Abstract
Given the choice of waiting for an adverse outcome or getting it over with quickly, many people choose the latter. Theoretical models of decision-making have assumed that this occurs because there is a cost to waiting—i.e., dread. Using functional magnetic resonance imaging, we measured the neural responses to waiting for a cutaneous electric shock. Some individuals dreaded the outcome so much that, when given a choice, they preferred to receive more voltage rather than wait. Even when no decision was required, these extreme dreaders were distinguishable from those who dreaded mildly by the rate of increase of neural activity in the posterior elements of the cortical pain matrix. This suggests that dread derives, in part, from the attention devoted to the expected physical response and not simply from fear or anxiety. Although these differences were observed during a passive waiting procedure, they correlated with individual behavior in a subsequent choice paradigm, providing evidence for a neurobiological link between the experienced disutility of dread and subsequent decisions about unpleasant outcomes.
Making decisions about gains and losses is one of the archetypal problems that all animals face, but when the outcome is temporally delayed from the decision, the problem becomes considerably more complex than simply choosing the course of action with the better expected outcome. Standard economic theory posits that preferences for outcomes that occur at different times can be represented by an expected utility of the future outcomes discounted by the amount of time one must wait for them (1). These theories typically apply discounting under the assumption that people care less about outcomes that are more remote in the future than those that are more imminent, which leads to the prediction that people should want to expedite desired experiences and delay undesirable experiences for as long as possible. A wide range of findings, however, shows that people often exhibit the opposite pattern: They prefer to delay gratifications and to speed up the occurrence of unpleasant outcomes. If people do, indeed, discount the future, then why do they so often exhibit patterns of preference that are the opposite of the predictions of time discounting? The answer, we suggest, lies in the fact that the act of waiting may itself bring subjective benefits or costs, such as the joyous anticipation of waiting for a birthday present or the misery of waiting for a dentist’s appointment. In the case of bad outcomes, the problem can be reduced to the utility of dread (2).
In contrast to standard discounted utility theory, another type of decision-making model posits that waiting enters the utility function separately from the outcome (3, 4). Here, an individual’s preference for waiting at any point in time reflects the relative weight of two considerations: the effect of time discounting on the present value of the outcome itself, and the effect of changes in timing on the length of the period of anticipation. The latter effect can explain why people sometimes delay pleasant outcomes and expedite unpleasant ones.
This is not the only possible reason that people might want to delay or expedite outcomes. It is also possible that delaying or speeding up an outcome could either increase the utility or disutility of an outcome at the time when it is experienced. For example, sensitization mechanisms in the central nervous system could modulate one’s hedonic reaction to an outcome, depending on how long one has to wait for it (5). If this were the case, then people might prefer to get unpleasant outcomes over with quickly, not because they dislike the dread associated with waiting, but because the outcome itself is more unpleasant after one has waited for it. Mechanisms producing anticipatory adaptation, on the other hand, could decrease one’s response to an outcome as a function of how long one waits for it, which would have the opposite effect on preferences for timing.
Although the cognitive and emotional processes of waiting are multidimensional, these economic models predict a specific shape for the time course of utility while an individual waits for an outcome, and each of the aforementioned theories makes a different prediction. Here, we used functional magnetic resonance imaging (fMRI) in the context of waiting for an adverse event—a cutaneous electrical shock—to identify which brain regions display time courses consistent with a theoretical model of dread and whether activity in these regions differentiates individuals based on their predilection to wait. Previous neuroimaging studies of pain have found evidence for anticipatory responses in nearly all elements of the “pain matrix”of the brain, although none has specifically linked these responses to the flow of dread in the context of an intertemporal choice (6–8). The pain matrix is a generally accepted network of brain regions that responds to noxious stimuli, and its elements have been variously associated with different aspects of the pain experience. For example, the somatosensory aspect of pain has been associated with activity in the primary somatosensory cortex (SI), the secondary somatosensory cortex (SII), and the posterior insula, whereas the visceral and emotional aspects of pain have been associated with activity in the anterior insula, rostral anterior cingulate cortex (ACC), and amygdala. Preparation for a withdrawal response has been linked to activity in mid-ACC and supplementary motor area (SMA), and the effects of attention have been observed in SII, the posterior insula, and the caudal ACC (9–11). Consequently, we hypothesized that dread would manifest in some components of the pain matrix and both the location and time course of these components would yield insight into the nature of dread itself.
