Abstract
Francisella tularensis causes tularemia, a highly contagious disease of animals and humans, but the virulence features of F. tularensis are poorly defined. F. tularensis and the related mouse pathogen Francisella novicida synthesize unusual lipid A molecules lacking the 4′-monophosphate group typically found in the lipid A of Gram-negative bacteria. LpxF, a selective phosphatase located on the periplasmic surface of the inner membrane, removes the 4′-phosphate moiety in the late stages of F. novicida lipid A assembly. To evaluate the relevance of the 4′-phosphatase to pathogenesis, we constructed a deletion mutant of lpxF and compared its virulence with wild-type F. novicida. Intradermal injection of 106 wild-type but not 108 mutant F. novicida cells is lethal to mice. The rapid clearance of the lpxF mutant is associated with a stronger local cytokine response and a greater influx of neutrophils compared with wild-type. The F. novicida mutant was highly susceptible to the cationic antimicrobial peptide polymyxin. LpxF therefore represents a kind of virulence factor that confers a distinct lipid A phenotype, preventing Francisella from activating the host innate immune response and preventing the bactericidal actions of cationic peptides. Francisella lpxF mutants may be useful for immunization against tularemia.
Keywords: antimicrobial peptides, outer membrane, innate immunity, polymyxin, tularemia
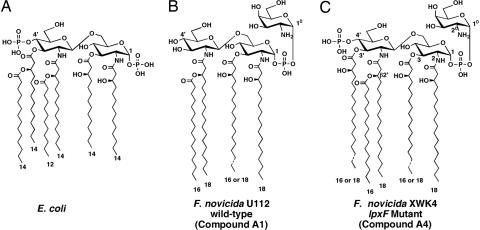
Gram-negative bacteria contain an asymmetric outer membrane with glycerophospholipids on the inside and lipid A (Fig. 1) on the outside (1, 2). Lipid A (endotoxin) is a structurally conserved molecule that is the membrane anchor for lipopolysaccharide (LPS) (2), and it is the component of the Gram-negative bacterial envelope specifically recognized by Toll-like receptor 4 (TLR4) on host immune cells (3, 4). TLR4 is one of several pattern recognition receptors that respond directly to conserved molecules present in microbial pathogens (5, 6). Upon engagement by lipid A or other microbial components, the TLRs initiate a series of intracellular signaling cascades, leading to the production of inflammatory cytokines and other immune responses (5, 6).
Fig. 1.
Structures of lipid A molecules synthesized by E. coli and F. novicida. (A) E. coli lipid A is synthesized by a system of nine constitutive enzymes (2), orthologs of which are present in Francisella (15). Numbers indicate the lengths of the predominant fatty acyl chains. (B) Structure of the predominant form of lipid A (compound A1) extracted from F. novicida wild-type U112 grown to late log phase at 37°C on 3% trypticase soy broth, supplemented with 0.1% cysteine (12, 48). Compound A2, which represents ≈10% of the total, is modified with a glucose residue at position 6′. (C) Structure of the predominant lipid A species (compound A4) present in XWK4, the lpxF mutant of F. novicida. When the 4′-phosphate moiety is not removed, the 3′-O-acyl chain stays in place, indicating an obligatory order of enzymatic processing. More than 90% of Francisella lipid A is free, in that it is not covalently linked to LPS (12).
There is growing evidence that pathogens attempt to modify their lipid A structures to avoid immune detection and early clearance. For instance, Francisella LPS and lipid A (7, 8) are poor immunostimulants compared with similar Escherichia coli or Salmonella preparations. Unlike the lipid A molecules found in most Gram-negative bacteria, which are hexaacylated disaccharides of glucosamine with phosphate groups at the 1 and 4′ positions (Fig. 1A) (2, 9), lipid A molecules of Francisella novicida (Fig. 1B) and Francisella tularensis (10–12) lack a 4′-phosphate group and a 3′-hydroxyacyl chain. Both of these substituents are necessary for robust signaling via TLR4 (13). Another unusual feature of both F. novicida and the live vaccine strain of F. tularensis is the fact that most of their lipid A is present in a “free” form (12), i.e., lacking the core oligosaccharide and O-antigen.
