Abstract
C2 domains are autonomously folded protein modules that generally act as Ca2+- and phospholipid-binding domains and/or as protein–protein interaction domains. We now report the primary structures and biochemical properties of a family of evolutionarily conserved mammalian proteins, referred to as E-Syts, for extended synaptotagmin-like proteins. E-Syts contain an N-terminal transmembrane region, a central juxtamembranous domain that is conserved from yeast to human, and five (E-Syt1) or three (E-Syt2 and E-Syt3) C-terminal C2 domains. Only the first E-Syt C2 domain, the C2A domain, includes the complete sequence motif that is required for Ca2+ binding in C2 domains. Recombinant protein fragments of E-Syt2 that include the first C2 domain are capable of Ca2+-dependent phospholipid binding at micromolar concentrations of free Ca2+, suggesting that E-Syts bind Ca2+ through their first C2 domain in a phospholipid complex. E-Syts are ubiquitously expressed, but enriched in brain. Expression of myc-tagged E-Syt proteins in transfected cells demonstrated localization to intracellular membranes for E-Syt1 and to plasma membranes for E-Syt2 and E-Syt3. Structure/function studies showed that the plasma-membrane localization of E-Syt2 and E-Syt3 was directed by their C-terminal C2C domains. This result reveals an unexpected mechanism by which the C2C domains of E-Syt2 and E-Syt3 functions as a targeting motif that localizes these proteins into the plasma membrane independent of their transmembrane region. Viewed together, our findings suggest that E-Syts function as Ca2+-regulated intrinsic membrane proteins with multiple C2 domains, expanding the repertoire of such proteins to a fourth class beyond synaptotagmins, ferlins, and MCTPs (multiple C2 domain and transmembrane region proteins).
Keywords: calcium, exocytosis, membrane traffic, protein targeting
C2 domains were identified as a conserved sequence motif in protein kinase C isoforms (1) and shown to represent autonomously folded Ca2+-binding domains in synaptotagmin-1 (2, 3). C2 domains are now recognized as the second most common Ca2+-binding module in the proteome after the smaller, more frequent EF-hand module (4). All C2 domains are composed of a stable eight-stranded β-sandwich that contains flexible loops at the top and bottom, with “top” and “bottom” defined by reference to the synaptotamin-1 C2A domain, the first C2 domain whose atomic structure and Ca2+-binding mode were determined (5, 6).
C2 domains come in two topological variations that are circular permutations of each other (reviewed in ref. 6). In type I C2 domains, e.g., those of synaptotagmin-1, the β-strands are arranged in a linear manner and the C and N termini emerge at the top of the domain (5). In contrast, in type II C2 domains as first found in the phospholipase Cδ C2 domain (7), the N terminus of the C2 domain is formed by β-strand 2 and the C terminus by β-strand 1, and the N and C termini emerge at the bottom of the C2 domain. In all C2 domains that bind Ca2+, Ca2+ binds exclusively to the top loops, coordinated by five conserved aspartate or asparagine residues (8). Although the Ca2+-binding modes of all C2 domains are the same, their precise Ca2+-binding properties differ. Some C2 domains, such as those of synaptotagmin-1, exhibit a low intrinsic Ca2+ affinity that is boosted several orders of magnitude by the presence of phospholipids (2, 8). In contrast, other C2 domains, such as those of rabphilin, display a high intrinsic Ca2+ affinity even in the absence of phospholipids (9). Moreover, not all C2 domains bind Ca2+. A subset of C2 domains lacks the residues involved in Ca2+ binding, making Ca2+ binding impossible (e.g., see refs. 10 and 11). More surprisingly, at least in the C2B domain of synaptotagmins 4 and 11, the canonical Ca2+-binding residues are present, but the domains nevertheless do not bind Ca2+ because a subtle change in the orientation of the β-strands makes it impossible for the top loops to actually coordinate Ca2+ (12).
Most but not all Ca2+-dependent, and some Ca2+-independent, C2 domains bind to phospholipids, as initially found for the synaptotagmin-1 C2A domain (2, 3) and the Pten C2 domain (10), respectively. In addition, several Ca2+-independent C2 domains constitute protein-interaction domains, as revealed in the crystal structure of the Munc13–1 C2A domain in a complex with the RIM zinc finger domain (13). Moreover, at least in synaptotagmin-1, Ca2+-binding to the C2 domains triggers their interaction with both phospholipids and SNARE complexes, suggesting a multifaceted functional activity at the phospholipid/protein interface (14, 15) and demonstrating that the same C2 domain can simultaneously bind to protein and phospholipids.