To test our hypothesis that dread follows a time course of activity in the pain matrix consistent with utility theory, we used a delay-conditioning paradigm with different levels of shock and delay. Participants (n = 32) were presented with a series of 96 passive trials inside the scanner (12). Each trial began with the presentation of a cue that indicated both the voltage level and the amount of time one would have to wait for the outcome (Fig. 1). Shocks were delivered to the dorsum of the left foot on a 100% reinforcement schedule (12). After the passive delay-conditioning procedure, but while still in the scanner, the utility of voltage and delay was estimated through a series of forced-choice options. In this phase, participants were presented with pairs of voltage and delay—e.g., “90% in 3 seconds”or “60% in 27 seconds”—and they had to choose which of the two offerings they would prefer to receive. The choices were real, not hypothetical, and participants received their preferred shock at the chosen voltage level and time. Choosing the shorter delay could not speed up the experiment, as each trial lasted the length of the longer of the two choices (when the shorter duration was chosen, the extra time was added to the intertrial interval after the shock).
Fig. 1.
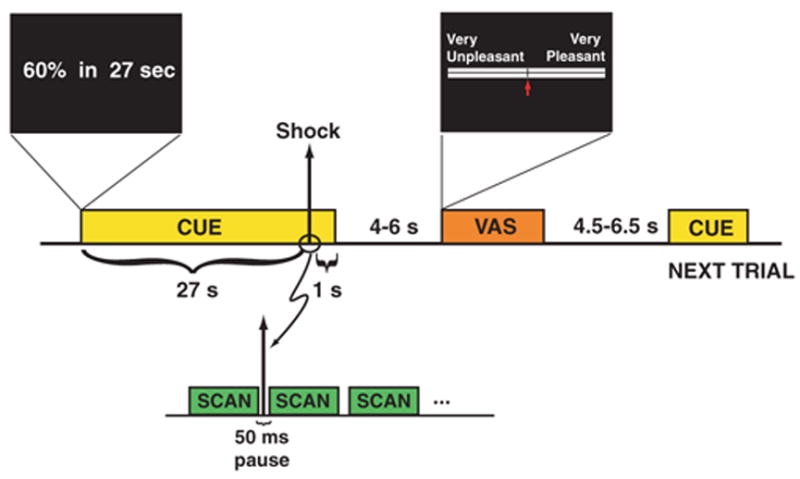
Functional MRI trial design. Each trial followed a delay-conditioning procedure, in which a cue was presented for the duration of the trial, up to and beyond the delivery of an aversive stimulus in the form of a brief cutaneous electric shock (10 to 15 ms in duration). At the beginning of each trial, a cue was displayed that indicated the level of shock (expressed as a percentage of the individual’s maximum tolerable voltage) and the time until that shock would be delivered. Four voltage levels [10, 30, 60 (shown), and 90%] and four time delays [1, 3, 9, and 27 s (shown)] were used in all 16 possible combinations. To avoid shock-induced artifacts on the fMRI images, a 50-ms pause between scan volumes was introduced, and each shock was delivered during this pause. Following the shock, the cue remained visible for another 1 s to prevent conditioning to the cue offset. A visual analog scale (VAS) was then presented in which the individual moved an arrow to indicate their subjective experience for the entire preceding trial, including the waiting time.
When the voltages between the choices were identical, participants generally chose the shorter delay (mean = 78.9% of these types of choices, range = 0 to 100%). Out of the 32 participants, 27 chose the shorter delay more than 50% of the time, indicating that the majority of individuals dreaded waiting for a shock. Some individuals dreaded so much that they were often willing to take the next higher voltage level to avoid waiting the longest delay, even though doing so would not cause the next trial to appear any sooner. Consistent with microeconomic theory, we take these revealed preferences as a measure of expected utility and then ask what neurobiological aspect of the passive experience correlates with this expected utility. Based on an individual’s preferences during the choice procedure, we constructed an ordinal ranking of voltage-delay combinations (Fig. 2A). The shape of the ranking curve tells us the relative importance of voltage and delay for each individual. A useful metric for characterizing this relationship is the marginal rate of substitution (MRS) of voltage for delay (13). The MRS tells us the value of time to that individual in terms of how much the voltage would have to be decreased for each added second of delay. The higher the MRS, the more a person dreads waiting (12). We used each individual’s MRS value as a behavioral metric of dread and then used a clustering procedure to divide the cohort of participants into two categories: extreme dreaders (n = 9) and mild dreaders (n = 23). The extreme dreaders were those individuals who preferred more voltage sooner to less voltage later, and the mild dreaders were those who dreaded only to the extent of shortening the delay at a given voltage but were not willing to take more voltage just to get the shock over with. Comparing the brain responses between these two groups during the shock-waiting period allowed us to test the predictions made by a utility-based theory of waiting about the biological flow of dread.