Despite their unusual lipid A structures and relatively low levels of intact LPS (12), F. novicida and F. tularensis encode the same lipid A biosynthetic enzymes present in E. coli (14, 15), suggesting that F. novicida cells initially synthesize lipid A precursors containing the 4′-phosphate group but thereafter remove it. We have recently discovered a selective lipid A 4′-phosphatase in F. novicida, designated LpxF (16). The active site of this phosphatase faces the periplasmic surface of the inner membrane (16). We hypothesized that F. novicida mutants lacking LpxF should synthesize lipid A molecules that retain the 4′-phosphate moiety and that LpxF might serve to enhance F. novicida virulence by conferring the capacity to avoid host immune recognition and/or killing by cationic antimicrobial peptides.
We now demonstrate that LpxF is the sole enzyme responsible for the removal of the 4′-phosphate moiety of lipid A in F. novicida. Deletion mutants with a kan insert replacing the lpxF gene synthesize lipid A molecules that retain both the 4′-phosphate group and the 3′-acyl chain (Fig. 1C). LpxF mutants are highly attenuated in a mouse infection model compared with wild-type F. novicida or with the live vaccine strain of F. tularensis. Mutants lacking the lpxF gene may therefore prove useful in the search for new vaccines against tularemia.
Results
Altered Lipid A Structure in a F. novicida lpxF Deletion Mutant.
We constructed a 4′-phosphatase deletion mutant of F. novicida (designated XWK4) by replacing the entire chromosomal lpxF gene, which encodes the lipid A 4′-phosphatase (16), with a kanamycin resistance cassette. An altered lipid A molecule is synthesized by the mutant (compound A4 in Fig. 2A). This substance retains the 4′-phosphate group, as judged by mass spectrometry [supporting information (SI) Fig. 6] and NMR spectroscopy (Fig. 3). The mutant lipid A also retains the 3′-hydroxyacyl chain (Fig. 1C), suggesting that the 4′-phosphate group must be removed before the 3′-hydroxyacyl chain is cleaved during lipid A maturation. The enzyme responsible for the cleavage of the 3′-hydroxyacyl chain is unknown.
Fig. 2.
Altered lipid A composition, lack of 4′-phosphatase activity, and polymyxin hypersensitivity of the F. novicida lpxF mutant. (A) Thin-layer chromatography of F. novicida lipids labeled uniformly with 32P and extracted by using a two-phase Bligh–Dyer system (16, 37). Samples containing ≈10,000 cpm were spotted in each lane. The plate was developed with chloroform/pyridine/88% formic acid/water (50:50:16:5, vol/vol), and the lipids were detected with a PhosphorImager. Most of the lipid A found in F. novicida is not linked to LPS (12), possibly because of the presence of an unusual Kdo-cleaving activity (37). Therefore, most of the lipid A of F. novicida is extracted directly with chloroform/methanol, together with the glycerophospholipids (12). The structure of compound A4 is shown in Fig. 1C. It accumulates in the lpxF mutant, whereas A1 and A2 disappear. (B) Absence of 4′-phosphatase activity in membranes of XWK4. (C) Kanamycin resistance and polymyxin hypersensitivity of mutant XWK4.
Fig. 3.
NMR spectroscopy of lipid A isolated from the lpxF mutant of F. novicida. (A) 31P NMR spectrum of compound A4. The 31P NMR signals at 0.53 and −2.5 ppm indicate the presence of two phosphate groups, designated P-4′ and P-1 according to the numbering scheme shown in Fig. 1C and described in detail previously (12). The 31P NMR spectrum of compound A1 from the wild-type (data not shown) shows only a single peak near −1.3 ppm, consistent with the absence of P-4′. (B) 1H NMR spectrum of component A4 in the anomeric and sugar proton regions. The relevant protons are labeled as in Fig. 1C (12). (C and D) Selective inverse decoupled difference 1H NMR spectra of compound A4, implemented by selective irradiation of the individual phosphorus atoms (12, 18), provide unequivocal evidence that a 4′-monophosphate group (0.53 ppm 31P signal) is present (C) and that two sugar residues are attached to the anomeric 1-phosphate group (−2.5 ppm 31P signal) (D), as in compound A1 of wild-type cells (12). The full assignments of the NMR spectra of compound A4, which are based on two-dimensional analyses, are summarized in SI Table 1.