C2 domains are primarily found in proteins that function in membrane traffic and/or signal transduction, with synaptotagmins and protein kinase C as the prime examples. In membrane trafficking proteins, C2 domains are generally present in multiple copies, whereas signal transduction proteins usually have only a single C2 domain. In the latter, the C2 domains usually function to localize the respective protein to the membrane, as shown for example for protein kinase C (16) or Pten (10). In contrast, in C2-domain proteins that function in membrane traffic, the C2 domains generally act as effector domains, with the proteins being attached to membranes either by a transmembrane region (TMR; e.g., see synaptotagmins) or by binding to a membrane protein [e.g., see rabphilin that binds to Rab3 (17)]. Few C2-domain proteins are conserved in yeast, for example the phosphatidylserine decarboxylase Psd2p (reviewed in ref. 18), protein kinase C (19), and the ubiquitin ligase Rsp5p (20).
Three families of putative trafficking proteins containing multiple C2 domains and a single TMR have been described: synaptotagmins, ferlins, and MCTPs (21–23). In addition, the sequence of a fourth type of protein with multiple C2 domains and a single TMR was reported from rat adipocytes (24). Interestingly, this adipocyte protein is evolutionarily related to a family of membrane proteins in yeast containing three C2 domains that were called tricalbins (25). Tricalbins may thus be noteworthy because they may represent the only proteins resembling synaptotagmins that are conserved in yeast, although the properties and expression of these proteins and their mammalian counterparts have not been examined.
In this study, we define a family of homologous proteins that we refer to as E-syts, for extended synaptotagmins, because of their similarity to synaptotagmins. E-Syts differ from yeast tricalbins, to which they are related, in that they are Ca2+-binding proteins. E-Syt1 contains five domains, and E-Syt2 and E-Syt3 contain three C2 domains. E-Syt1 is localized to intracellular membranes, whereas E-Syt2 and E-Syt3 are localized to the plasma membrane. Interestingly, E-Syt2 and E-Syt3 are targeted to the plasma membrane by a mechanism that does not depend on their TMR, but is instead determined by their Ca2+-independent third C2C domain. Thus, E-Syts represent a family of Ca2+-binding membrane proteins that form a heterogeneous group of proteins, two of which are targeted to the plasma membrane by a unique mechanism.
Results
Structure and Expression of E-Syts.
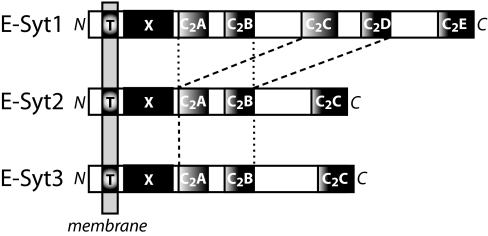
We identified E-Syts by databank searches for C2 domain proteins that contain a TMR (26). Analyses of vertebrate sequences uncovered three evolutionarily conserved and closely related E-Syt proteins that are distantly homologous to yeast tricalbins (25). One of these proteins, named here E-Syt1, was originally described by Morris et al. (24) as an anonymous membrane protein isolated from intracellular vesicles. We assembled full-length sequences for E-Syt1, E-Syt2, and E-Syt3 from EST clones and verified their primary structures by sequencing multiple independent clones and comparing the resulting sequences with database entries. The domain structures of E-Syts emerging from these analyses are shown in Fig. 1, and an alignment of the human E-Syt sequences and of their worm and insect homologs is depicted in [supporting information (SI) Fig. 5].
Fig. 1.
Domain structures of E-Syts. T, TMR; X, X domain unique to E-Syts; C2A to C2E, C2 domains (note that the C2C and C2D domains of E-Syt1 are highly homologous to the C2A and C2B domains of all E-Syts). For an alignment of E-Syt sequences, see SI Fig. 5.
E-Syts consist of a short, nonconserved N-terminal sequence, a single N-terminal TMR, a conserved domain that differs from other identified domains in the databanks (here referred to as “X”), and either five C2 domains (E-Syt1) or three C2 domains (E-Syt2 and E-Syt 3). The TMR, X domain, and C2A domain closely follow each other without a discernable linker sequence separating them. In contrast, the C2A and C2B domains in all E-Syts are connected by a short variable linker (≈20 residues), and the C2B and C2C domains are connected by a long variable linker (>100 residues). Apart from the X domain, the C2 domains, and the linker sequences, E-Syts have no recognizable domains, suggesting that their functions are mediated by the X and C2 domains. In E-Syt1, the third and fourth C2 domains (the C2C and C2D domains that are absent from E-Syt2 and E-Syt3) are highly homologous to the first and second C2 domains (the C2A and C2B domains), respectively (SI Fig. 5). This suggests that evolutionarily, the C2C and C2D domains were duplicated in E-Syt 1 (Fig. 1 and SI Fig. 5).
