic instability in DNA-PKcs mutant cells with telomere dysfunction.
To study the impact of DNA-PKcs deficiency on the chromosome fusion and cellular apoptotic responses associated with telomere dysfunction in the mouse, a series of cohorts were generated with various telomere lengths and functions by breeding the DNA-PKcs-null allele (33) through successive generations of mTerc−/− mice (8, 57) (see Materials and Methods). Several different primary cell cultures were derived from the various mouse cohorts to evaluate telomere and chromosomal integrity in relation to mTerc and DNA-PKcs status.
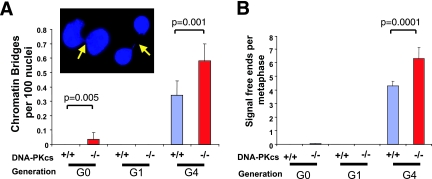
As a first step, we measured the presence of chromatin bridges in ear fibroblast cell cultures as a proxy for telomere dysfunction-associated genomic instability that manifests in the form of chromosome fusions and anaphase bridges. Primary ear fibroblasts derived from mTerc+/+ or mTerc+/− (designated G0) and first-generation (G1) mTerc−/− mice that were either the wild type or null for DNA-PKcs harbored few chromatin bridges, although a small but significant number were detected in G0 DNA-PKcs−/− cells, an observation in line with previous reports linking DNA-PKcs deficiency to elevated anaphase bridging (36) (Fig. 1). Consistent with the notion that telomere dysfunction engenders chromosome instability, cells derived from G4 mTerc−/− DNA-PKcs+/+ mice harbored substantial levels of chromatin bridges between cells (0.35 bridges per 100 nuclei) (Fig. 1A) and, upon the addition of DNA-PKcs deficiency, these littermate control cultures exhibited a significant increase in chromatin bridges (0.6 per 100 cells; P = 0.001) (Fig. 1A). These results suggested that loss of DNA-PKcs function results in moderately increased genomic instability in the background of telomerase deficiency.
FIG. 1.
Late-generation (G4) mTerc−/− DNA-PKcs−/− mice harbor genomic instability and telomere dysfunction. (A) Chromatin bridges between nuclei were scored in cultures of ear fibroblasts derived from at least three individual mice of the indicated genotypes. At least 1,000 nuclei were scored from each cell line. The ratio is presented as an average incidence per 100 nuclei. A representative image of chromatin bridges between two sets of two nuclei from a G4 mTerc−/− DNA-PKcs−/− culture is presented in the inset. (B) Telomere signal-free ends were scored on metaphases from at least three independently derived bone marrow cultures from mice of the indicated genotypes.
NHEJ components DNA-PKcs and Lig4 are not required for the generation of chromosomal fusions in mTerc−/− cells with telomere dysfunction.
Metaphase spreads of various types of primary cell cultures were examined to assess telomere reserves and to characterize the types of genomic instability. Telomere FISH documented increased telomere signal-free ends in G4 mTerc−/− DNA-PKcs−/− metaphases compared with G4 mTerc−/− DNA-PKcs+/+ controls (6.3 versus 4.3 per metaphase, respectively; P = 0.0001) (Fig. 1B). Metaphase spreads of primary whole bone marrow and ear fibroblast cell cultures derived from G0 mTerc+/− and G1 mTerc−/− mice that were either the wild type or null for DNA-PKcs were largely diploid and showed minimal chromosomal structural abnormalities (Tables 1 and 2), whereas G4 mTerc−/− DNA-PKcs+/+ metaphases harbored end-to-end fusions of p-arm to p-arm (p-p fusions) typical of telomere-dysfunctional cells, and these fusions generally lacked telomeres at their junction as documented previously (8, 48) (Tables 1 and 2). Notably, in contrast to previous reports of a requirement of DNA-PKcs for the generation of such telomere end-to-end fusions (25), abundant p-p and q-q fusions lacking telomere signals were readily detected in G4 mTerc−/− DNA-PKcs−/− primary bone marrow and ear fibroblast metaphases (Tables 1 and 2) as well as in early passage G4 mTerc−/− DNA-PKcs−/− MEFs (Table 3; Fig. 2A and B). Thus, in three different primary cell types examined, DNA-PKcs deficiency in late-generation mTerc−/− cells with telomere dysfunction is associated with an increased level of genomic instability. These studies establish that DNA-PKcs is not required for chromosomal end-to-end fusions brought about by progressive telomere erosion in cells null for mTerc.
TABLE 1.
Cytogenetic analysis of primary bone marrow cells
| Generation | Genotype | No. of:
|
|||||
|---|---|---|---|---|---|---|---|
| Mice | Metaphases analyzed | p-p fusions (%)a | q-q fusions (%)a | Samples with aneuploidy (%) | Fragmented chromatids or chromosomes (%) | ||
| G0 | mTerc+/−DNA-PKcs+/+ | 3 | 120 | 0 | 0 | 0 | 0 |
| mTerc+/−DNA-PKcs−/− | 3 | 120 | 0 | 0 | 0 | 1 (0.8) | |
| G1 | mTerc−/−DNA-PKcs+/+ | 2 | 100 | 0 | 0 | 0 | 0 |
| mTerc−/−DNA-PKcs−/− | 2 | 100 | 0 | 0 | 0 | 1 (1.0) | |
| G4 | mTerc−/−DNA-PKcs+/+ | 6 | 150 | 5 (3.3) | 0 | 4 (2.6) | 1 (0.6) |
| mTerc−/−DNA-PKcs−/− | 7 | 175 | 9 (5.1) | 2 (1.1) | 11 (4.5) | 5 (2.8) | |
Number of metaphases with at least one fusion event. Numbers in parentheses indicate percentage of metaphases with at least one event.
TABLE 2.
Cytogenetic analysis of primary ear fibroblast cells
| Generation | Genotype | No. of:
|
|||||
|---|---|---|---|---|---|---|---|
| Mice | Metaphases analyzed | p-p fusions (%)a | q-q fusions (%)a | Samples with aneuploidy (%) | Fragmented chromatids or chromosomes (%) | ||
| G0 | mTerc+/−DNA-PKcs+/+ | 3 | 75 | 0 | 0 | 0 | 0 |
| mTerc+/−DNA-PKcs−/− | 3 | 75 | 0 | 0 | 0 | 1 (1.3) | |
| G1 | mTerc−/−DNA-PKcs+/+ | 2 | 60 | 0 | 0 | 0 | 0 |
| mTerc−/−DNA-PKcs−/− | 2 | 60 | 0 | 0 | 0 | 2 (3.3) | |
| G4 | mTerc−/−DNA-PKcs+/+ | 4 | 125 | 8 (6.4) | 0 | 3 (2.4) | 2 (1.6) |
| mTerc−/−DNA-PKcs−/− | 5 | 150 | 10 (6.7) | 1 (0.6) | 10 (6.7) | 8 (5.3) | |
Number of metaphases with at least one fusion event. Numbers in parentheses indicate percentage of metaphases with at least one event.
TABLE 3.
