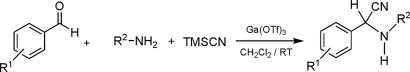Abstract
The synthesis of α-aminonitriles and their fluorinated analogs has been carried out in high yield and purity by the Strecker reaction from the corresponding ketones and amines with trimethylsilyl cyanide using gallium triflate in dichloromethane. Monofluoro-, difluro-, or trifluoromethyl groups can be incorporated into the α-aminonitrile product by varying the nature of the fluorinated ketones. Study with various fluorinated and nonfluorinated ketones reveals that the choice of proper catalyst and the solvent system (suitable metal triflates as a catalyst and dichloromethane as a solvent) plays the key role in the direct Strecker reactions of ketones.
Keywords: three-component reaction, α-aminonitriles
One of the most important multicomponent reactions is the Strecker reaction to synthesize α-amino acids via the formation of α-aminonitriles (1). However, successful three component Strecker reactions using ketones and fluorinated ketones are rare (2–14). Fluorinated amino acids are becoming increasingly important in pharmaceuticals and other biological applications (15–21), such as the development of anticancer drugs for the control of tumor growth and drugs for the control of blood pressure and allergies (22). They have been shown as irreversible inhibitors of pyridoxal phosphate-dependent enzymes (23). Also, recent studies with fluorinated amino acids have shown the possibilities for the design and construction of hyperstable protein folds and studies of the protein–protein interaction for unnatural amino acids (24–30). Fluorinated amino acids are also a valuable tool for the screening of protein dynamics by NMR studies (24–30). Consequently, fluorinated amino acids have become the object of intense synthetic activity in recent years.
The importance of Lewis acid catalysis in organic synthetic reactions has been well documented (31, 32). However, most of the strong and efficient Lewis acids such as AlCl3, AlBr3, SbF5, etc., are prone to fast hydrolysis and consequent deactivation. They are used in stoichiometric amounts and are not reusable in many cases. Therefore, reactions involving these catalysts generally require water free conditions and large amounts of the catalysts. We have found that gallium (III) trifluoromethanesulfonate [Ga(OTf)3, gallium triflate], acts as an effective but mild and nonhydrolysable Lewis acid catalyst for many organic synthetic transformations such as Friedel–Crafts alkylations, dehydration of oximes to the corresponding nitriles, Beckman rearrangement, etc. (33–36). This catalyst can be easily recovered from the reaction mixture and reused, showing its significant potential as a safe and environmentally benign catalyst. Herein, we report the results of the synthesis of both fluorinated and nonfluorinated α-aminonitriles from the corresponding ketones and amines with trimethylsilyl cyanide (TMSCN) using a catalytic amount (5 mol%) of gallium triflate as a catalyst in dichloromethane. These reactions are fast and clean, with no further purification required in most of the cases.
Results and Discussion
The Strecker reaction with aldehydes has been studied extensively with a variety of catalysts (37–46) including a number of metal triflates (47–49). However, the reactions are not feasible for ketones. Efficient, clean, and direct three-component Strecker reaction using ketones is difficult. Quite often, these reactions have to be carried out stepwise (preparation of imines first followed by cyanide addition) (2,3) or under high pressure conditions (6,7). Use of ammonia or ammonium salts in the presence of cyanides has been described (8–13). As a first step, therefore, we performed the Strecker reaction of aldehydes with different types of amines to check the potential of gallium triflate as a catalyst in dichloromethane as a solvent (Scheme 1). The reaction is found to be clean and simple, giving the products in good to excellent yields (Table 1.
Scheme 1.
Ga(OTf)3 catalyzed Strecker reaction using different aldehydes and amines.
Table 1.