All E-Syt C2 domains are composed of a type 2 topology as defined originally for the phospholipase Cδ C2 domain (7). In mammalian, worm, and insect E-Syts, only the C2A domain contains the canonical Ca2+-binding residues in the top loops between β-strands 2/3, 4/5, and 6/7. The other C2 domains lack several of the critical Ca2+-binding asparate/aparagine residues in these loops, suggesting that they are unlikely to bind Ca2+. The sequences of the C2 domains do not otherwise contain noticeable features except for the presence of a relatively long sequence in the top loop between β-strands 2/3 in the C2B domains of the vertebrate E-Syts, suggesting that this loop may have a more extensive structure. Interestingly, this long loop sequence is alternatively spliced, at least in E-Syt1 and E-Syt2 (SI Fig. 5).
To determine the tissue distribution of E-Syt expression, we performed RT-PCR analyses on various human tissues using E-Syt1-, E-Syt2-, and E-Syt3-specific primers and a normal human tissue cDNA panel (PrimGen, Bothell, WA) (see SI Experimental Procedures). We observed ubiquitous expression of all E-Syts, with an enrichment of E-Syt2 and E-Syt3 in cerebellum SI Fig. 6.
Phospholipid Binding by E-Syt2.
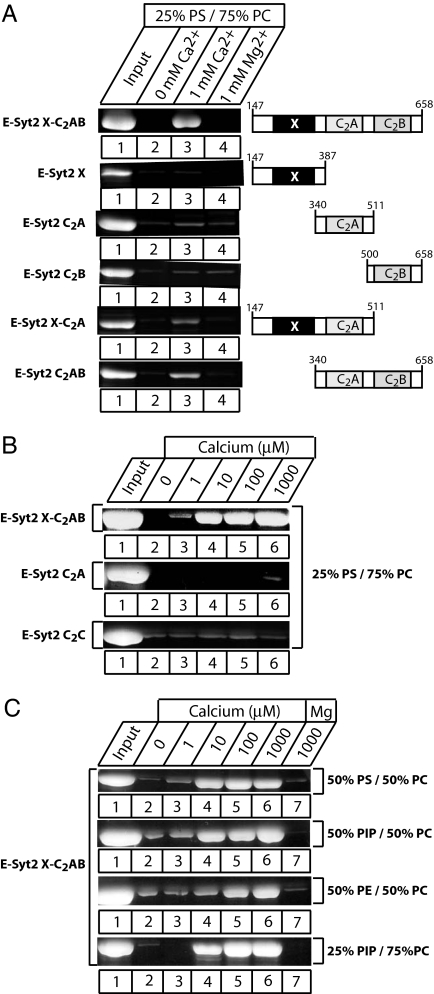
Because sequence analyses suggested that the C2A domain of E-Syts bind Ca2+, we investigated their potential Ca2+-binding properties using a series of recombinant proteins containing various fragments from E-Syt2 expressed as GST fusion proteins (Fig. 2A). We tested the binding of these protein fragments to liposomes composed of 25% phosphatidylserine (PS)/75% phosphatidylcholine (PC) in the absence or presence of divalent cations, using a sensitive centrifugation assay. We detected no phospholipid binding in the absence of divalent cations or in the presence of Mg2+ with any E-Syt2 fragments. In contrast, fusion proteins of the C2A domain, with either the X domain or the C2B domain or both, bound to liposomes in a Ca2+-specific manner. Although the only common denominator of these different E-Syt2 fragments was the presence of the C2A domain (Fig. 2A), the isolated C2A domain by itself only weakly bound to phospholipids in a Ca2+-dependent manner (Fig. 2B), possibly because the fragment used did not exactly include the precise domain boundaries.
Fig. 2.
Ca2+- and phospholipid-dependent membrane binding by E-Syt2 C2 domains. (A) Recombinant GST-fusion proteins containing the E-Syt2 domains indicated on the right were used in phospholipid-binding assays. Proteins were incubated in the absence of divalent cations, in 1 mM Ca2+, or in 1 mM Mg2+ with liposomes composed of 25% PS/75% PC (wt/wt). Liposomes were pelleted by centrifugation, and bound proteins were analyzed by SDS/PAGE and Coomassie staining. (B) Ca2+ dependence of phospholipid binding. Recombinant GST-fusion proteins containing either the E-Syt juxtamembranous X domain together with the C2Aand C2B domains (XC2AB), or only the C2A or C2C domain from E-Syt2, were incubated with liposomes composed of 25% PS/75% PC in the presence of the indicated concentrations of free Ca2+. Proteins bound to the liposomes were analyzed by centrifugation, followed by SDS/PAGE and Coomassie blue staining. (C) Phospholipid dependence of Ca2+-dependent membrane binding of the E-Syt2 C2AB domains. Phospholipid binding of a GST fusion protein containing the E-Syt2 C2AB domains was tested at the indicated Ca2+ concentrations and in the presence of 1 mM Mg2+ (as a negative control) in liposomes with the four different phospholipid compositions shown on the right. (PIP, phosphatidylinositol phosphate; PE, phosphatidylethanolamine).