Cytogenetic analysis of MEFs
| Generation | Genotype | No. of:
|
|||||
|---|---|---|---|---|---|---|---|
| Cell lines | Metaphases analyzed | p-p fusions (%)a | q-q fusions (%)a | Fragmented chromatids or chromosomes (%) | Other abnormalities (description) | ||
| G4 | mTerc−/−DNA-PKcs+/+ | 3 | 60 | 2 (3.3) | 0 | 0 | 1 (1 triradial) |
| G4 | mTerc−/−DNA-PKcs−/− | 4 | 80 | 8 (10) | 2 (2.5) | 2 (2.5) | |
Number of metaphases with at least one fusion event. Numbers in parentheses indicate percentage of metaphases with at least one event.
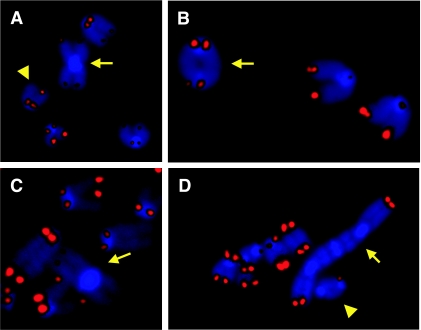
FIG. 2.
G4 mTerc−/− DNA-PKcs−/− and Lig4−/− cells harbor telomeric fusions. (A) p-p arm telomere end-to-end fusion (arrow) and a telomere signal-free end (arrowhead) in late-generation (G4) mTerc−/− DNA-PKcs−/− MEFs. (B) q-q arm telomere end-to-end fusion (arrow) in late-generation (G4) mTerc−/− DNA-PKcs−/− MEFs. (C) p-p arm telomere end-to-end fusion (arrow) in a metaphase from a late-generation (G4) mTerc−/− Lig4−/− MEF cell line. (D) Multicentric end-to-end fusion (arrow) and q-q fusion (arrowhead) in a metaphase derived from a late-generation (G4) mTerc−/− Lig4−/− p53−/− MEF cell line. The blue staining is DAPI, and the red signal is the telomere probe; separate images for each were overlaid by using Adobe Photoshop.
The ability to generate chromosomal end fusions in the absence of the key NHEJ component, DNA-PKcs, prompted further assessment of the classical NHEJ pathway in this DNA repair process. As DNA ligase IV (Lig4) activity is an absolute requirement for NHEJ, mediating the last joining step of the NHEJ process (58), we assessed the capacity of late-generation mTerc−/− Lig4−/− cells to generate chromosomal end fusions at eroded telomeres. To this end, we bred a null allele of Lig4 (31) through successive mTerc−/− generations to produce MEFs representing all relevant experimental genotypes. In this context, 40% of G4 mTerc−/− Lig4+/+ MEF metaphases harbored at least one p-p fusion, and the end-to-end fusions that lacked detectable telomere sequences at their junction were still readily apparent with G4 mTerc−/− Lig4−/− MEFs (55% of metaphases) (Table 4; Fig. 2C). To further facilitate cytogenetic analysis of these cultures, we alleviated the p53-dependent checkpoint that causes Lig4−/− cells to senesce early (30). In the setting of p53 deficiency or simian virus 40 T-antigen expression, p-p and q-q fusions lacking telomere sequences and other abnormalities continued to be readily detected in G4 mTerc−/− Lig4−/− MEFs, and some cells now harbored up to eight p-p fusions that consistently lacked telomere sequences at their junction per metaphase, suggesting that these fusion events were formed de novo (Table 4; Fig. 2D). Thus, given the combined results for DNA-PKcs and Lig4 mutants, we conclude that NHEJ is not required for the creation of chromosomal fusions arising in cells with denuded telomeres produced by mTerc deficiency in the mouse and that other repair pathways can apparently be utilized to generate chromosomal fusions in this setting.
TABLE 4.
Cytogenetic analysis of Lig4 mTerc MEFs
| Generation and genotype | No. of:
|
|||||
|---|---|---|---|---|---|---|
| Metaphases analyzed | Chr/Mete (avg) | p-p fusions (%)a | q-q fusions (%)a | Fragmented chromatids or chromosomes | Other abnormalities (description) | |
| G4 Lig4+/+ p53+/+ | 18 | 36.9 | 8 (44) | 0 | 0 | |
| G4 Lig4−/− p53+/+ | 20 | 39 | 11 (55) | 0 | 4 fragments, 2 chromosome breaks | 3 (1 p-q fusion, 1 tri-radial, 1 quadriradial) |
| G4 Lig4−/− p53−/− | 14 | 61 | 12 (86)b | 6 | 2 chromatid breaks | 3 (3 p-q fusions) |
| G4 Lig4−/− T antigend | 9 | 50.5 | 6 (67)c | 0 | 3 chromatid breaks | |
| G3 Lig4−/− T antigen | 15 | 55.5 | 10 (67) | 5 | ||
Number of metaphases with at least one fusion event. Numbers in parentheses indicate percentage of metaphases with at least one event.
Up to 8 p-p fusions were observed per metaphase in this cell line.
Up to 7 p-p fusions were observed per metaphase in this cell line.
This T-antigen cell line is a derivative of the G4 Lig4−/− p53+/+ primary cell line; the G3 Lig4−/− T-antigen cell line (last column) was independently derived.
Number of chromosomes per metaphase.
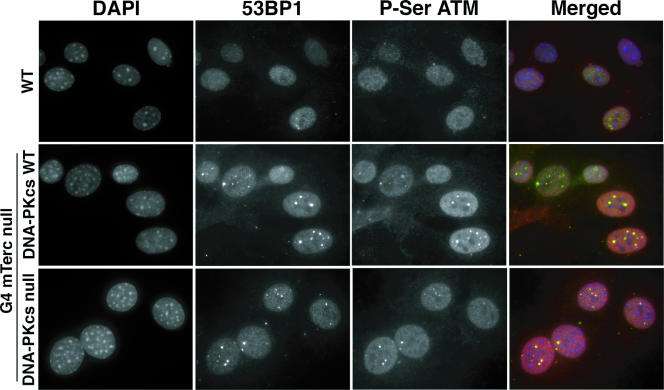
The presence of dysfunctional telomeres can result in the activation of the DNA damage response (DDR) (20, 49, 85). The observation of fusions between chromosomes that lack telomere sequences in G4 mTerc−/− DNA-PKcs-null cells suggested that the DDR machinery was functioning properly in these cells. To further substantiate this point, we assessed the functional state of the DDR by immunofluorescence staining for two members of the DDR pathway, 53BP1 and activated Atm (ser1981). We observed a significant increase in the numbers of detectable 53BP1 and phospho-ser1981 Atm foci in G4 mTerc−/− MEFs compared to those seen in G0 mTerc+/− MEFs (Table 5). Notably, the average number of detectable foci was further increased in G4 DNA-PKcs-null MEFs (Table 5), which was concomitant with the increase in signal-free ends described earlier (Table 3). These foci were also readily detectable in spontaneously immortalized G4 mTerc−/− DNA-PKcs−/− MEFs (Fig. 3). These biochemical studies confirm that telomere attrition in this background leads to activation of DDR pathways that operate in a DNA-PK-independent manner.