Fig. 2.
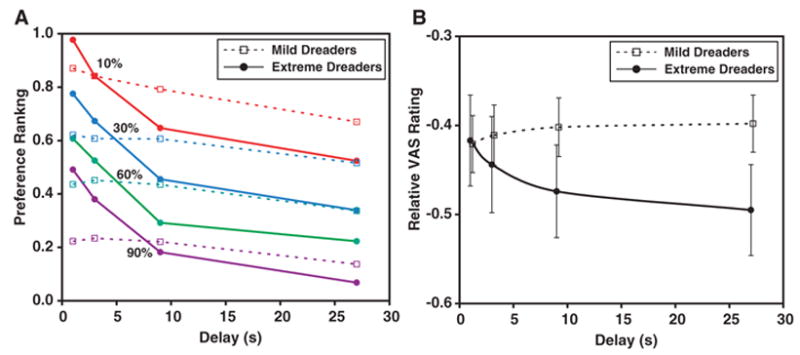
Ratings of aversive experience. (A) Ratings, as a function of voltage and delay, obtained by forced-choice preference procedure after the fMRI session. Participants were offered a series of choice pairs in which they had to choose between different voltage and delay combinations. An ordinal ranking was computed based on these choices (0 is worst and 1 is best), and participants were categorized as either ‘‘mild dreaders’’ (prefer to receive shock as soon as possible, but not so much as to take more voltage to do so) and ‘‘extreme dreaders’’ (really dislike waiting, as evidenced by choosing more voltage to receive the shock quickly). There was a significant effect on preference by both voltage [F(3,90) = 709.9, P < 0.0001] and delay [F(3,90) = 32.4, P < 0.0001] as well as the interaction of group (mild versus extreme dreader) and delay [F(3,90) = 12.0, P < 0.0001]. (B) Visual analog scale (VAS) ratings as a function of delay, normalized to each individual’s minimum rating (–1 is the worst rating and 0 is neutral) and averaged across the four voltage levels. Error bars show SEM across participants. There was a significant interaction between group and delay [repeated measures analysis of variance: F(3,90) = 4.4, P = 0.007], with the extreme dreaders indicating that the shock experience after a longer delay was significantly worse than the equivalent voltage at a shorter delay. This was not the case for the mild dreaders.
Although MRS was calculated based on the forced-choice procedure, it was possible that the act of choosing changed the subjective experience of each trial from the passive condition. To verify the generalizability of the choice-based categorization of the individuals outside a decision-making paradigm, we examined how the two groups rated their experiences on the passive trials. Confirming the subjective equivalence of passive and active experiences, extreme dreaders rated trials with long waits as significantly more unpleasant than trials with shorter waits, but mild dreaders did not show this effect (Fig. 2B).
To determine whether dreading behavior that resulted from waiting altered the response to the outcome, we examined the fMRI response to the shock itself. We identified brain regions sensitive to shock amplitude by a linearly increasing contrast across voltage levels and then subjected 12 subregions of this map that intersected the pain matrix to further analysis on the ex-post effect of waiting on the shock itself (Fig. 3). A voltage-weighted contrast on the response to the instantaneous shock revealed a map consistent with previous reports of the pain matrix. Although a significant effect of the length of delay was observed in the right SII, the predominant pattern in the pain matrix was that waiting did not change the response to the shock itself, nor was there a differential voltage sensitivity between mild and extreme dreaders. Therefore, whatever differentiated the two groups must have occurred during the waiting period. It does not appear that the preference for expediting negative outcomes results from any impact of waiting on the utility of the outcome itself.
Fig. 3.