The 31P NMR spectrum of compound A4 (Fig. 3A) shows the presence of two phosphorus atoms. In contrast, the lipid A present in wild-type cells contains only a single phosphorus atom (12). Selective decoupling (17, 18) of the individual phosphorus atoms present in compound A4 demonstrates that one phosphate group is attached to the 4′ position of the glucosamine disaccharide and the other to the 1 position of the proximal unit (Fig. 3 B–D). The assignments of key 1H, 13C, and 31P resonances of compound A4, which further support the structure shown in Fig. 1C, are summarized in SI Table 1.
The 4′-phosphatase activity that is characteristic of wild-type F. novicida U112 membranes is absent in XWK4 (Fig. 2B), indicating that lpxF is the sole gene encoding lipid A 4′-phosphatase activity in this organism. The mutant is resistant to kanamycin, as judged by a disk diffusion assay, because of the kan cassette that replaces the lpxF gene (Fig. 2C). The mutant grows at about one-third the rate of the wild-type cells, but it is not temperature-sensitive (SI Fig. 7).
The F. novicida lpxF Mutant Is Avirulent.
To test the virulence of the lpxF mutant, we compared the survival of mice injected with increasing numbers of live wild-type or mutant bacteria. For comparative purposes, we also included the live vaccine strain of F. tularensis, which grows at about half the rate of F. novicida at 37°C. Although the underlying basis is unknown, the live vaccine strain is significantly attenuated in humans, and it was used in some countries for the prevention of tularemia (19). Because Francisella infections are often initiated after dermal inoculation by infected insects (20), we chose the footpad as the site for bacterial challenge. In pilot experiments, we found that 106 (or more) wild-type bacteria killed the mice within 2 days, but up to 108 mutant bacteria failed to kill any mice (data not shown). Spleens of the surviving mice inoculated with low sublethal doses of wild-type F. novicida were enlarged 3- to 4-fold (as judged by their weight) and contained viable bacteria. Spleens of mice injected with up to 108 lpxF mutant bacteria were not enlarged and were free of live bacteria (data not shown).
These preliminary findings were confirmed with an additional group of 30 mice. Ten mice received 106 wild-type F. novicida, another 10 received 106 cells of the live vaccine strain, and the remaining 10 received 106 lpxF mutant bacteria (Fig. 4). The mice injected with the wild-type or live vaccine strain died within 2 or 5 days, respectively, whereas all of the animals injected with the lpxF mutant bacteria survived (Fig. 4). None of the lpxF mutant survivors contained viable bacteria in their spleens (data not shown), demonstrating that the lpxF mutant bacteria are indeed greatly attenuated.
Fig. 4.
Loss of virulence in lpxF mutants of F. novicida. Three groups of 10 mice were injected into their right rear footpads with 106 viable wild-type F. novicida U112, 106 live vaccine strain (LVS), or 106 lpxF XWK4 mutant F. novicida. Survival of the animals was recorded once a day. (A) Average weights of the surviving animals. (B) Percentage of surviving animals in each treatment group.
Enhanced Cytokine Response to the F. novicida lpxF Mutant.
The marked in vivo attenuation of lpxF mutant bacteria could be attributable to increased recognition and clearance by the innate immune system of the host. To determine whether this possibility was the case, 106 viable wild-type or mutant bacteria, suspended in 3 ml of normal saline, were injected into the peritoneal cavities of mice (three per group), where both mediator responses and neutrophil emigration could be assessed conveniently. After 4 h, the peritoneal fluid was collected and analyzed for the presence of a panel of seven chemokines and cytokines (Fig. 5A). Remarkably, wild-type F. novicida failed to induce any detectable mediator responses under these conditions, whereas the F. novicida lpxF mutant stimulated significant production of the proinflammatory cytokine IL-6 and the two potent chemoattractants, monocyte chemoattractant protein 1 (MCP1) and keratinocyte-derived chemokine (KC) (Fig. 5A) (21, 22). As anticipated from the induction of IL-6, MCP1 and KC (Fig. 5A), there was a markedly higher neutrophil response elicited in the peritoneal cavities by the lpxF mutant compared with wild-type F. novicida (Fig. 5B). The total number of leukocytes in the peritoneal fluid of mice was 2.4 × 106 with the mutant versus 5.4 × 105 with the wild-type. The number of viable F. novicida cells remaining in the peritoneal fluid after 4 h was also determined. The number of surviving mutant bacteria was at least 100 times lower than that observed with the wild-type (Fig. 5C). The increased clearance of the mutant is consistent with the enhanced recruitment of neutrophils (Fig. 5B). The data in Fig. 5 are the averages of three experiments.