TABLE 5.
DDR foci in mTerc DNA-PKcs MEFs
| Generation and genotype | No. of:
|
||||
|---|---|---|---|---|---|
| Cell lines | 53BP1 nuclei | 53BP1 foci/nucleus (avg)a | P-Ser Atm nuclei | P-ser Atm foci/nucleus (avg)a | |
| G0 mTerc+/−DNA-PKcs WT or heterozygous | 4 | 481 | 1.20 ± 2.36 | 496 | 0.4 ± 1.29 |
| G0 mTerc+/−DNA-PKcs | 1 | 124 | 0.93 ± 2.87 | 124 | 0.47 ± 1.64 |
| G4 mTerc−/−DNA-PKcs WT or heterozygous | 4 | 483 | 2.80 ± 3.27b | 496 | 1.17 ± 1.54b |
| G4 mTerc−/−DNA-PKcs null | 2 | 240 | 3.54 ± 4.27c | 248 | 2.28 ± 2.58d |
Number is the average ± standard deviation.
P value of < 0.001 compared to G0 mTerc+/− DNA-PKcs+/+ or DNA-PKcs+/− MEFs (two-sample t test).
P value of 0.01 compared to G4 mTerc−/− DNA-PKcs+/+ or DNA-PKcs+/− MEFs (two-sample t test).
P value of < 0.001 compared to G4 mTerc−/− DNA-PKcs+/+ or DNA-PKcs+/− MEFs (two-sample t test).
FIG. 3.
G4 mTerc−/− DNA-PKcs−/− MEFs harbor hallmark DNA damage response foci. Immunofluorescence images from representative telomerase-intact (wild type [WT], top row), G4 mTerc−/− DNA-PKcs+/+ (middle row), and G4 mTerc−/− DNA-PKcs−/− (bottom row) immortalized MEFs stained with anti-53BP1 and anti-phospho-ser1981 ATM antibodies and counterstained with DAPI, with merged images from each. Magnification, ×600.
DNA-PKcs is not required for the telomere checkpoint response in vivo.
DNA-PK has been postulated to mediate the apoptotic response to telomere dysfunction in several tissues in telomerase-deficient mice (24-26). To assess the impact of DNA-PKcs deficiency on classical tissue phenotypes associated with telomere dysfunction in our cohort, we carefully audited anaphase bridging, apoptosis, and tissue integrity in the gastrointestinal and male germ cell compartments—organ systems that are known to display prominent progressive degenerative phenotypes with increasing telomere dysfunction (17, 47, 50, 57, 76, 92).
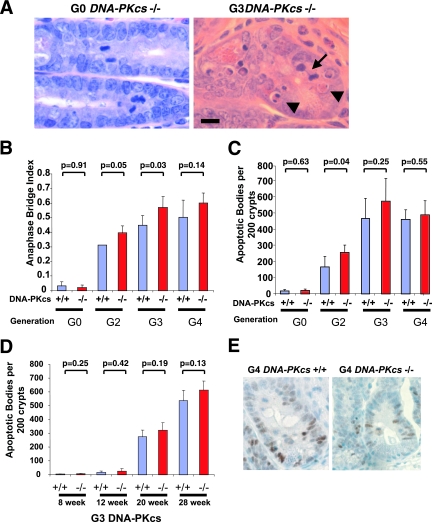
In the gastrointestinal epithelium, which sustains marked cell turnover throughout life (9, 70), the proliferating crypt precursor cells undergo apoptotic cell death in the setting of telomere dysfunction, and the level of apoptosis correlates tightly with the degree of telomere dysfunction, as measured by anaphase bridge index (92). Consistent with the high degree of telomere dysfunction (as indicated by chromosome fusion and anaphase bridging) found in the above-mentioned G4 mTerc−/− primary cell cultures, the anaphase bridge index in the crypt cell compartment increased progressively as a function of successive mTerc−/− generations in DNA-PKcs+/+ and DNA-PKcs−/− mice (Fig. 4A and B). The dispensability of DNA-PKcs in the creation of telomere attrition-dependent anaphase bridges was also evident in G2 and G3 mTerc−/− DNA-PKcs−/− crypts, which showed increased anaphase bridge index relative to G2 and G3 mTerc−/− DNA-PKcs+/+ controls (Fig. 4B).
FIG. 4.
Apoptosis and anaphase bridging in mTerc DNA-PKcs mice. (A) Representative images of small intestinal crypts from a G0 DNA-PKcs−/− and a G3 mTerc−/− DNA-PKcs−/− mouse stained with H&E, demonstrating an anaphase bridge (arrow) and several apoptotic bodies (arrowheads). Magnification, ×800. Bar, 0.01 mm. (B) Quantification of anaphase bridging index in small intestine crypts from adult mice of the indicated genotypes, represented as incidence per 100 scorable mitoses. H&E-stained sections of intestines from at least three mice of each genotype were scored. (C) Apoptotic body index from small intestine crypts of adult mice of the indicated genotypes, represented as the mean incidence per 200 crypts. At least 200 crypts from H&E-stained sections from three individual mice of the indicated genotypes were scored. (D) Apoptotic body index in small intestine crypts as a function of age and DNA-PKcs status in G3 mTerc−/− mice. (E) p53 immunohistochemistry from histological sections from G4 mTerc−/− mice of either the wild type (left) or null (right) for DNA-PKcs. p53-positive nuclei appear brown. Hematoxylin (blue) counterstain. Magnification, ×800.
DNA-PK has been postulated to play a role in the activation of apoptosis in response to telomere erosion in several tissues, including the intestine (25, 26); thus, we examined the level of apoptosis in small intestinal crypts in mTerc DNA-PKcs mice. While crypt apoptotic bodies were rare in 20-week-old G0/G1 mTerc−/− DNA-PKcs+/+ and DNA-PKcs−/− mice (Fig. 4C), the number of crypt apoptotic bodies increased progressively from G1 through G4 mTerc−/− DNA-PKcs+/+ generations as previously described (92). Notably, DNA-PKcs deficiency failed to prevent apoptosis in any of the generations of mTerc−/− mice analyzed (Fig. 4C). The possibility that DNA-PKcs function may vary in other regions of the intestine was ruled out by the finding that similar levels of crypt cell apoptosis occur in large intestines of G4 mTerc−/− DNA-PKcs+/+ and DNA-PKcs−/− mice (data not shown). In addition, G3 mTerc−/− DNA-PKcs+/+ and DNA-PKcs−/− crypts showed a comparable progressive increase in apoptotic bodies as a function of advancing age (Fig. 4D). Finally, an examination of the rate of proliferation by Ki67 staining or BrdU incorporation assays did not reveal any differences between G3 mTerc−/− DNA-PKcs+/+ and DNA-PKcs−/− intestinal crypts (data not shown). Correspondingly, in keeping with the importance of p53 in inducing telomere dysfunction-associated apoptosis (17), anti-p53 immunohistochemical staining showed comparably robust reactivity in G4 mTerc−/− DNA-PKcs+/+ and mTerc−/− DNA-PKcs−/− crypt cells, consistent with DNA-PKcs-independent activation of p53 in response to telomere dysfunction (Fig. 4E). We conclude that DNA-PK is dispensable for activation of the apoptotic program in response to telomere dysfunction in the intestinal crypt stem cell compartment.