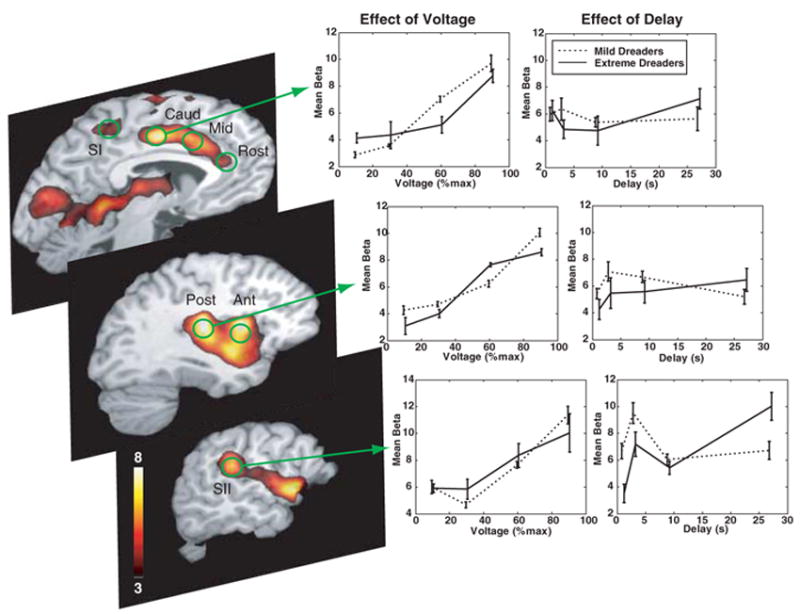
Effect of voltage and delay on the brain response to the shock itself. Statistical parametric map of the voltage-sensitive response to shock (left), identified by a linearly weighted contrast across the four voltage levels (P < 0.001, uncorrected). ROIs (green) were defined on the basis of this functional map in conjunction with anatomical masks within the cortical pain matrix (6–11): SI for the foot, SII (32, 33), anterior (Ant) and posterior (Post) insular cortex, caudal ACC (Caud), middle ACC (Mid), rostral ACC (Rost), and amygdala (not shown). There was a significant positive effect of voltage on the amplitude of response to the shock itself in all of the ROIs (middle, shown for caudal ACC, right posterior insula, and right SII), and this was not significantly different for the mild and extreme dreaders. With the possible exception of the right SII, the length of the preceding delay had minimal, if any, effect on the response to the shock itself (right) and was not significantly different between mild and extreme dreaders (12). The trials with 1-s and 3-s delays, however, did not allow complete separation of the cue response from the shock response, and so these beta values are not exactly equivalent to the 9-s and 27-s values. The general lack of an effect of delay on the instantaneous response to the shock itself suggested that the utility of the outcome was not affected by how long one had to wait for it. Given this evidence, the differentiation of mild and extreme dreaders must have occurred during the waiting period (Fig. 4).
To understand how the brain response differed between mild and extreme dreaders during the waiting period, we performed a time-series analysis on the regions of interest (ROIs). We used Loewenstein’s model for the utility of anticipation to test the hypothesis that the distinguishing characteristic between mild and extreme dreaders lies in the prospective response to future outcomes (3). In this model, the present value of a delayed act of consumption is divided into two components: the utility from consumption and the utility from anticipation (dread). Assuming instantaneous consumption at the time (T ) of shock delivery, the present value at time (t) of a future act of consumption is the utility of consumption U discounted by an exponential function with rate r = Ue−r(T – t) (1). In addition to the discounted consumption utility, anticipation—i.e., dread—confers utility in and of itself. For the sake of simplicity, we assumed that the instantaneous intensity of dread was constant and that the present value was this constant, α , multiplied by the time remaining until the shock. Thus, combining the terms for dread and discounted consumption, the present value U(V,t) = U(V) × [α (T – t) + e−r(T – t)], where U(V ) is the utility of the shock (a function of voltage V ) occurring at time T; α is the dread factor, and r is the discount rate. According to this theory, differences in the utility of dread should be measurable as differences in the dread factor α . A dread factor that is significantly positive would manifest as an early increase in the time course of activity (as opposed to a slow increase as the shock approached in time).