Ga(OTf)3 catalyzed Strecker reaction using different aldehydes and amines
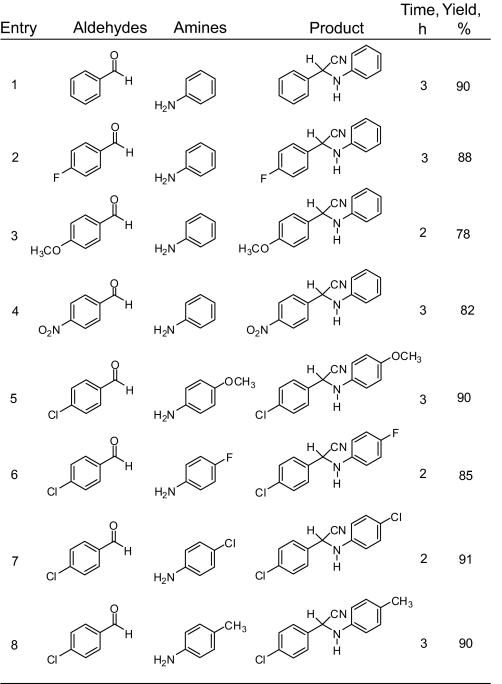
Encouraged by our results of the Strecker reaction with aldehydes, we directed our study toward ketones and performed the Strecker reaction under similar conditions (Scheme 2). It has been reported that the Strecker reaction of acetophenone even with the activated amine 3,4,5-trimethylaniline using metal triflates and acetonitrile as the solvent gave very poor yield of the product (47–49). We found that a similar reaction of acetophenone with aniline and TMSCN (cyanide source) in dichloromethane using Ga(OTf)3 as a catalyst proceeds smoothly under mild conditions (room temperature, 5 h) giving the corresponding α-aminonitrile in excellent yield and high purity (Table 2, entry 1), despite that the direct Strecker reaction using aromatic ketones and aromatic amines has been repeatedly cited in the literature as a challenge (6, 7, 37–46). We performed the reaction with a variety of ketones and anilines at room temperature in dichloromethane, and in all cases high yields of the nitrile products were obtained emphasizing the generality of our methodology. The results are shown in Table 2.
Scheme 2.
Ga(OTf)3 catalyzed Strecker reaction using ketones and amines.
Table 2.
Ga(OTf)3 catalyzed Strecker reaction using ketones and amines
*Heated at 50°C with 2 equivalents of TMSCN and 1.5 equivalents of aniline.
We also screened other metal triflate catalysts for their catalytic activity and found them to be effective in most cases (Table 3). However, gallium triflate was found to be the most useful giving the highest yield of products (Table 3, entry 1). The reaction with neodymium triflate (Table 3, entry 8) was incomplete, giving a mixture of the corresponding imine and the α-aminonitrile product. These results point toward the need of the proper catalyst and solvent system (suitable metal triflate as a catalyst and dichloromethane as a solvent in present cases), which play the key role for the success of the reaction. In earlier studies (37–49), acetonitrile and toluene were used as solvents; however, these are not suitable for the Lewis acid catalyzed direct Strecker reaction of ketones due to their interaction with the catalyst. The use of dichloromethane minimizes such interaction, resulting in enhanced catalytic activity of the catalyst toward ketones and providing a suitable environment for the reaction.
Table 3.
Strecker reaction of acetophenone and aniline catalyzed by various metal triflates
| Entry | Catalyst | Yield, %* |
|---|---|---|
| 1 | Ga(OTf)3 | 98 |
| 2 | Yb(OTf)3 | 92 |
| 3 | Y(OTf)3 | 85 |
| 4 | Sc(OTf)3 | 89 |
| 5 | Sm(OTf)3 | 90 |
| 6 | La(OTf)3 | 75 |
| 7 | Cu(OTf)2 | 80 |
| 8 | Nd(OTf)3 | 75† |
Time, 5 h; Amount of catalyst, 5 mol%.
*Isolated yield.
†Determined by NMR analysis.