Fig. 5.
Enhanced recruitment of cytokines and neutrophils into mouse peritoneum injected with the lpxF mutant XWK4. In these experiments, 106 viable bacteria were injected into each mouse peritoneum. After 4 h, the peritoneal fluid was withdrawn. (A) Cytokines tested were TNFα, macrophage inflammatory protein 2 (MIP2), MCP1, IL6, IL13, IL1β, and keratinocyte-derived chemokine. The cytokine levels were determined by the Elisa Tech Company (Aurora, CO). Six mice were used in each experiment; three were infected with U112 and three with XWK4. The experiment was repeated three times and the data averaged. (B) The percentage of neutrophils in the peritoneal fluids was determined. Six mice were used in each experiment; three were injected with U112 and three with XWK4. The experiment was repeated twice and the data averaged. (C) The titers of viable bacteria in the fluids were determined by serial dilution plating. The P value was <0.02 for all observed differences.
Hypersensitivity of the F. novicida lpxF Mutant to Polymyxin.
A significant proportion of the neutrophil bactericidal activity is attributed to the repertoire of secreted cationic antimicrobial peptides, such as the defensins (23). Because structural modifications that reduce the net negative charge of lipid A have been shown to alter susceptibility to cationic antimicrobial peptides (24–26), we compared the sensitivity of the mutant and wild-type with the cationic antimicrobial peptide polymyxin B (16, 27). Employing a disk diffusion assay, we observed that wild-type F. novicida U112 bacteria are highly resistant to polymyxin (Fig. 2C), whereas the lpxF mutant cells are hypersensitive (Fig. 2C), consistent with the phosphorylation status of their lipid A (Fig. 1C). We have not yet examined the sensitivity of the lpxF mutant to other cationic antimicrobial peptides.
TLR4 Is Not Activated by Lipid A from the F. novicida lpxF Mutant.
Despite the presence of 4′-phosphate group, the mutant bacteria failed to evoke a TNFα response (Fig. 5A), suggesting that TLR4 does not play a significant role in the immune recognition of mutant XWK4. In fact, purified lipid A preparations from both the wild-type and lpxF mutant bacteria were completely inactive in triggering IL-8 production in HEK293 cells expressing recombinant TLR2 or TLR4/MD2 (SI Fig. 8). TLR4 may not be able to recognize F. novicida lipid A, irrespective of the presence or absence of the 4′-phosphate group, because of its relatively long fatty acyl chains (Fig. 1). However, the presence of the 4′-phosphate group in the lipid A of the lpxF mutant markedly affects the electrostatic properties of the bacterial envelope, enabling cationic antimicrobial peptides to bind and damage the mutant envelope (23). Membrane damage would account for the rapid i.p. killing of the lpxF mutant, and it would expose microbial ligands recognized by other pattern recognition receptors, such as bacterial lipoproteins or peptidoglycan by TLR2 and bacterial DNA by TLR9 (5).
Discussion
For decades, microbial virulence has been linked primarily to factors such as microbial adherence (28), invasion (29, 30), toxin production (31, 32), or iron-chelating capacity (33). With the awareness of the critical roles played by pattern recognition receptors of host cells in immune recognition and in eliciting host responses against pathogens (5), there is growing interest in the notion of immune avoidance as a critical virulence factor. For instance, Montminy et al. (34) recently constructed a strain of Yersinia pestis that synthesizes hexaacylated lipid A (a potent TLR4 agonist) instead of wild-type, tetraacylated Y. pestis lipid A (a weak agonist or an antagonist, depending on the system) (35). This change in lipid A structure abolished Y. pestis virulence in the mouse, presumably because of an enhanced innate immune response mediated by TLR4 (34). Y. pestis cells that synthesize hexaacylated lipid A also provided protective immunity against subsequent challenges with wild-type, virulent Y. pestis (34).