All of the contralateral (right hemisphere) ROIs and the caudal ACC displayed time courses with dread factors significantly different from zero, but this was an effect observed primarily in the extreme dreaders and not the mild dreaders (compare with the early, sustained, increases in Fig. 4). Both SI and SII showed marked elevations in activity after the presentation of the cue—an elevation which continued to rise in advance of the shock. But the initial elevation in SI, SII, and right posterior insula, which was measured by the dread factor, was significantly greater in the extreme dreaders (12). The time course in the caudal ACC displayed a significant dread factor for only the extreme dreaders. The right amygdala had a significant dread factor for both groups but was not significantly different between mild and extreme dreaders. From the time course of the response in these regions, coupled with its predominance in individuals who showed the most extreme behavioral evidence of not wanting to wait, we conclude that the component of anticipation that can be specifically attributed to dread is manifest in the posterior elements of the cortical pain matrix (SI, SII, the posterior insula, and the caudal ACC) and not the anterior ones (the anterior insula and the rostral ACC).
Fig. 4.
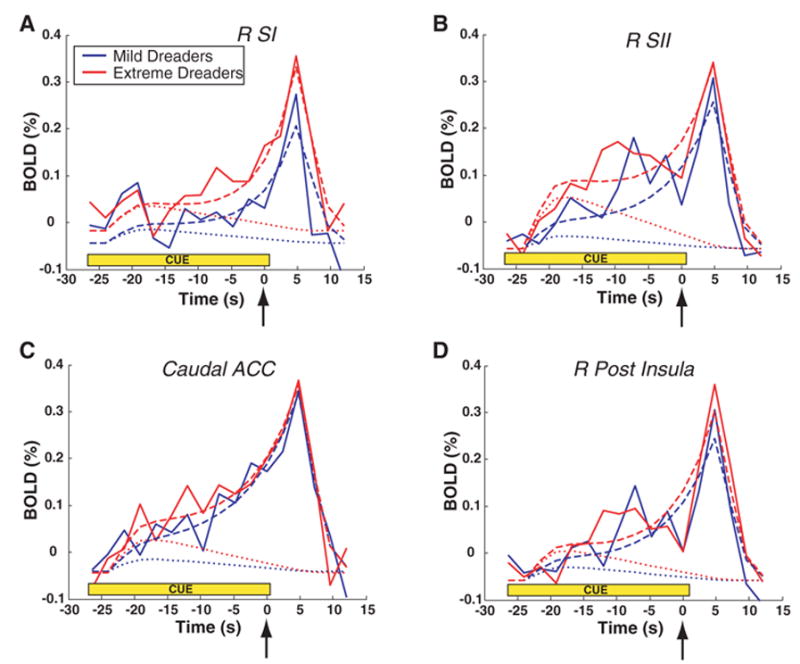
Flow of dread in selected brain regions while waiting for shocks. Solid lines were averaged from 60 and 90% voltage trials (27-s trials only) in mild dreaders (solid blue) and extreme dreaders (solid red). The trial began with the cue at t = –27 s, and the shock occurred at t = 0 (arrow). During the waiting period (cue), the extreme dreaders displayed earlier and more sustained activity increases than the mild dreaders. BOLD, blood oxygenation level–dependent response as percentage change from baseline. To determine whether these differences were based predominately in an early prospective response or a later anticipation of consumption, a theoretical model of waiting was fit to the data (dashed lines). This model was comprised of two terms that were convolved with a hemodynamic response function: a declining dread term (dotted lines) and an exponentially increasing time-discounted consumption term (not shown for clarity). The dread term was calculated as the forward-looking integral from time t to the shock (i.e., –α t), which has the characteristic of being maximal at the beginning of the trial and decreasing linearly to zero at the time of the shock. Significantly positive values for the dread factor α are associated with the experience of disutility from waiting itself. The four ROIs that had significantly greater (P ≤ 0.001) dread factors in the extreme dreaders compared with those of the mild dreaders were (A) the right SI; (B) the right SII; (C) the caudal ACC; and (D) the right posterior insula. The difference between mild and extreme dreaders is seen most clearly by the early increase in activity, especially in the right SII and the caudal ACC and noted by the difference between the two dotted lines.