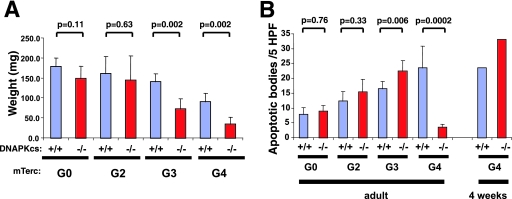
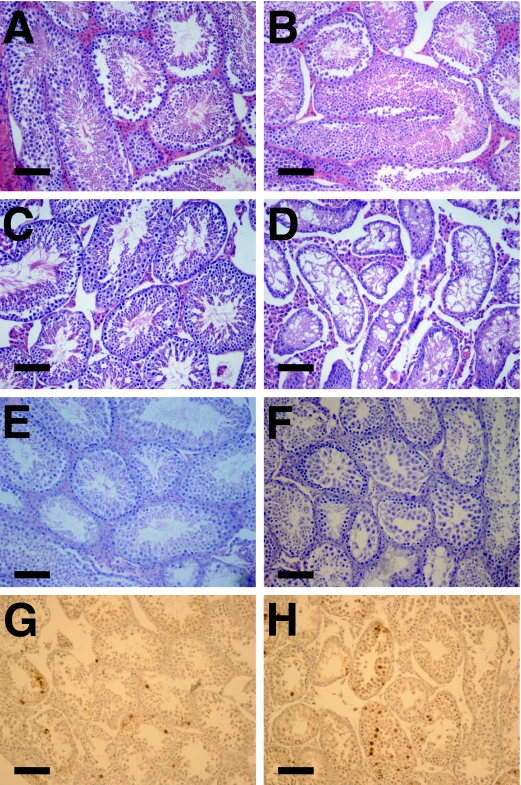
The proliferating germ cell compartment in the testes is also exquisitely sensitive to telomere dysfunction in a p53-dependent manner (17), prompting an analysis of DNA-PKcs and telomere interactions in this tissue. Consistent with previous reports (50, 57), testis weights were reduced progressively in adult G3 and G4 mTerc−/− DNA-PKcs+/+ mice relative to early generation controls or wild-type mice (Fig. 5A). Mutation of DNA-PKcs resulted in a significantly greater loss of testis weight than those seen with G3 and G4 mTerc−/− DNA-PKcs+/+ controls, although the loss of DNA-PKcs did not significantly affect testis weight in telomerase-intact mice (P = 0.11) (Fig. 5A). Previous studies have shown that testicular atrophy in late-generation mTerc−/− mice results from apoptotic depletion of germ cells (17, 50, 57). As the level of telomere dysfunction increased as a function of successive mTerc−/− generations from G2 to G4, testicular germ cells showed the expected corresponding increase in the level of apoptosis, as assessed by TUNEL staining, in 18-week-old mice (Fig. 5B). The G4 mTerc−/− DNA-PKcs−/− testes showed significantly fewer apoptotic cells than did G4 mTerc−/− DNA-PKcs+/+ mice (P = 0.0002), prompting the authors of a previous study to conclude that DNA-PKcs participates in the telomere dysfunction checkpoint response and that its loss results in decreased apoptosis in this compartment (25). However, histological analyses showed that the seminiferous tubules in adult G4 mTerc−/− DNA-PKcs−/− mice are nearly devoid of germ cells (Fig. 6). This finding, coupled with the higher level of apoptosis (P = 0.006) but significantly diminished weight of testes (P = 0.002) in G3 mTerc−/− DNA-PKcs−/− mice (Fig. 5A and B), indicated that the significantly diminished weight of G4 mTerc−/− DNA-PKcs−/− mice might be a result of a higher apoptosis rate at an earlier time point, resulting in germ cell depletion that precludes an accurate analysis of germ cell apoptosis in adult G4 mTerc−/− DNA-PKcs−/− mice. To directly address this possibility, we examined apoptosis in younger G4 mTerc−/− DNA-PKcs−/− testes that still possess adequate cellularity (i.e., that have yet to deplete their germ cell reserves). In contrast to the adult mice, 4-week-old G4 mTerc−/− DNA-PKcs−/− testes, which still retained active meiotic tubules (Fig. 6E and F), exhibited florid apoptosis at a level at least comparable to that observed with G4 mTerc−/− DNA-PKcs+/+ littermates (Fig. 5B; Fig. 6G and H). Thus, these results are similar to those from the intestinal studies, and they indicate that DNA-PKcs does not play a role in attenuating the apoptotic response to telomere dysfunction as previously proposed; instead, its absence appears to modestly accelerate apoptosis in the germ compartment.
FIG. 5.
Testis atrophy and apoptosis is accelerated in G4 mTerc−/− DNA-PKcs−/− mice. (A) Testis weights from adult mice of the indicated genotypes. At least three mice per genotype were examined. (B) Apoptosis (measured by TUNEL staining) in testes from adult mice of the indicated genotypes. Data are represented as the mean of TUNEL-positive nuclei per high-power field (HPF). At least five HPFs from at least three independent mice were examined.
FIG. 6.
Testicular atrophy and apoptosis in G4 DNA-PKcs−/− mice. Histological sections of testis samples stained with H&E. Magnification, ×200. Bar, 0.1 mm. (A) G0 DNA-PKcs+/+; samples are from week 37. (B) G0 DNA-PKcs−/−, week 37. (C) G4 DNA-PKcs+/+, week 8. (D) G4 DNA-PKcs−/−, week 8. (E) G4 DNA-PKcs+/+, week 4. (F) G4 DNA-PKcs−/−, week 4. (G and H) TUNEL staining of 4-week-old (G) G4 DNA-PKcs+/+ and (H) G4 DNA-PKcs−/− testis samples. Magnification, ×200. Bar, 0.1 mm.
DISCUSSION
In this genetic study, we have examined the role of DNA-PKcs in response to telomere dysfunction brought about by progressive telomere erosion in the telomerase-deficient mouse. A cytogenetic examination of three primary cell types revealed that DNA-PKcs is not required for the production of chromosomal end-to-end fusions that are commonplace in telomere-eroded late-generation mTerc−/− cells. That the classical NHEJ pathway is indeed not required for the production of telomere fusions resulting from telomere erosion is further supported by similar cytogenetic profiles (including fusions) for late-generation mTerc−/− cells in the presence or absence of LIG4. Finally, a detailed serial morphological analysis of two tissues indicates that DNA-PKcs plays a limited role, if any, in the telomere checkpoint response in vivo.