Here, we demonstrate that inactivation of the periplasmic phosphatase, previously shown to be involved in Francisella lipid A 4′ dephosphorylation in vitro (16), results in a mutant that retains the 4′-phosphate moiety in its lipid A in living cells (Figs. 1 and 3). Although viable, this mutant is severely incapacitated in its ability to kill mice compared with wild-type F. novicida or the F. tularensis live vaccine strain (Fig. 4). Because the lpxF gene is not present in most other Gram-negative bacteria (16), its acquisition by Francisella species may represent an evolutionary adaptation for avoiding the host's innate immune system, especially the bactericidal action of cationic antimicrobial peptides. However, unlike the situation in Y. pestis (34), the modification of lipid A structure seen in the F. novicida lpxF mutant does not enhance the ability of F. novicida lipid A to activate TLR4 (SI Fig. 8) or activate RAW cells to produce TNF. Instead, we propose that the hypersensitivity of the lpxF mutant to cationic peptides (Fig. 2C) damages the outer membrane and exposes ligands for other TLRs, such as lipoproteins and/or CpG-rich bacterial DNA (5, 36). Another possibility is that the mutant might be more sensitive to killing by serum and/or complement.
As in wild-type F. novicida (12), >90% of the lipid A present in the lpxF mutant is in the free form, i.e., not covalently linked to 3-deoxy-d-manno-octulosonic acid (Kdo) and core oligosaccharides. The mechanism by which free lipid A is generated in F. novicida is uncertain (12). The F. novicida genome does encode a Kdo transferase (15), suggesting that a normal core domain is first synthesized and attached to nascent lipid A but then is removed at a later stage. An unusual Kdo hydrolase activity (37), present in membranes of F. novicida but not E. coli, may be responsible for releasing the lipid A from newly synthesized LPS. Other mechanisms cannot yet be excluded, such as the rapid export of newly synthesized lipid A molecules not yet modified with Kdo (12). The significance of free lipid A in strains of Francisella is uncertain but might be revealed by the isolation and characterization of mutants lacking the Kdo hydrolase.
Targeting of the lpxF gene or gene product may represent an interesting approach for the prevention of human tularemia. An inhibitor of LpxF would not have antibiotic activity per se, but it might be useful for preventing the spread of tularemia by rendering the host resistant to infection. Alternatively, potent inhibitors of the second step of lipid A biosynthesis, such as CHIR-090, have recently been described (38). F. novicida cells are very sensitive to killing by CHIR-090 (data not shown), suggesting that LpxC inhibitors should be very effective for both the prevention and the treatment of tularemia.
Because the structure of lipid A in the F. tularensis pathogen (11) is identical to that in F. novicida (12) (Fig. 1), F. tularensis lpxF mutants will likely be avirulent and should be tested as human vaccines. Other bacteria that synthesize lipid A without a 4′-phosphate group include Rhizobium etli (39), Rhizobium leguminosarum (40), Helicobacter pylori (41, 42), Porphyromonas gingivalis (43), and Leptospira interrogans (44). An ortholog of lpxF is present in the genomes of the plant endosymbionts R. etli and R. leguminosarum, consistent with their lipid A structures (16). Deletion mutants in R. etli lpxF make lipid A species that retain the 4′-phosphate moiety (C. Ingram, C. Sohlenkamp and C.R.H.R., unpublished data), as is observed in F. novicida. Interestingly, the genomes of H. pylori, P. gingivalis, and L. interrogans do not contain orthologs of lpxF (16). However, 4′-phosphatase activity can nevertheless be demonstrated in cell extracts of some of these bacteria (N. Que, S. Basu, and C.R.H.R., unpublished data), suggesting that structurally diverse lipid A 4′-phosphatases may exist. Because safe and effective vaccines against H. pylori, P. gingivalis, and L. interrogans are not available, lipid A 4′-phosphatase mutants of these organisms should be characterized with regard to changes in pathogenesis and immune stimulation.