We next examined the apparent Ca2+ affinity and phospholipid specificity of Ca2+-dependent phospholipid binding by the E-Syt2 X C2AB domain fragment (Fig. 2C). Binding was observed at micromolar concentrations of free Ca2+, similar to that observed for the synaptotagmin-1 C2 domains. Different from the synaptotagmin-1 C2 domains, however, E-Syt2 Ca2+-dependent phospholipid binding was not specific for negatively charged phospholipid but was also detectable with liposomes composed of neutral phospholipids (PE and PC; Fig. 2C), although negatively charged phospholipids exhibited a higher apparent Ca2+ affinity.
Subcellular Localization of E-Syts.
To determine the subcellular localization of E-Syts, we raised antibodies to various E-Syts by using recombinant protein fragments but were unable to generate antibodies of sufficiently high affinity to detect endogenous E-Syt proteins (data not shown). Thus, to circumvent this problem, we constructed vectors that express E-Syts with an N-terminal myc-epitope tag, either as full-length proteins or as N-terminally truncated proteins lacking the TMR. Expression of all of these proteins from the respective vectors was confirmed by immunoblotting in transfected HEK293 and COS cells (data not shown).
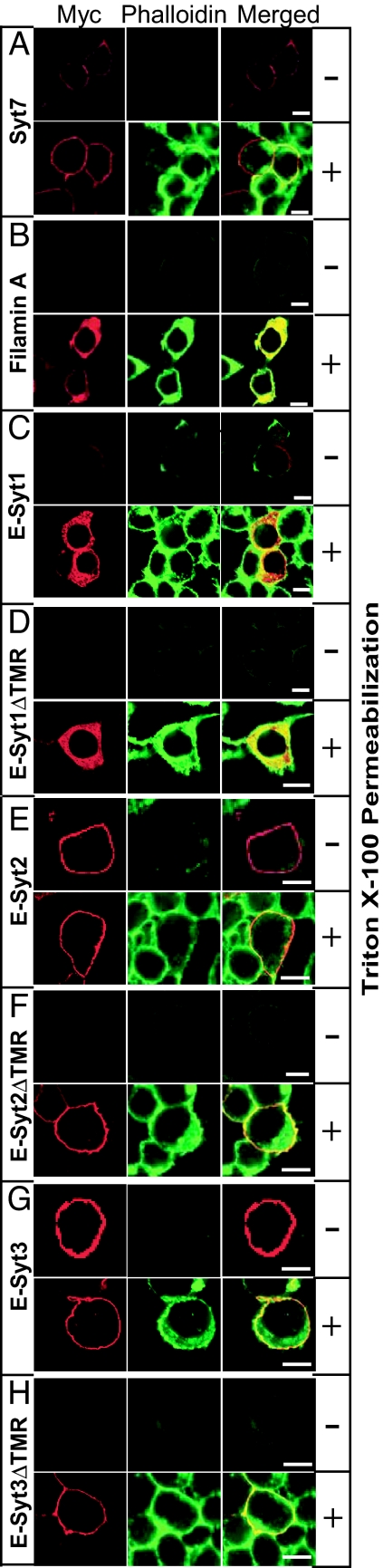
To immunolocalize E-Syts, we transfected HEK293 cells with the expression vectors and stained the cells with anti-myc epitope antibodies either without or with permeabilization (Fig. 3). This experiment was designed to test whether E-Syts are plasma membrane proteins, because plasma membrane localization would expose their N-terminal myc-tagged sequences on the cell surface and thus make them accessible to immunolabeling without permeabilization. As a positive control, we used N-terminally myc-tagged synaptotagmin-7 that is quantitatively deposited into the plasma membrane (27); as a negative control, we used N-terminally myc-tagged filamin A, an intracellular protein. Moreover, the N-terminally truncated E-Syts served as a further control because they do not contain noncytoplasmic sequences and thus should not be exposed on the cell surface. All transfected cells were probed with myc antibodies and with fluorescent phalloidin (to label the actin cytoskeleton).
Fig. 3.
Subcellular localization of E-Syts in HEK293 cells. Confocal images of HEK293 cells transfected with various constructs expressing the proteins indicated on the left. (A) Synaptotagmin-7 (Syt-7). (B) Filamin-A. (C, E, and G) full-length E-Syt1, E-Syt2, or E-Syt3. (D, F, and H) truncated E-Syt1 (E-Syt1ΔTMR), E-Syt2 (E-Syt2ΔTMR), or E-Syt3 (E-Syt3ΔTMR) lacking the N-terminal TMR. All proteins contained an N-terminal myc-epitope. Transfected cells were fixed and stained with anti-myc antibodies (Left, red) and fluorescein-labeled phalloidin (Center, green). (Right) Merged images). Cells were analyzed either without detergent permeabilization to probe surface-exposed epitopes or after detergent solubilization with Triton X-100 as indicated on the right. (Scale bars: 5 μm.)