The results of this study run contrary to the notion that DNA-PK and, by extension, NHEJ, are primary mediators of the in vitro and in vivo response to telomere dysfunction in mammalian cells. One of these cellular responses to experimentally induced telomere dysfunction is the initiation of a DNA damage response that ultimately results in the fusion of denuded chromosomes, effectively “repairing” the telomere damage. In one such experimental system, the expression of the telomere binding protein TRF2 in a dominant negative mutant (86) in human and mouse cells induces an “uncapping” response, leading to recognition of the damaged telomeres as DNA damage (85). These uncapped telomeres are eventually resolved by the processing of the 3′ G-rich overhang by ERCC1/XPF (96) and the eventual fusion of chromosomes retaining substantial telomere repeat sequences in a Lig4 (NHEJ)-dependent manner (81). We speculate that the contrasting observations of fusions in our model system might be reconciled with those represented by the dominant-negative TRF2 approach by the nature of the different experimental systems used. It seems likely that the repair processes that are capable of recognizing the loss of telomere repeats stemming from natural telomere erosion (caused in our system by telomerase deficiency) are distinct from those recognizing the uncapping of telomere ends that results from the alteration of chromatin structure, perhaps regulated by the cell cycle phase in which they occur. Such a distinction between these systems was also observed with S. pombe, for which deletion of Taz1 resulted in Ku- and Lig4-dependent chromosome fusions (28), whereas chromosome circularization in the absence of the telomerase catalytic subunit Trt1 functions independently of Lig4 (4). In human cells, normal telomeres are recognized by the Atm-MRN repair and checkpoint pathway during the G2 phase of the cell cycle in primary human fibroblasts, which is likely reflective of a need for postreplicative processing of telomeres (88). As such, it seems likely that telomere erosion that has exceeded that necessary for function may be first recognized at this time point, leading to the activation of a repair pathway that functions independently of classical DNA-PK-mediated NHEJ. The latter notion fits with the observation that end-to-end fusions resulting from telomere erosion in mTerc−/− mice occur by microhomology-mediated DNA repair events (48). Aberrant ligation of two chromosomes retaining telomere DNA following dominant negative TRF2 expression utilizes processing by the ERCC1/XPF nuclease (96), indicating that fusions likely occur between blunt or nearly blunt telomeres. Our data indicate that a nonclassical NHEJ-mediated repair system creates telomere erosion-associated chromosome fusions. The presence of aberrant chromosome fusions in classical NHEJ-deficient mammalian cells (62), as well as evidence for a low-efficiency NHEJ system that repairs DNA damage after radiation in the absence of classical NHEJ (54), points to the existence of alternative NHEJ pathways, although they remain poorly characterized for mammals and controversial (58). Evidence clearly exists that the MRE11-RAD50-XRS2 complex in yeast regulates distinct types of NHEJ repair events (63), in some cases separable from Ku-mediated NHEJ (16, 59), and mutation of both Mre11 and Ku70 in the background of Tert deficiency in A. thaliana also revealed that distinct pathways of chromosome fusions exist (45). Although the function of its mammalian counterparts (MRE11-RAD50-NBS1) in this process is currently unclear, its close association with normal telomeres (88, 95) makes this complex an attractive one for further genetic study with the mouse.
We must also reconcile our results with those of others who have implicated DNA-PKcs specifically in mediating, in part, the effects of telomere erosion in the background of telomerase deficiency (25, 26). First, although independently derived strains of DNA-PKcs mutant mice were used in our study and in previous work (25, 26) they are phenotypically similar and share an immunological phenotype (33, 84) that is more severe and less leaky than that of the classical SCID mouse harboring a point mutation in DNA-PKcs (10, 11). This suggests that differences in experimental conditions and interpretations, rather than intrinsic differences in mouse strains, are responsible for the discrepancies between results of our study and those previously reported (25, 26). Here, we have established in three separate cell systems, including primary cells from the bone marrow, that the incidence of chromosome fusions was not diminished in the late-generation mTerc−/− DNA-PKcs−/− cells relative to littermate DNA-PKcs wild-type controls. In addition to these cell culture systems, we have documented comparable anaphase bridge indices (an in vivo correlate of telomere dysfunction) in the late-generation mTerc−/− DNA-PKcs−/− and DNA-PKcs+/+ crypts. In the previous report by Espejel et al. (25) implicating DNA-PKcs in this process, we note that the authors used only one cell type derived from a single generation, in which even the mTerc−/− DNA-PKcs+/+ control cells failed to show telomere fusions. We further report here that loss of Lig4 has no impact on telomere erosion-induced fusion. As Lig4 (and its partner XRCC4) have perhaps the most specific role in NHEJ (58), this evidence supports the view that classical DNA-PK- mediated NHEJ is not absolutely required for fusion of telomere-eroded chromosomes.
A further distinction between our results and those previously reported by others relates to the purported function of NHEJ members in facilitating telomere dysfunction-induced apoptosis (24-26). Telomere dysfunction in the germ compartment in the testis results largely in a p53-dependent apoptotic process (17). We found that, although adult G4 DNA-PKcs−/− mice have drastically reduced apoptosis, this observation may be susceptible to artifactual interpretation, as a result of the extreme depletion of germ cells, rather than being seen as an intrinsic function in signaling the apoptotic mechanism in this tissue. Supporting our interpretations are the significant increases in apoptosis in older G3 mTerc−/− DNA-PKcs−/− testes and in young G4 mTerc−/− DNA-PKcs−/− testes that maintain sufficient cellularity to derive an accurate quantification of apoptosis. In agreement with our observations, DNA-PKcs-deficient mice exhibit increased testicular apoptosis and p53 activation following exposure to ionizing radiation (41), consistent with a large body of evidence to date that DNA-PK plays a limited role in DNA damage signaling to p53 (discussed below). Whether these effects reflect upon a direct function for DNA-PKcs in monitoring telomere and genomic integrity in meiosis or a whether loss of DNA-PKcs and associated accelerated instability causes enhanced activation of the normal meiotic telomere checkpoint pathway is not clear, and additional experiments are required to more fully understand these functions.
Consistent with our data that DNA-PKcs is not required for the telomere erosion-dependent checkpoint in vivo, we have also found that DNA-PKcs was dispensable for activation of p53-dependent apoptosis in either the small or large intestine stem/progenitor compartment and may accelerate, rather than prevent, telomere dysfunction-induced apoptosis in the testis. Here again, we believe that differences in experimental conditions and interpretations are responsible for the differences observed. For the intestine, we have used validated morphological criteria for the identification of apoptotic bodies in the small and large intestinal progenitor compartment responsible for long-term epithelial renewal in this organ (92). A prior report examined a surrogate of apoptosis via active caspase-3 in intestinal epithelia rather than the more critical crypt compartment (26). Our studies indicate that it is unlikely that DNA-PKcs is relevant for maintaining homeostasis in this compartment in response to telomere dysfunction. Furthermore, our data that DNA-PKcs is dispensable for this p53-mediated process are in agreement with the overwhelming evidence that DNA-PKcs is not required for activation of the radiation- and V(D)J-induced DNA DSB p53 checkpoint in vitro (1, 15, 32, 56, 71) and in vivo (39-41, 55) and, although some evidence exists that DNA-PKcs may exert some regulatory action on p53 in certain apoptotic contexts (93), our evidence suggests that telomere dysfunction signals to p53 independently of DNA-PKcs in vivo.