Materials and Methods
Construction of F. novicida Mutant XWK4 Lacking the Lipid A 4′-Phosphatase.
The lpxF gene with 2 kb of flanking DNA was amplified by PCR from genomic F. novicida DNA and cloned into the XbaI and BamHI sites of pWSK29 (45). The forward PCR primer (5′-GCGCGTCTAGATCGGCAAACTCATCTGCGACAAC-3′) was designed with a clamp region, a XbaI restriction site (underlined), and a match to the coding strand of chromosomal DNA ≈2kb upstream of lpxF. The reverse primer (5′-CCGGATCCTATTATTTTTTTTGCTGTTGAATTGTGTT-3′) was designed with a clamp region, a BamHI restriction site (underlined), and a match to the anticoding strand of chromosomal DNA ≈2 kb downstream from lpxF. The resulting hybrid plasmid, pWSK-F5000, was transformed into E. coli DY330 (46). A kan cassette (47) with 50 bp of DNA flanking the lpxF gene was electroporated into DY330/pWSK-F5000. The forward and reverse primers are 5′-TTAGACTATACTACTAATTTATTAAGCTTGAAGGTTTAGA-GAGGTAAATTATGAGCCATATTCAACGGGAAACG-3′ and 5′-TCTTTTAATATATTCAATTTTTATTATCTATTTTTATCAAAAATAAATCATTAGAAAAACTCATCAAGCATCAATG-3′, respectively. The sequences of the ends of the kanamycin resistance cassette are underlined. This kan cassette was electroporated into DY330/pWSK-F5000. The lpxF gene in pWSK-F5000 was replaced with the kan resistance cassette by homologous recombination to generate pWSK-K5000. The linear insert in pWSK-K5000 was isolated and transformed at 0.1 μg/ml into F. novicida under conditions described previously (12). The mutant XWK4, derived by recombination of the transforming DNA with the chromosome, was selected on plates containing 20 μg/ml kanamycin.
Lipid A 4′-Phosphatase Assay.
The 4′-phosphatase was assayed by using 4′-[32P]lipid IVA as the substrate with direct detection of 32Pi release, as described (16). The assay mixture contained 50 mM potassium phosphate (pH 6), 0.1% Triton X-100, 0.1 mg/ml E. coli phospholipids, and 10 μM tetraacylated E. coli 4′-[32P]lipid IVA (3,000–6,000 cpm/nmol) (16).
Antibiotic Disk Diffusion Assays.
Disk diffusion tests were used to determine antibiotic susceptibility (16). U112 and XWK4 were grown to late log phase and diluted into trypticase soy broth containing cysteine to A600 = 0.2. A lawn of cells was spread onto a trypticase soy broth/cysteine agar plate with a sterile cotton swab. Sterile filter paper disks (6-mm diameter) were placed on top of the lawn, and 20 μg of each antibiotic was spotted onto a disk (2 μl of a 10 mg/ml stock). Plates were incubated overnight at 37°C to assess antibiotic sensitivity.
Purification and Mass Spectrometry of Lipid A Species.
Lipid A species were purified by column chromatography and preparative thin-layer chromatography, as described previously (12, 18). Mass spectra were acquired on a QSTAR-XL quadrupole time-of-flight tandem mass spectrometer (MS/MS) (ABI/MDS-Sciex, Toronto, ON, Canada), equipped with an electrospray ionization (ESI) source. Lipid A samples were dissolved at ≈25 μg/ml in a mixture of chloroform and methanol (2:1, vol/vol) and were immediately analyzed by ESI/MS in the negative ion mode (12). Nitrogen was used as collision gas for MS/MS experiments. Data acquisition and analysis were performed by using the instrument's Analyst QS software.
NMR Spectroscopy.