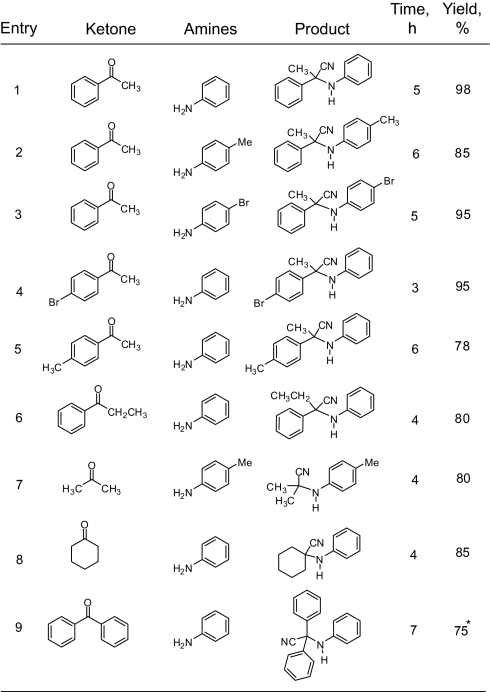
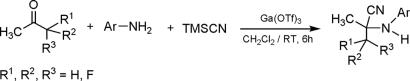
For different amines and ketones, feasibility of the reaction and selectivity of the expected product depend on various electronic as well as steric factors. For example, reaction of benzophenone required higher temperature and excess of TMSCN to afford the desired α-aminonitrile (Table 2, entry 9). Reactions with aliphatic amines under ambient conditions led to mixtures with considerable drop in selectivity. However, benzylamine gave significant amount of the Strecker product. Therefore, it is indicated that gallium triflate provides the best Lewis acidity required for successful reactions (Table 2). Any significant change in the basicity of the amines and the steric environment of the amines/ketones results in a significant change in the rate of the three component reaction and the selectivity of α-aminonitriles. Encouraged by these results, we extended our methodology to fluorinated ketones. We found that mono-, di-, and trifluoromethylated ketones react smoothly with a variety of amines under mild conditions to provide the corresponding fluorinated α-aminonitriles in high yield and purity. One of the significant aspects of this methodology is that we can incorporate mono-, di-, or trifluoromethyl moiety in the α-aminonitrile product by simply varying the nature of the fluorinated ketones (Scheme 3). Fluorinated ketones, of course, are much more reactive than nonfluorinated ones.
Scheme 3.
Ga(OTf)3 catalyzed Strecker reaction of fluorinated ketones.
Due to the specific properties of F atom such as small size, high electronegativity, C F bond strength, etc., the introduction of F atom into many biologically active molecules can bring about remarkable and profound changes in their physical, chemical, and biological behavior (50–57). Fluorine-containing amino acids have been widely used in biological tracers, mechanistic probes, enzyme inhibitors, and in many medical applications.
F bond strength, etc., the introduction of F atom into many biologically active molecules can bring about remarkable and profound changes in their physical, chemical, and biological behavior (50–57). Fluorine-containing amino acids have been widely used in biological tracers, mechanistic probes, enzyme inhibitors, and in many medical applications.
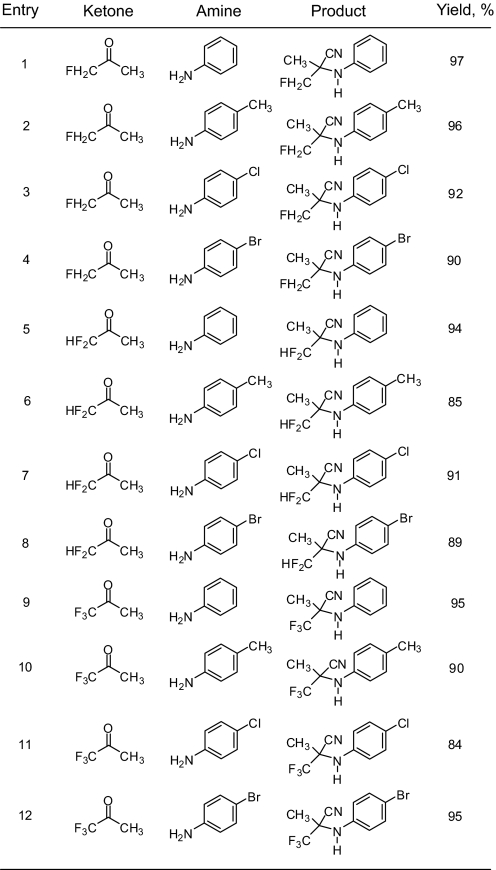
Our method is also feasible with aliphatic fluorinated ketones. However, it is interesting to note that, with aromatic trifluoromethyl ketones such as 1,1,1-trifluoroacetophenone, instead of the expected three-component reaction product, the trimethylsilyl-protected fluorinated cyanohydrin derivative (TMSCN addition product from 1,1,1-trifluoroacetophenone) was obtained. Hence, success of the overall reaction depends on the rate of the two possible routes; initial aminal/imine formation and the TMSCN addition to ketones. It is probable that, in the case of the aliphatic fluorinated ketones, the rate of initial aminal/imine formation is fast compared with the rate of the cyanohydrin adduct formation and subsequently the desired products from three-component reaction were formed predominantly. However, in the case of 1,1,1-trifluoroacetophenone and its derivatives, the rate of the cyanohydrin adduct formation is higher compared with the rate of aminal or imine formation; hence, the TMS protected cyanohydrin adduct was observed instead of the three-component reaction product. Table 4 shows the results of the Strecker reaction for different fluorinated ketones and a variety of amines. One major advantage of this procedure is that no further purification is needed, thus avoiding tedious chromatography and loss of products during purification. The products are obtained in very high yield and purity.