We made three principal observations: (i) E-Syt1 was not detected in unpermeabilized cells but was observed in permeabilized cells in an unidentified intracellular compartment that did not resemble the Golgi apparatus, endoplasmic reticulum, lysosomes, or mitochondria (Fig. 3); (ii) E-Syt2 and E-Syt3 were fully stained on the cell surface in unpermeabilized transfected cells and appeared to be completely inserted into the plasma membrane, because permeabilization did not uncover additional intracellular staining for these E-Syts (Fig. 3); and (iii) the localization of E-Syts did not change upon removal of the TMR. As expected, E-Syt2 and E-Syt3 without a TMR could no longer be detected on the cell surface in unpermeabilized cells; nevertheless, they were still found to be quantitatively associated with the plasma membrane (Fig. 3). Thus the localization of E-Syt2 and E-Syt3 into close proximity to the plasma membrane does not depend on the TMR.
Plasma Membrane Targeting of E-Syt2 and E-Syt3 Is Mediated by Its C-Terminal C2 Domain.
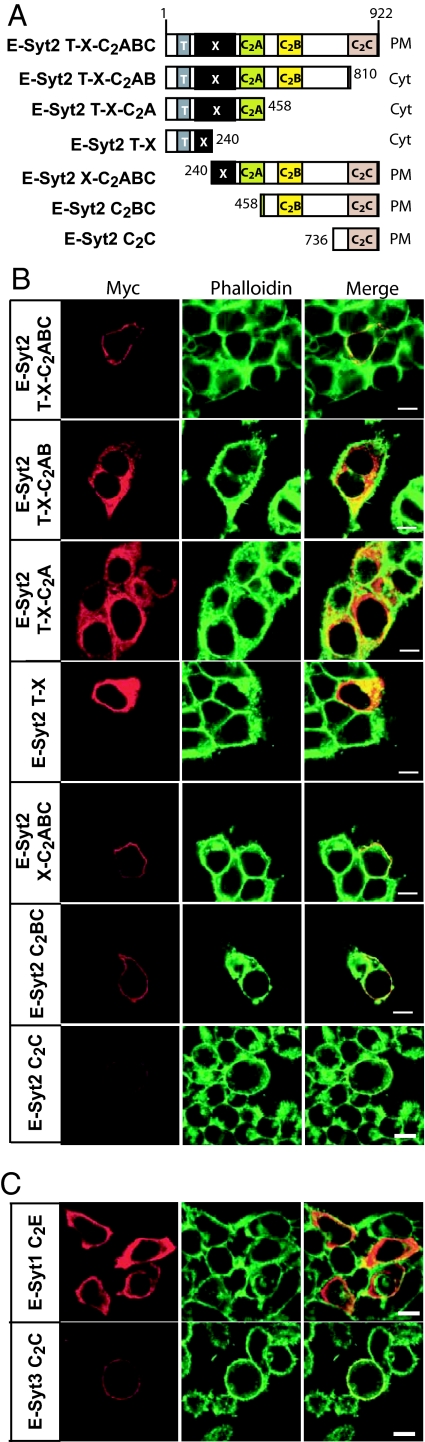
To investigate which sequences target E-Syt2 and E-Syt3 to the plasma membrane even in the absence of the TMR, we produced E-Syt2 deletion constructs that contained an N-terminal myc epitope and were analyzed by transfection into HEK293 cells (Fig. 4A). We found that deletion of the C2C domain abolished the plasma membrane localization of E-Syt2. In contrast, partial or complete deletion of the TMR, X domain, and/or the C2A domain had no effect on the plasma membrane localization of E-Syt2 (Fig. 4B). We next tested whether the C2C domain of E-Syt2 is sufficient for plasma membrane localization and whether this property is shared with the C-terminal C2 domains of other E-Syts by examining the localization of isolated C-terminal C2 domains in transfected HEK293 cells (Fig. 4 B and C). Both the E-Syt2 and the E-Syt3 C2C domains were localized to the plasma membrane, whereas the E-Syt1 C2E domain was not, thus mirroring the localizations of the full-length proteins. Therefore, although the C-terminal C2 domains of E-Syt1, E-Syt2, and E-Syt3 share a high degree of sequence homology, only the latter two include targeting information that deposits them on the intracellular surface of the plasma membrane.
Fig. 4.