Compound A1 (2 mg) (12) was dissolved in 0.3 ml of CDCl3/CD3OD/D2O (4:4:1, vol/vol), and 1 mg of compound A4 was dissolved in 0.3 ml of CDCl3/CD3OD/D2O (6:5:1, vol/vol). Proton and carbon chemical shifts were referenced to internal tetramethylsilane at 0.00 ppm. The 2H signal of CD3OD was used as a field frequency lock with the residual signal of CD3OD serving as the secondary reference at 49.5 ppm for carbon spectra. 1H NMR spectra were recorded at the Duke University NMR Center on an Inova 800 NMR spectrometer (Varian, Palo Alto, CA) equipped with an 8-mm cryogenic probe. 1H NMR spectra at 800 MHz were obtained with a 7.2-kHz spectral window, a 67° pulse field angle (4.5 μs), a 4.5-s acquisition time, and a 1-s relaxation delay. The spectra were digitized by using 64,000 points to obtain a digital resolution of 0.225 Hz per point. Two-dimensional NMR experiments (COSY, NOESY, TOCSY, HMQC) were performed at 800 MHz as described previously (12, 17, 18). 1H-decoupled 31P NMR spectra were recorded at 202.3 MHz on a Varian Inova 500 spectrometer with a spectral window of 12,143.3 Hz digitized into 25,280 data points (digital resolution of 1 Hz per point or ≈0.005 ppm/point), a 600 pulse flip angle (8 μs), and a 1.6-s repeat time. 31P chemical shifts were referenced to 85% H3PO4 at 0.00 ppm. Inverse decoupled difference spectra were recorded as 1H-detected 31P-decoupled heteronuclear NMR experiments, as described previously (17, 18).
Mouse Infection Studies.
Six-week-old C57BL/6 mice, weighing ≈23 g, were obtained from the National Cancer Institute, Bethesda, MD. Each mouse challenged with F. novicida cells was injected intradermally into the right rear footpad with 20 μl of PBS, containing the indicated number of washed viable bacteria, derived from a late log phase culture grown at 37°C on 3% trypticase soy broth, supplemented with 0.1% cysteine (12, 48). Survival of the animals was recorded once a day, and each animal was weighed. After 2 weeks, the animals were killed. Spleens were weighed and cultured to determine the presence or absence of viable bacteria. Under these conditions, mice are relatively resistant to infection by F. novicida, but this route of exposure simulates a common route of infection in humans (20, 49). All animal studies were approved by Duke University Institutional Animal Care and Use Committee, and experiments followed its guidelines and regulations.
Supplementary Material
Acknowledgments
We thank Dr. Fanny N. Lauw of the University of Massachusetts at Worcester for the TLR2 and TLR4 activation assays and Dr. Francis Nano of the University of Victoria, British Columbia, for providing strain U112 and the conditions for its transformation with DNA. This work was supported by National Institutes of Health (NIH) Grants R37 GM51796 (to C.R.H.R.) and R37 DK050814 (to S.N.A.). The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. were supported by LIPID MAPS Large-Scale Collaborative Grant GM069338 from the NIH. The Duke NMR Center was supported in part by NIH/National Cancer Institute Grant P30 CA14236 (to A.A.R.). NMR instrumentation was funded by the National Science Foundation, the NIH, the North Carolina Biotechnology Center, and Duke University.
Abbreviation
- TLR
Toll-like receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611606104/DC1.