Table 4.
Ga(OTf)3 catalyzed Strecker reaction of mono-, di-, and trifluoromethyl ketones
In summary, the application of Ga(OTf)3 as an effective water tolerable, reusable catalyst for the Strecker reaction has been demonstrated. Our studies show that not only aldehydes but also ketones and fluorinated ketones can efficiently undergo the Strecker reaction under very mild conditions using Ga(OTf)3 as the catalyst in dichloromethane. Various metal triflates show good catalytic activity for the Strecker reaction. However, Ga(OTf)3 is the preferred catalyst of choice in the series due to its nonhydrolysable and reusable nature as additional advantages. Simple and clean reaction, high yields, and high purity of the products are the salient features of this methodology. Our method also provides an efficient alternate route for the existing high pressure and stepwise methodologies for the Strecker reaction of ketones. Furthermore, it provides a general reaction for the synthesis of mono-, di-, and trifluoromethylated amino acids via the formation of the corresponding aminonitrile intermediates. Further studies are required to render the reaction stereoselective by employing chiral ligands in conjunction with Ga(OTf)3 and related Lewis acid catalysts.
Materials and Methods
Ga metal (99.999%) was purchased from Aldrich (Milwaukee, WI), and trifluoromethanesulfonic acid (99.5%) was available from 3M (St. Paul, MN). Most of the aldehydes, ketones and amines were from Aldrich. TMSCN and the fluorinated ketones were also available from different commercial sources.
Preparation of Ga(OTf)3.
Ga(OTf)3 has been pepared following a reported procedure (58). Gallium metal (2.45 g, 35 mmol) was placed in a 100-ml round-bottomed flask, and trifluoromethanesulfonic acid (30 g, 200 mmol) was then added and stirred at 150°C for 24 h. After cooling the mixture to 0°C, the mixture was poured into 200 g of ice, then filtered to remove any unreacted Ga. Water and excess triflic acid was removed by evaporation, and the mixture was dried by heating at 200°C for 5 h using a P2O5 trap under vacuum. Ga(OTf)3 was obtained as a white powder (12.2 g, 67%).
General Method for the Ga(OTf)3 Catalyzed Strecker Reactions of Aldehydes, Ketones, and Fluorinated Ketones.
Adehyde or ketone (2 mmol)/fluorinated ketone (3 mmol) and amine (2 mmol) dissolved in 4 ml of CH2Cl2 was added to Ga(OTf)3 (52 mg, 5 mol %) in a pressure tube. TMSCN (3 mmol) was then added to the reaction mixture and pressure tube was closed. The mixture was stirred at room temperature until the completion of the reaction with monitoring at different time intervals by TLC and NMR. The mixture was then filtered and the residue was washed with CH2Cl2 (three times, 15 ml). The filtrate was collected, and the solvent was removed under reduced pressure to obtain the product. Further purification can be carried out by tituration of the residue with excess hexane followed by evaporation of hexane. Products were characterized by spectral analysis (1H NMR, 13C NMR, 19F NMR, and HRMS), and the spectral data and representative NMR images are included in the supporting information (SI).
Supplementary Material
Acknowledgments
This work is dedicated to Prof. Horst Prinzbach with admiration and friendship on the occasion of his 75th birthday. Support by Loker Hydrocarbon Research Institute is gratefully acknowledged.
Abbreviation
- TMSCN
trimethylsilyl cyanide.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611316104/DC1.