The C-terminal C2 domain of E-Syt2 and E-Syt3 but not E-Syt1 direct plasma membrane localization. (A) Schematic diagram of the E-Syt2 deletion constructs and summary of their subcellular localization in transfected HEK293 cells (on right; PM, plasma membrane; Cyt, cytoplasmic). Numbers display residue numbers at the N and C termini of the various fragments. (B) Confocal images of HEK293 cells transfected with various constructs as indicated on the left, permeabilized, and stained with myc antibodies (red) and fluorescent phalloidin (green); merged images are depicted on the right (yellow indicates red/green overlap). (C) Confocal images of HEK293 cells transfected with myc-tagged C-terminal C2 domains of E-Syt1 and E-Syt3, permeabilized, and stained with myc antibodies (red) and fluorescent phalloidin (green); merged images are depicted on the right (yellow indicates red/green overlap). (Scale bars for B and C, 5 μm.)
To investigate how it is possible for the E-Syt2 and E-Syt3 C2C domains to be localized to the plasma membrane, we evaluated their biochemical properties. The E-Syt2 fragment containing the C2B and C2C domains is strongly expressed in HEK293 cell and quantitatively localized to the plasma membrane (Fig. 4B). Subcellular fractionations revealed that the C2B/C2C domain fragment was insoluble even after extraction of the cells with 1% Triton X-100 (SI Fig. 7; see SI Experimental Procedures). Disrupting the actin cytoskeleton with latrunculin or the microtubule cytoskeleton with nocodazole, however, had no effect on the solubility of the E-Syt2 C2B/C2C domain fragment or its plasma membrane localization, despite the fact that, at least in the case of latrunculin-A, the actin cytoskeleton was disrupted (SI Fig. 7). Thus, the E-Syt2 C2B/C2C domain fragment is not simply anchored to the plasma membrane by the cortical cytoskeleton.
Discussion
E-Syts as Membrane-Tethered Ca2+-Binding Proteins.
We here describe a family of membrane proteins with multiple C2 domains, referred to as E-Syts because, like synaptotagmins, these proteins contain an N-terminal TMR and C-terminal cytoplasmic C2 domains. With the E-Syts, four families of evolutionarily conserved membrane proteins containing C2 domains that bind Ca2+ have now been defined: synaptotagmins, ferlins, MCTPs, and E-Syts (26, 28, 29). All of these proteins contain a single TMR and multiple C2 domains, from two C2 domains for synaptotagmins to at least six C2 domains for ferlins. E-Syts share other properties with synaptotagmins, ferlins, and MCTPs: two of the three E-Syt isoforms are localized to the plasma membrane, as are some of the synaptotagmin and ferlin isoforms, and E-Syts are ubiquitously expressed, although enriched in brain, as again shown for some synaptotagmin and ferlin isoforms. However, two of these protein families, synaptotagmins and E-Syts, contain an N-terminal TMR, whereas the other two, ferlins and MCTPs, contain a C-terminal TMR. Moreover, as described here, E-Syts differ from the other proteins, with multiple C2 domains and a single TMR in three key properties:
The cytoplasmic sequences of E-Syts include an additional, highly conserved domain that is not found in other proteins with multiple C2 domains, here referred to as the X domain for want of a better name (Fig. 1).
E-Syts are the only proteins with a single TMR and multiple C2 domains that are conserved in yeast, where they are closely related to tricalbins. However, in all E-Syts, the C2A domain contains a signature Ca2+-binding motif that is characteristic of Ca2+-binding C2 domains and that mediates Ca2+ binding (Fig. 2), whereas in tricalbins, none of the C2 domains contains a Ca2+-binding motif, making it highly unlikely that they bind Ca2+.
Although the C2A domain of E-Syt2 appears to mediate Ca2+-dependent phospholipid binding, its Ca2+-dependent phospholipid binding properties are unusual compared with those of other proteins containing a single TMR and multiple C2 domains. The E-Syt2 phospholipid binding specificity is such that it does not require negatively charged phospholipids but also works with neutral phospholipids (Fig. 2C). Moreover, the isolated C2A domain does not appear to work efficiently but exhibits a significant amount of Ca2+-dependent phospholipid binding only when expressed in conjunction with either the X domain or the C2B domain (Fig. 2A), a property that we do not currently understand.
E-Syts have no recognizable domains besides their TMR, X domain, and C2 domains, suggesting that their function is mediated by the combination of these three sequence elements. The overall characteristics of E-Syts suggest that E-Syts are Ca2+-regulatory proteins that function in conjunction with membranes. At present, the physiological roles of E-Syts are unclear, but both synaptotagmins and ferlins are involved in the Ca2+-dependent regulation of exocytosis (reviewed in refs. 29 and 30), suggesting that MCTPs and E-Syts may also have a role in membrane traffic. It seems likely that E-Syts have an important role, because they are evolutionarily conserved, and because yeast tricalbins appear to be essential for survival (25). Moreover, the distinct localizations of E-Syt1 vs. E-Syt2 and E-Syt3 point to divergent functions, consistent with their different domain structures. Detailed genetic and cell-biological studies will be required, however, to determine the functions of E-Syts and compare them with those of synaptotagmins, ferlins, and MCTPs.