In summary, our results challenge the assumption that classical NHEJ mediates the response to telomere erosion that culminates in chromosome fusion and apoptosis. At the same time, our studies do not exclude a role for classical NHEJ in chromosome fusions brought about by telomere attrition, but they do underscore the idea that additional repair pathways can operate on eroded telomere ends. These observations and this model system set the stage for studies exploring the repair and checkpoint response pathways that respond to telomere dysfunction and mediate its detrimental effects on mammalian cells and tissues.
Acknowledgments
We thank F. Alt for critically reading the manuscript and for providing the DNA-PKcs and Lig4 mutant mice; S. Jiang, Y. Zhang, and A. Yu for animal husbandry; and N. Simon for statistical advice. The authors also thank the reviewers for insightful comments.
R.S.M. was supported by a Howard Ringold Fellowship from the Damon Runyon Cancer Research Fund. K.-K.W. is supported by NIH grant K08AG 2400401 and the Sidney Kimmel Foundation for Cancer Research. R.A.D. is an American Cancer Society Research Scholar and an Ellison Medical Foundation Senior Scholar and is supported by NIH grants R01CA84628, P01CA95616, and U01CA84313 and by the Robert A. and Renee E. Belfer Foundation Institute for Innovative Cancer Science.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Abraham, J., D. Spaner, and S. Benchimol. 1999. Phosphorylation of p53 protein in response to ionizing radiation occurs at multiple sites in both normal and DNA-PK deficient cells. Oncogene 18:1521-1527. [DOI] [PubMed] [Google Scholar]
- 2.Ahnesorg, P., P. Smith, and S. P. Jackson. 2006. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell 124:301-313. [DOI] [PubMed] [Google Scholar]
- 3.Bailey, S. M., J. Meyne, D. J. Chen, A. Kurimasa, G. C. Li, B. E. Lehnert, and E. H. Goodwin. 1999. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc. Natl. Acad. Sci. USA 96:14899-14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann, P., and T. R. Cech. 2000. Protection of telomeres by the Ku protein in fission yeast. Mol. Biol. Cell 11:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baur, J. A., Y. Zou, J. W. Shay, and W. E. Wright. 2001. Telomere position effect in human cells. Science 292:2075-2077. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi, A., and T. de Lange. 1999. Ku binds telomeric DNA in vitro. J. Biol. Chem. 274:21223-21227. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn, E. H. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579:859-862. [DOI] [PubMed] [Google Scholar]
- 8.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 9.Booth, C., and C. S. Potten. 2000. Gut instincts: thoughts on intestinal epithelial stem cells. J. Clin. Investig. 105:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527-530. [DOI] [PubMed] [Google Scholar]
- 11.Bosma, M. J. 1992. B and T cell leakiness in the scid mouse mutant. Immunodefic. Rev. 3:261-276. [PubMed] [Google Scholar]
- 12.Boulton, S. J., and S. P. Jackson. 1996. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 24:4639-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck, D., L. Malivert, R. de Chasseval, A. Barraud, M. C. Fondaneche, O. Sanal, A. Plebani, J. L. Stephan, M. Hufnagel, F. le Deist, A. Fischer, A. Durandy, J. P. de Villartay, and P. Revy. 2006. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell 124:287-299. [DOI] [PubMed] [Google Scholar]
- 14.Bundock, P., H. van Attikum, and P. Hooykaas. 2002. Increased telomere length and hypersensitivity to DNA damaging agents in an Arabidopsis KU70 mutant. Nucleic Acids Res. 30:3395-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burma, S., A. Kurimasa, G. Xie, Y. Taya, R. Araki, M. Abe, H. A. Crissman, H. Ouyang, G. C. Li, and D. J. Chen. 1999. DNA-dependent protein kinase-independent activation of p53 in response to DNA damage. J. Biol. Chem. 274:17139-17143. [DOI] [PubMed] [Google Scholar]
- 16.Chen, C., and R. D. Kolodner. 1999. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 23:81-85. [DOI] [PubMed] [Google Scholar]
- 17.Chin, L., S. E. Artandi, Q. Shen, A. Tam, S. L. Lee, G. J. Gottlieb, C. W. Greider, and R. A. DePinho. 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527-538. [DOI] [PubMed] [Google Scholar]
- 18.Custer, R. P., G. C. Bosma, and M. J. Bosma. 1985. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am. J. Pathol. 120:464-477. [PMC free article] [PubMed] [Google Scholar]
- 19.d'Adda di Fagagna, F., M. P. Hande, W. M. Tong, D. Roth, P. M. Lansdorp, Z. Q. Wang, and S. P. Jackson. 2001. Effects of DNA nonhomologous end-joining factors on telomere length and chromosomal stability in mammalian cells. Curr. Biol. 11:1192-1196. [DOI] [PubMed] [Google Scholar]
- 20.d'Adda di Fagagna, F., P. M. Reaper, L. Clay-Farrace, H. Fiegler, P. Carr, T. Von Zglinicki, G. Saretzki, N. P. Carter, and S. P. Jackson. 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194-198. [DOI] [PubMed] [Google Scholar]
- 21.de Lange, T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100-2110. [DOI] [PubMed] [Google Scholar]
- 22.Difilippantonio, M. J., S. Petersen, H. T. Chen, R. Johnson, M. Jasin, R. Kanaar, T. Ried, and A. Nussenzweig. 2002. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J. Exp. Med. 196:469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espejel, S., and M. A. Blasco. 2002. Identification of telomere-dependent “senescence-like” arrest in mouse embryonic fibroblasts. Exp. Cell Res. 276:242-248. [DOI] [PubMed] [Google Scholar]
- 24.Espejel, S., S. Franco, S. Rodriguez-Perales, S. D. Bouffler, J. C. Cigudosa, and M. A. Blasco. 2002. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 21:2207-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espejel, S., S. Franco, A. Sgura, D. Gae, S. M. Bailey, G. E. Taccioli, and M. A. Blasco. 2002. Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. EMBO J. 21:6275-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espejel, S., P. Klatt, J. Menissier-de Murcia, J. Martin-Caballero, J. M. Flores, G. Taccioli, G. de Murcia, and M. A. Blasco. 2004. Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86, or DNA-PKcs. J. Cell Biol. 167:627-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espejel, S., M. Martin, P. Klatt, J. Martin-Caballero, J. M. Flores, and M. A. Blasco. 2004. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 5:503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira, M. G., and J. P. Cooper. 2001. The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol. Cell 7:55-63. [DOI] [PubMed] [Google Scholar]
- 29.Fisher, T. S., and V. A. Zakian. 2005. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amsterdam) 4:1215-1226. [DOI] [PubMed] [Google Scholar]