References
- 1.Strecker A. Liebigs Ann Chim. 1850;75:27–51. [Google Scholar]
- 2.Warmuth R, Munsch TE, Stalker RA, Li B, Beatty A. Tetrahedron. 2001;57:6383–6397. [Google Scholar]
- 3.Surendra K, Krishnaveni NS, Mahesh A, Rao KR. J Org Chem. 2006;71:2532–2534. doi: 10.1021/jo052510n. [DOI] [PubMed] [Google Scholar]
- 4.Suginome M, Yamamoto A, Ito Y. Chem Commun. 2002:1392–1393. [Google Scholar]
- 5.Fetterly BM, Jana NK, Verkade JG. Tetrahedron. 2006;62:440–456. [Google Scholar]
- 6.Matsumoto K, Kim JC, Iida H, Hamana H, Kumamoto K, Kotsuki H, Jenner G. Helv Chim Acta. 2005;88:1734–1754. [Google Scholar]
- 7.Jenner G, Salen RB, Kim JC, Matsumoto K. Tetrahedron Lett. 2003;44:447–449. [Google Scholar]
- 8.Rousset A, Lasperas M, Taillades J, Commeyras A. Tetrahedron. 1980;36:2649–2661. [Google Scholar]
- 9.Pascal R, Taillades J, Commeyras A. Tetrahedron. 1978;34:2275–2281. [Google Scholar]
- 10.Bejaud M, Mion L, Commeyras A. Tetrahedron Lett. 1975;34:2985–2986. [Google Scholar]
- 11.Taillades J, Commeyras A. Tetrahedron. 1974;30:127–132. [Google Scholar]
- 12.Taillades J, Commeyras A. Tetrahedron. 1974;30:2493–2501. [Google Scholar]
- 13.Taillades J, Commeyras A. Tetrahedron. 1974;30:3407–3417. [Google Scholar]
- 14.McConathi J, Martarello L, Malveaux EJ, Camp VM, Simpson NE, Simpson CP, Bowers GD, Olson JJ, Goodman MM. J Med Chem. 2002;45:2240–2249. doi: 10.1021/jm010241x. [DOI] [PubMed] [Google Scholar]
- 15.Qiu X-L, Meng W-D, Quing F-L. Tetrahedron. 2004;60:6711–6745. [Google Scholar]
- 16.Sutherland A, Willis CL. Nat Prod Rep. 2000;17:621–631. doi: 10.1039/a707503k. [DOI] [PubMed] [Google Scholar]
- 17.Haufe G, Kroger S. Amino Acids. 1996;11:409–424. doi: 10.1007/BF00807945. [DOI] [PubMed] [Google Scholar]
- 18.Tolman V. Amino Acids. 1996;11:15–36. doi: 10.1007/BF00805718. [DOI] [PubMed] [Google Scholar]
- 19.Kelly NM, Sutherland A, Willis CL. Nat Prod Rep. 1997;14:205–219. [Google Scholar]
- 20.Dev R, Badet B, Meffre P. Amino Acids. 2003;24:245–261. doi: 10.1007/s00726-002-0410-9. [DOI] [PubMed] [Google Scholar]
- 21.Kukhar VP, Soloshonok VA. Fluorine Containing Amino Acids: Synthesis and Properties. New York: Wiley; 1995. [Google Scholar]
- 22.Filler R, Kobayashi Y, Yagupolskii LM. Biomedical Aspects of Fluorine Chemistry. Amsterdam: Elsevier; 1993. [Google Scholar]
- 23.Yoder NC, Kumar K. Chem Soc Rev. 2002;31:335–341. doi: 10.1039/b201097f. [DOI] [PubMed] [Google Scholar]
- 24.Marsh ENG. Chem Biol. 2000;7:R153–R157. doi: 10.1016/s1074-5521(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 25.Salopek-Sondi B, Vaughan MD, Skeels MC, Honek JF, Luck LA. J Biomol Struct Dyn. 2003;21:235–246. doi: 10.1080/07391102.2003.10506919. [DOI] [PubMed] [Google Scholar]
- 26.Bai P, Luo L, Peng Z. Biochemistry. 2000;39:372–380. doi: 10.1021/bi992056f. [DOI] [PubMed] [Google Scholar]
- 27.Fischer M, Schott A-K, Kemter K, Feicht R, Ritcher G, Illarinov B, Eisenreich W, Gerhardt S, Cushman M, Steinbacher S, et al. BMC Biochem. 2003;4(18):1–19. doi: 10.1186/1471-2091-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danielson MA, Falke JJ. Annu Rev Biophys Biomol Struct. 1996;25:163–195. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ropson IJ, Frieden C. Proc Natl Acad Sci USA. 1992;89:7222–7226. doi: 10.1073/pnas.89.15.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cairi M, Gerig JT, Hammond SJ, Klinkenborg JC, Nieman RA. Bull Magn Reson. 1983;5:157–160. [Google Scholar]