Unusual Targeting Mechanism of E-Syts.
As assayed with myc-tagged transfected proteins, E-Syt1 is localized to intracellular membranes, whereas E-Syt2 and E-Syt3 are quantitatively deposited into the plasma membrane (Fig. 3). Unexpectedly, the intracellular vesicular localization of E-Syt1 and the plasma membrane localization of E-Syt2 and E-Syt3 were independent of their TMRs, whereas the C2C domains of these proteins were sufficient for their localizations (Fig. 4).
We are not aware of a previous example in which a C2 domain directs the localization of a protein, suggesting that the mechanism described here is previously uncharacterized. This mechanism presumably involves interaction of the E-Syt2 C2C domain with another plasma membrane protein, similar to the interaction of the Munc13 C2A domain with RIM (13), as suggested by two observations. First, the E-Syt2 C2B/C2C domain, localized to the plasma membrane, is detergent-insoluble and thus not phospholipid-bound (SI Fig. 7). Second, although the detergent-insolubility of the E-Syt2 C2B/C2C domain suggests a possible role for the cytoskeleton in its localization, drugs that disrupt either the actin or the microtubule cytoskeleton did not alter the plasma membrane localization or insolubility of the E-Syt2 C2B/C2C domain fragment (SI Fig. 7). These results suggest that although the E-Syt2 and E-Syt3 TMR is anchored in the plasma membrane as evidenced by the extracellularly exposed N-terminal sequence, E-Syt2 and E-Syt3 may be involved in an independent interaction with the plasma membrane via their C-terminal C2C domain that presumably binds to a detergent-insoluble, as yet unidentified plasma membrane component. This binding likely not only mediates the targeting of E-Syt2 and E-Syt3 to the plasma membrane but is also probably involved in their unknown functions at the plasma membrane.
Experimental Procedures
Cloning, Sequence Analyses, and Data Bank Searches.
E-Syts were identified by databank searches of genomic and cDNA sequences. Their full-length human sequences were then assembled by sequencing of expressed-sequence tag clones, and submitted to GenBank (accession nos. DQ993200, DQ993201, and DQ993202).
Expression and Purification of Recombinant GST Fusion Proteins.
The cDNA sequences encoding various domains of the human E-Syts were amplified by PCR, subcloned into pGEX-KG vector, and expressed and purified as recombinant GST fusion proteins essentially as described (19). The following GST fusion proteins were produced: E-Syt1 X C2AB (residues 91–600), E-Syt1 C2A (residues 278–449), E-Syt1 C2E (residues 918-1105), E-Syt2 X C2AB (residues 147–658), E-Syt2 X C2A (residues 147–511), E-Syt2 X (residues 147–387), E-Syt2 C2A (residues 340–511), E-Syt2 C2AB (residues 340–658), E-Syt2 C2B (residues 500–658), and E-Syt2 C2C (residues 736–922).
Construction and Expression of Vectors Encoding Various myc-Tagged E-Syt Fusion Proteins.
The following gene constructs were subcloned into pCMV vector with an N-terminal myc-tag, and transfected into HEK293 cells for subcellular localization experiments: E-Syt1 full-length (residues 1–1105), E-Syt2 full-length (residues 1–922), E-Syt3 full-length (residues 1–887), E-Syt1 ΔTM (residues 92–1105), E-Syt2 ΔTM (residues 150–922), E-Syt3 ΔTM (residues 73–887), E-Syt2 deletion constructs (residues 1–810, 1–458, 1–240, 240–922, 458–922, and 755–922), E-Syt1 deletion construct (residues 965-1105), and E-Syt3 deletion construct (residues 748–887).
Phospholipid binding assays were carried out with purified soluble GST fusion proteins in buffer A (50 mM Hepes-NaOH, pH 6.8/0.1 M NaCl/4 mM sodium EGTA). The GST fusion C2 domain proteins were incubated with liposomes of defined phospholipid composition in buffer A containing variable amounts of CaCl2 or MgCl2 to provide defined concentrations of free Ca2+ and Mg2+,respectively (calculated by using EqCal for Windows software from Biosoft, Ferguson, MO). After incubations, liposomes with bound C2-domain proteins were isolated by centrifugation essentially as described (31, 32) and bound proteins were precipitated, resuspended in 30 μl of 2× SDS sample buffer, and analyzed by SDS/PAGE and Coomassie blue staining.
Immunostaining and Confocal Imaging.