References
- 1.Raetz CRH. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 2.Raetz CRH, Whitfield C. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.van Duin D, Medzhitov R, Shaw AC. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Cowley SC, Myltseva SV, Nano FE. Mol Microbiol. 1996;20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 8.Conlan JW, Vinogradov E, Monteiro MA, Perry MB. Microb Pathog. 2003;34:39–45. doi: 10.1016/s0882-4010(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 9.Raetz CRH, Dowhan W. J Biol Chem. 1990;265:1235–1238. [PubMed] [Google Scholar]
- 10.Vinogradov E, Perry MB, Conlan JW. Eur J Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 11.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. Infect Immun. 2004;72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ribeiro AA, Guan Z, McGrath S, Cotter R, Raetz CRH. Biochemistry. 2006;45:14427–14440. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, et al. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 14.Garrett TA, Que NL, Raetz CRH. J Biol Chem. 1998;273:12457–12465. doi: 10.1074/jbc.273.20.12457. [DOI] [PubMed] [Google Scholar]
- 15.Larsson P, Oyston PC, Chain P, Chu MC, Duffield M, Fuxelius HH, Garcia E, Halltorp G, Johansson D, Isherwood KE, et al. Nat Genet. 2005;37:153–159. doi: 10.1038/ng1499. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, McGrath SC, Cotter RJ, Raetz CRH. J Biol Chem. 2006;281:9321–9330. doi: 10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro AA, Zhou Z, Raetz CRH. Magn Res Chem. 1999;37:620–630. [Google Scholar]
- 18.Zhou Z, Ribeiro AA, Raetz CRH. J Biol Chem. 2000;275:13542–13551. doi: 10.1074/jbc.275.18.13542. [DOI] [PubMed] [Google Scholar]
- 19.Oyston PC, Quarry JE. Antonie Van Leeuwenhoek. 2005;87:277–281. doi: 10.1007/s10482-004-6251-7. [DOI] [PubMed] [Google Scholar]
- 20.Ellis J, Oyston PC, Green M, Titball RW. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouwy M, Struyf S, Proost P, Van Damme J. Cytokine Growth Factor Rev. 2005;16:561–580. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Ley K. Microcirculation. 2003;10:289–295. doi: 10.1038/sj.mn.7800194. [DOI] [PubMed] [Google Scholar]
- 23.Zasloff M. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 24.Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 2001;276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- 25.Karbarz MJ, Kalb SR, Cotter RJ, Raetz CRH. J Biol Chem. 2003;278:39269–39279. doi: 10.1074/jbc.M305830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller SI, Ernst RK, Bader MW. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 27.Breazeale SD, Ribeiro AA, McClerren AL, Raetz CRH. J Biol Chem. 2005;280:14154–14167. doi: 10.1074/jbc.M414265200. [DOI] [PubMed] [Google Scholar]
- 28.Boyle EC, Finlay BB. Curr Opin Cell Biol. 2003;15:633–639. doi: 10.1016/s0955-0674(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 29.Finlay BB, Falkow S. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cossart P, Sansonetti PJ. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 31.Collier RJ, Young JA. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 32.O'Neal CJ, Jobling MG, Holmes RK, Hol WG. Science. 2005;309:1093–1096. doi: 10.1126/science.1113398. [DOI] [PubMed] [Google Scholar]
- 33.Fischbach MA, Lin H, Liu DR, Walsh CT. Nat Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 34.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 35.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CRH. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 36.Akira S, Takeda K. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CRH. J Biol Chem. 2004;279:49470–49478. doi: 10.1074/jbc.M409078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClerren AL, Endsley S, Bowman JL, Andersen NH, Guan Z, Rudolph J, Raetz CRH. Biochemistry. 2005;44:16574–16583. doi: 10.1021/bi0518186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Que NLS, Ribeiro AA, Raetz CRH. J Biol Chem. 2000;275:28017–28027. doi: 10.1074/jbc.M004009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhat UR, Forsberg LS, Carlson RW. J Biol Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]
- 41.Suda Y, Ogawa T, Kashihara W, Oikawa M, Shimoyama T, Hayashi T, Tamura T, Kusumoto S. J Biochem (Tokyo) 1997;121:1129–1133. doi: 10.1093/oxfordjournals.jbchem.a021705. [DOI] [PubMed] [Google Scholar]
- 42.Tran AX, Karbarz MJ, Wang X, Raetz CRH, McGrath SC, Cotter RJ, Trent MS. J Biol Chem. 2004;279:55780–55791. doi: 10.1074/jbc.M406480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa T. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 44.Que-Gewirth NLS, Ribeiro AA, Kalb SR, Cotter RJ, Bulach DM, Adler B, Saint Girons I, Werts C, Raetz CRH. J Biol Chem. 2004;279:25420–25429. doi: 10.1074/jbc.M400598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RF, Kushner SR. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 46.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. Proc Natl Acad Sci USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds CM, Kalb SR, Cotter RJ, Raetz CRH. J Biol Chem. 2005;280:21202–21211. doi: 10.1074/jbc.M500964200. [DOI] [PubMed] [Google Scholar]
- 48.Kieffer TL, Cowley S, Nano FE, Elkins KL. Microbes Infect. 2003;5:397–403. doi: 10.1016/s1286-4579(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 49.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.