- 31.Yamamoto H. Lewis Acids in Org Synthesis. Vols 1 and 2. Weinheim, Germany: Wiley; 2000. [Google Scholar]
- 32.Pons J-M, Santelli M. Lewis Acids and Selectivity in Org Synthesis. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 33.Olah GA, Farooq O, Farnia SMF, Olah JA. J Am Chem Soc. 1988;110:2560–2565. [Google Scholar]
- 34.Olah GA, Farooq O, Cheng LX, Farnia MAF, Aklonis JJ. J Appl Polym Sci. 1992;45:1355–1360. [Google Scholar]
- 35.Yan P, Batamack P, Prakash GKS, Olah GA. Catal Lett. 2005;101:141–143. [Google Scholar]
- 36.Prakash GKS, Yan P, Török B, Bucsi I, Tanaka M, Olah GA. Catal Lett. 2003;85:1–6. [Google Scholar]
- 37.Heydari A, Fatemi P, Alizadeh A-A. Tetrahedron Lett. 1998;39:3049–3050. [Google Scholar]
- 38.Kobayashi S, Nagayama S, Busujima T. Tetrahedron Lett. 1996;37:9221–9224. [Google Scholar]
- 39.De SK. J Mol Catal A. 2005;232:123–125. [Google Scholar]
- 40.Horenstein BA, Nakanishi K. J Am Chem Soc. 1989;111:6242–6246. [Google Scholar]
- 41.Mulzer J, Meier A, Buschmann J, Luger P. Synthesis. 1996:123–132. [Google Scholar]
- 42.De SK. Synth Commun. 2005;35:653–656. [Google Scholar]
- 43.De SK, Gibbs RA. Tetrahedron Lett. 2004;45:7407–7408. [Google Scholar]
- 44.Yadav JS, Reddy BVS, Eshwaraiah B, Sreenivas M. Tetrahedron. 2004;60:1767–1771. [Google Scholar]
- 45.Martínez R, Ramón DJ, Yus M. Tetrahedron Lett. 2005;46:8471–8474. [Google Scholar]
- 46.Chen W-Y, Lu J. Synlett. 2005:2293–2296. [Google Scholar]
- 47.Yadav JS, Reddy BVS, Eshwaraiah B, Sreenivas M, Vishnumurthy P. New J Chem. 2003;27:462–465. [Google Scholar]
- 48.Royer L, De SK, Gibbs RA. Tetrahedron Lett. 2005;46:4595–4597. [Google Scholar]
- 49.Kobayashi S, Busujima T, Nagayama S. Chem Commun. 1998:981–982. [Google Scholar]
- 50.Kirsch P. Modern Fluoroorganic Chemistry. Weinheim, Germany: Wiley; 2004. [Google Scholar]
- 51.Chambers RD. Fluorine in Organic Chemistry. Oxford: Blackwell; 2004. [Google Scholar]
- 52.Smart RE, Banks BE, Tatlow JC. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum; 1994. [Google Scholar]
- 53.Welch JT, Eswarakrishnan S. Fluorine in Bioorg Chem. New York: Wiley; 1991. [Google Scholar]
- 54.Ojima I, McCarthy JR, Welch JT. Biomedical Frontiers of Fluorine Chemistry. Vol. 369. Washington, DC: Am Chem Soc; 1996. ACS Symposium Series. [Google Scholar]
- 55.Banks RE. Organofluorine Chemicals and their Industrial Applications. New York: Ellis Harwood; 1979. [Google Scholar]
- 56.Peters R. Carbon-Fluorine Coimpounds Chemistry, Biochemistry and Biological Activities: A Ciba Foundation Symposium. Amsterdam: Elsevier; 1972. [PubMed] [Google Scholar]
- 57.Walsh CT. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]
- 58.Boumizane K, Herzog-Cance MH, Jones DJ, Pascal JL, Potier J, Roziere J. Polyhedron. 1991;10:2757–2769. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.