HEK293 cells plated on cover slips in 12-well plates were transfected with E-Syt expression and control vectors by using FuGENE (Roche Applied Science, Indianapolis, IN). Two days after transfection, cells were washed in PBS and fixed with 3.7% formaldehyde in PBS. After fixation, cells were blocked and nonpermeabilized or permeabilized by 3% nonfat milk with 0.1% Nonidet P-40 in PBS. Cells were then incubated with monoclonal antibodies against the myc epitope (Santa Cruz Biotechnology, Santa Cruz, CA) and FITC-conjugated Phalloidin (Molecular Probes, Eugene, OR) for 1 h, washed three times with PBS, and reacted with Alexa Fluor 488-labeled secondary antibody (Invitrogen, Carlsbad, CA) for 1 h. After three washes with PBS, cells were briefly immersed in water, and mounted with Vectashield (Vector Laboratories, Burlingame, CA). Images were acquired on a TCS2 laser-scanning confocal microscope (Leica, Bannockburn, IL) using a magnification ×62 oil objective.
Miscellaneous Procedures.
SDS/PAGE and immunoblotting were performed by using standard procedures (22, 23). Immunoblots were developed by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Supplementary Material
Acknowledgments
We thank Ms. Iza Kornblum, Ewa Borowicz, and Andrea Roth for excellent technical assistance.
Abbreviations
- PC
phosphatidylcholine
- PS
phosphatidylserine
- TMR
transmembrane region.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. DQ993200–DQ993202).
This article contains supporting information online at www.pnas.org/cgi/content/full/0611725104/DC1.
References
- 1.Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. Science. 1986;233:859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- 2.Davletov BA, Südhof TC. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 3.Chapman ER, Jahn R. J Biol Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- 4.Persechini A, Moncrief ND, Kretsinger RH. Trends Neurosci. 1989;12:462–467. doi: 10.1016/0166-2236(89)90097-0. [DOI] [PubMed] [Google Scholar]
- 5.Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 6.Rizo J, Südhof TC. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 7.Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- 8.Ubach J, Zhang X, Shao X, Südhof TC, Rizo J. EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubach J, Garcia J, Nittler MP, Südhof TC, Rizo J. Nat Cell Biol. 1999;1:106–112. doi: 10.1038/10076. [DOI] [PubMed] [Google Scholar]
- 10.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 11.Dai H, Tomchick DR, Garcia J, Südhof TC, Machius M, Rizo J. Biochemistry. 2005;44:13533–13542. doi: 10.1021/bi0513608. [DOI] [PubMed] [Google Scholar]
- 12.Dai H, Shin O-H, Machius M, Tomchick DR, Südhof TC, Rizo J. Nat Struct Mol Biol. 2004;11:844–849. doi: 10.1038/nsmb817. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Machius M, Dulubova I, Dai H, Südhof TC, Tomchick D, Rizo J. PLOS Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Maximov A, Shin O-H, Dai H, Rizo J, Südhof TC. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Pang ZP, Shin O-H, Meyer AC, Rosenmund C, Südhof TC. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgione JR, Lin JH, McCammon JA, Newton AC. J Biol Chem. 2006;281:1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl B, Chou JH, Li C, Südhof TC, Jahn R. EMBO J. 1996;15:1799–1809. [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JY, Riekhof WR, Wu WI, Voelker DR. Biochem Soc Trans. 2006;34:404–408. doi: 10.1042/BST0340404. [DOI] [PubMed] [Google Scholar]
- 19.Denis V, Cyert MS. Eukaryot Cell. 2005;4:36–45. doi: 10.1128/EC.4.1.36-45.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morvan J, Froissard M, Haguenauer-Tsapis R, Urban-Grimal D. Traffic. 2004;5:383–392. doi: 10.1111/j.1398-9219.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 21.Südhof TC. J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 22.Bansal D, Campbell KP. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Shin O-H, Han W, Wang Y, Südhof TC. J Biol Chem. 2005;280:1641–1651. doi: 10.1074/jbc.M407305200. [DOI] [PubMed] [Google Scholar]
- 24.Morris NJ, Ross SA, Neveu JM, Lane WS, Lienhard GE. Biochim Biophys Acta. 1999;1431:525–530. doi: 10.1016/s0167-4838(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 25.Creutz CE, Snyder SL, Schulz TA. Cell Mol Life Sci. 2004;61:1208–1220. doi: 10.1007/s00018-004-4029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin O-H, Han W, Wang Y, Südhof TC. J Biol Chem. 2005;280:1641–1651. doi: 10.1074/jbc.M407305200. [DOI] [PubMed] [Google Scholar]
- 27.Han W, Rhee JS, Maximov A, Lin W, Hammer RE, Rosenmund C, Südhof TC. J Biol Chem. 2005;280:5089–5100. doi: 10.1074/jbc.M408757200. [DOI] [PubMed] [Google Scholar]
- 28.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 29.Bansal D, Campbell KP. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Südhof TC. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez I, Arac D, Ubach J, Gerber SH, Shin O-H, Gao Y, Anderson RGW, Südhof TC, Rizo J. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 32.Shin O-H, Rizo J, Südhof TC. Nat Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.