- 30.Frank, K., N. E. Sharpless, Y. Gao, J. M. Sekiguchi, D. O. Ferguson, C. Zhu, J. P. Manis, J. W. Horner, R. A. DePinho, and F. W. Alt. 2000. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 5:993-1002. [DOI] [PubMed] [Google Scholar]
- 31.Frank, K. M., J. M. Sekiguchi, K. J. Seidl, W. Swat, G. A. Rathbun, H. L. Cheng, L. Davidson, L. Kangaloo, and F. W. Alt. 1998. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature 396:173-177. [DOI] [PubMed] [Google Scholar]
- 32.Fried, L. M., C. Koumenis, S. R. Peterson, S. L. Green, P. van Zijl, J. Allalunis-Turner, D. J. Chen, R. Fishel, A. J. Giaccia, J. M. Brown, and C. U. Kirchgessner. 1996. The DNA damage response in DNA-dependent protein kinase-deficient SCID mouse cells: replication protein A hyperphosphorylation and p53 induction. Proc. Natl. Acad. Sci. USA 93:13825-13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao, Y., J. Chaudhuri, C. Zhu, L. Davidson, D. T. Weaver, and F. W. Alt. 1998. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity 9:367-376. [DOI] [PubMed] [Google Scholar]
- 34.Gao, Y., Y. Sun, K. M. Frank, P. Dikkes, Y. Fujiwara, K. J. Seidl, J. M. Sekiguchi, G. A. Rathbun, W. Swat, J. Wang, R. T. Bronson, B. A. Malynn, M. Bryans, C. Zhu, J. Chaudhuri, L. Davidson, R. Ferrini, T. Stamato, S. H. Orkin, M. E. Greenberg, and F. W. Alt. 1998. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell 95:891-902. [DOI] [PubMed] [Google Scholar]
- 35.Gilley, D., H. Tanaka, M. P. Hande, A. Kurimasa, G. C. Li, M. Oshimura, and D. J. Chen. 2001. DNA-PKcs is critical for telomere capping. Proc. Natl. Acad. Sci. USA 98:15084-15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goytisolo, F. A., E. Samper, S. Edmonson, G. E. Taccioli, and M. A. Blasco. 2001. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol. Cell. Biol. 21:3642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandin, N., C. Damon, and M. Charbonneau. 2000. Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment. Mol. Cell. Biol. 20:8397-8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 39.Guidos, C. J., C. J. Williams, I. Grandal, G. Knowles, M. T. Huang, and J. S. Danska. 1996. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 10:2038-2054. [DOI] [PubMed] [Google Scholar]
- 40.Gurley, K. E., and C. J. Kemp. 1996. p53 induction, cell cycle checkpoints, and apoptosis in DNAPK-deficient scid mice. Carcinogenesis 17:2537-2542. [DOI] [PubMed] [Google Scholar]
- 41.Hamer, G., H. L. Roepers-Gajadien, A. van Duyn-Goedhart, I. S. Gademan, H. B. Kal, P. P. van Buul, T. Ashley, and D. G. de Rooij. 2003. Function of DNA-protein kinase catalytic subunit during the early meiotic prophase without Ku70 and Ku86. Biol. Reprod. 68:717-721. [DOI] [PubMed] [Google Scholar]
- 42.Hande, P., P. Slijepcevic, A. Silver, S. Bouffler, P. van Buul, P. Bryant, and P. Lansdorp. 1999. Elongated telomeres in scid mice. Genomics 56:221-223. [DOI] [PubMed] [Google Scholar]
- 43.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 44.Hasty, P., J. Campisi, J. Hoeijmakers, H. van Steeg, and J. Vijg. 2003. Aging and genome maintenance: lessons from the mouse? Science 299:1355-1359. [DOI] [PubMed] [Google Scholar]
- 45.Heacock, M., E. Spangler, K. Riha, J. Puizina, and D. E. Shippen. 2004. Molecular analysis of telomere fusions in Arabidopsis: multiple pathways for chromosome end-joining. EMBO J. 23:2304-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hefferin, M. L., and A. E. Tomkinson. 2005. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amsterdam) 4:639-648. [DOI] [PubMed] [Google Scholar]
- 47.Hemann, M. T., K. L. Rudolph, M. A. Strong, R. A. DePinho, L. Chin, and C. W. Greider. 2001. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol. Biol. Cell 12:2023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemann, M. T., M. A. Strong, L. Y. Hao, and C. W. Greider. 2001. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107:67-77. [DOI] [PubMed] [Google Scholar]
- 49.Herbig, U., W. A. Jobling, B. P. Chen, D. J. Chen, and J. M. Sedivy. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14:501-513. [DOI] [PubMed] [Google Scholar]
- 50.Herrera, E., E. Samper, J. Martin-Caballero, J. M. Flores, H. W. Lee, and M. A. Blasco. 1999. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18:2950-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu, H. L., D. Gilley, E. H. Blackburn, and D. J. Chen. 1999. Ku is associated with the telomere in mammals. Proc. Natl. Acad. Sci. USA 96:12454-12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu, H. L., D. Gilley, S. A. Galande, M. P. Hande, B. Allen, S. H. Kim, G. C. Li, J. Campisi, T. Kohwi-Shigematsu, and D. J. Chen. 2000. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 14:2807-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hug, N., and J. Lingner. 2006. Telomere length homeostasis. Chromosoma 115:413-425. [DOI] [PubMed] [Google Scholar]
- 54.Iliakis, G., H. Wang, A. R. Perrault, W. Boecker, B. Rosidi, F. Windhofer, W. Wu, J. Guan, G. Terzoudi, and G. Pantelias. 2004. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 104:14-20. [DOI] [PubMed] [Google Scholar]
- 55.Jhappan, C., T. M. Yusufzai, S. Anderson, M. R. Anver, and G. Merlino. 2000. The p53 response to DNA damage in vivo is independent of DNA-dependent protein kinase. Mol. Cell. Biol. 20:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jongmans, W., M. Artuso, M. Vuillaume, H. Bresil, S. P. Jackson, and J. Hall. 1996. The role of ataxia telangiectasia and the DNA-dependent protein kinase in the p53-mediated cellular response to ionising radiation. Oncogene 13:1133-1138. [PubMed] [Google Scholar]
- 57.Lee, H. W., M. A. Blasco, G. J. Gottlieb, J. W. Horner II, C. W. Greider, and R. A. DePinho. 1998. Essential role of mouse telomerase in highly proliferative organs. Nature 392:569-574. [DOI] [PubMed] [Google Scholar]
- 58.Lieber, M. R., Y. Ma, U. Pannicke, and K. Schwarz. 2004. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair (Amsterdam) 3:817-826. [DOI] [PubMed] [Google Scholar]
- 59.Ma, J. L., E. M. Kim, J. E. Haber, and S. E. Lee. 2003. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol. Biol. Cell 23:8820-8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maser, R. S., and R. A. DePinho. 2004. Telomeres and the DNA damage response: why the fox is guarding the henhouse. DNA Repair (Amsterdam) 3:979-988. [DOI] [PubMed] [Google Scholar]
- 61.Mieczkowski, P. A., J. O. Mieczkowska, M. Dominska, and T. D. Petes. 2003. Genetic regulation of telomere-telomere fusions in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100:10854-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills, K. D., D. O. Ferguson, and F. W. Alt. 2003. The role of DNA breaks in genomic instability and tumorigenesis. Immunol. Rev. 194:77-95. [DOI] [PubMed] [Google Scholar]
- 63.Moore, J. K., and J. E. Haber. 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2164-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nacht, M., A. Strasser, Y. R. Chan, A. W. Harris, M. Schlissel, R. T. Bronson, and T. Jacks. 1996. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 10:2055-2066. [DOI] [PubMed] [Google Scholar]
- 65.Olovnikov, A. M. 1973. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41:181-190. [DOI] [PubMed] [Google Scholar]
- 66.Pedram, M., C. N. Sprung, Q. Gao, A. W. Lo, G. E. Reynolds, and J. P. Murnane. 2006. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol. Cell. Biol. 26:1865-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perrod, S., and S. M. Gasser. 2003. Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cell. Mol. Life Sci. 60:2308-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perryman, L. E. 2004. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet. Pathol. 41:95-100. [DOI] [PubMed] [Google Scholar]
- 69.Porter, S. E., P. W. Greenwell, K. B. Ritchie, and T. D. Petes. 1996. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 24:582-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potten, C. S., and M. Loeffler. 1990. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110:1001-1020. [DOI] [PubMed] [Google Scholar]
- 71.Rathmell, W. K., W. K. Kaufmann, J. C. Hurt, L. L. Byrd, and G. Chu. 1997. DNA-dependent protein kinase is not required for accumulation of p53 or cell cycle arrest after DNA damage. Cancer Res. 57:68-74. [PubMed] [Google Scholar]
- 72.Riha, K., J. M. Watson, J. Parkey, and D. E. Shippen. 2002. Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J. 21:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rooney, S., F. W. Alt, D. Lombard, S. Whitlow, M. Eckersdorff, J. Fleming, S. Fugmann, D. O. Ferguson, D. G. Schatz, and J. Sekiguchi. 2003. Defective DNA repair and increased genomic instability in Artemis-deficient murine cells. J. Exp. Med. 197:553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rooney, S., J. Chaudhuri, and F. W. Alt. 2004. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol. Rev. 200:115-131. [DOI] [PubMed] [Google Scholar]
- 75.Rooney, S., J. Sekiguchi, S. Whitlow, M. Eckersdorff, J. P. Manis, C. Lee, D. O. Ferguson, and F. W. Alt. 2004. Artemis and p53 cooperate to suppress oncogenic N-myc amplification in progenitor B cells. Proc. Natl. Acad. Sci. USA 101:2410-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudolph, K. L., S. Chang, H. W. Lee, M. Blasco, G. J. Gottlieb, C. Greider, and R. A. DePinho. 1999. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96:701-712. [DOI] [PubMed] [Google Scholar]
- 77.Rudolph, K. L., M. Millard, M. W. Bosenberg, and R. A. DePinho. 2001. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 28:155-159. [DOI] [PubMed] [Google Scholar]
- 78.Samper, E., F. A. Goytisolo, P. Slijepcevic, P. P. van Buul, and M. A. Blasco. 2000. Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep. 1:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao, C., L. Deng, O. Henegariu, L. Liang, N. Raikwar, A. Sahota, P. J. Stambrook, and J. A. Tischfield. 1999. Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice. Proc. Natl. Acad. Sci. USA 96:9230-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharpless, N. E., D. O. Ferguson, R. C. O'Hagan, D. H. Castrillon, C. Lee, P. A. Farazi, S. Alson, J. Fleming, C. C. Morton, K. Frank, L. Chin, F. W. Alt, and R. A. DePinho. 2001. Impaired nonhomologous end-joining provokes soft tissue sarcomas harboring chromosomal translocations, amplifications, and deletions. Mol. Cell 8:1187-1196. [DOI] [PubMed] [Google Scholar]
- 81.Smogorzewska, A., J. Karlseder, H. Holtgreve-Grez, A. Jauch, and T. de Lange. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12:1635-1644. [DOI] [PubMed] [Google Scholar]
- 82.Song, K., D. Jung, Y. Jung, S. G. Lee, and I. Lee. 2000. Interaction of human Ku70 with TRF2. FEBS Lett. 481:81-85. [DOI] [PubMed] [Google Scholar]
- 83.Stellwagen, A. E., Z. W. Haimberger, J. R. Veatch, and D. E. Gottschling. 2003. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17:2384-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taccioli, G. E., A. G. Amatucci, H. J. Beamish, D. Gell, X. H. Xiang, M. I. Torres Arzayus, A. Priestley, S. P. Jackson, A. Marshak Rothstein, P. A. Jeggo, and V. L. Herrera. 1998. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity 9:355-366. [DOI] [PubMed] [Google Scholar]
- 85.Takai, H., A. Smogorzewska, and T. de Lange. 2003. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13:1549-1556. [DOI] [PubMed] [Google Scholar]
- 86.van Steensel, B., A. Smogorzewska, and T. de Lange. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401-413. [DOI] [PubMed] [Google Scholar]
- 87.Vaziri, H., F. Schachter, I. Uchida, L. Wei, X. Zhu, R. Effros, D. Cohen, and C. B. Harley. 1993. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 52:661-667. [PMC free article] [PubMed] [Google Scholar]
- 88.Verdun, R. E., L. Crabbe, C. Haggblom, and J. Karlseder. 2005. Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol. Cell 20:551-561. [DOI] [PubMed] [Google Scholar]
- 89.Vogel, H., D. S. Lim, G. Karsenty, M. Finegold, and P. Hasty. 1999. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl. Acad. Sci. USA 96:10770-10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watson, J. D. 1972. Origin of concatemeric T7 DNA. Nat. New Biol. 239:197-201. [DOI] [PubMed] [Google Scholar]
- 91.Wei, C., R. Skopp, M. Takata, S. Takeda, and C. M. Price. 2002. Effects of double-strand break repair proteins on vertebrate telomere structure. Nucleic Acids Res. 30:2862-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong, K. K., S. Chang, S. R. Weiler, S. Ganesan, J. Chaudhuri, C. Zhu, S. E. Artandi, K. L. Rudolph, G. J. Gottlieb, L. Chin, F. W. Alt, and R. A. DePinho. 2000. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 26:85-88. [DOI] [PubMed] [Google Scholar]
- 93.Woo, R. A., M. T. Jack, Y. Xu, S. Burma, D. J. Chen, and P. W. Lee. 2002. DNA damage-induced apoptosis requires the DNA-dependent protein kinase, and is mediated by the latent population of p53. EMBO J. 21:3000-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu, C., K. D. Mills, D. O. Ferguson, C. Lee, J. Manis, J. Fleming, Y. Gao, C. C. Morton, and F. W. Alt. 2002. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell 109:811-821. [DOI] [PubMed] [Google Scholar]
- 95.Zhu, X. D., B. Kuster, M. Mann, J. H. Petrini, and T. de Lange. 2000. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25:347-352. [DOI] [PubMed] [Google Scholar]
- 96.Zhu, X. D., L. Niedernhofer, B. Kuster, M. Mann, J. H. Hoeijmakers, and T. de Lange. 2003. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell 12:1489-1498. [DOI] [PubMed] [Google Scholar]