The manifestation of dread in the more posterior elements of the pain matrix informs our understanding of what dread is and how it impacts decision-making. The pain matrix can be divided broadly into somatosensory, attentive, movement, and emotional divisions. Although dread is usually thought of as an emotion based on fear and anxiety (14), our localization of dread to the posterior elements of the matrix suggests that dread has a substantial attentive component. Both the mild and extreme dreaders displayed time courses of activity in SI, SII, the caudal ACC, and the posterior insula that were consistent with the utility-based theory of dread. The more anterior, “emotional”components (e.g., the anterior insula, the rostral ACC, and the amygdala) did not have such time courses. Moreover, it was the significantly different dread factor in the posterior divisions that most clearly distinguished mild from extreme dreading behavior when individuals subsequently had to make decisions regarding wait times. Both SI and SII have generally been associated with the physical intensity of noxious stimulation (9, 10, 15), whereas the caudal ACC has been associated with the attentive component of pain (16). With regard to nociceptive inputs, both SI and SII receive afferent signals from the posterior portion of the ventromedial nucleus of the thalamus, whereas the ACC receives input from the mediodorsal nucleus (10). As the terminal fields from the spinothalamic system, these regions naturally show activations that track stimulation voltage. But increasing stimulation intensity also elicits increased attention, and SII has been associated with the spatial localization of noxious stimuli (17). In the context of waiting, however, increased activity in this region suggests increased attention toward the location of the impending shock. The caudal ACC (also termed the posterior midcingulate cortex) is a key region for the modulation of inputs coming from the spinothalamic pathway through both SI and SII, and the caudal ACC is closely associated with sensory orientation and preparatory motor responses (16, 18). We found that the caudal ACC showed a significantly greater early response in the extreme dreaders than in the mild dreaders. Interestingly, the amygdala, whose role in aversive conditioning is well known (19), displayed a significant dread response on the right side, but this was not significantly different between the mild and extreme dreaders. This suggests that although the amygdala may contribute to the emotional component of dread, it does not differentiate mild from extreme dreaders.
Taken together, the anatomical locations of dread responses suggest that the subjective experience of dread that ultimately drives an individual’s behavior comes from the attention devoted to the expected physical response (SI, SII, the caudal ACC, and the posterior insula) and not simply a fear or anxiety response. Indeed, this finding would be consistent with the theory that dread comes from the integral of future expected utility—a cognitive operation that would depend on attentional resources to make such a projection possible. In contrast, distracting an individual’s attention from the affected part of the body would be predicted to decrease dread, a finding supported by the use of hypnotic suggestion to decrease pain (20).
Because we collected fMRI data during the passive experience and not during the choice procedure, any correlations with dread cannot be due to the decision-making process itself. Unlike previous reports of neurobiological processes during intertemporal choice (21), the imaging data reported here were acquired passively—when no choices were offered and no decisions were required. Thus, the regions of fMRI activity that differentiated two patterns of decision-making must be related to the experiential utility of dread. To our knowledge, this is the first time that experiential utility has been linked directly, and biologically, to decision utility, even though the two forms are assumed to be related (22).
Although the idea of utility is fundamental to rational choice theories, utility has been surprisingly difficult to measure, other than through the act of choosing. Thus, the demonstration of activity traces in the brain that follow a time course consistent with that predicted by a model based on utility theory is a notable validation for one of the basic constructs of economics (23). However, specifically attributing such patterns to the flow of utility, versus some other time-dependent process, depends both on the specificity of the model’s predictions and how well the data fit these predictions.
For the subjective experience of dread, the model used here is quite specific. The distinguishing feature of this model is the additional utility (or disutility) conferred by the act of waiting (3). Simpler models of decision-making that do not account for dread cannot explain why people should hasten the occurrence of an unpleasant outcome. Nearly all of the individuals studied in our experiment, however, exhibited this behavior, and the degree to which they did so was correlated with the early increase in activity in the posterior parts of the pain matrix. As instantiated in our modification of the Loewenstein model, anticipated dread is computed as the forward-looking integral from the present moment to the time of the expected outcome, which is maximal at the beginning of a trial and decreases monotonically to zero at the outcome. The outcome, even if unpleasant, thus affords relief from the dread. This type of time course is not generally accounted for by other theories of anticipation. Indeed, apart from the requirement that an expectation of an outcome is formed, few theories predict the nature of anticipation. Trial-based models of learning, such as Rescorla-Wagner (24) and temporal difference (25) suggest that the learning of an association between cue and outcome is driven by the mismatch between expectation and outcome but say little about what form the expectation should take leading up to the outcome. Other theories suggest that anticipation is, in part, based on the recollection of past experience, but again, say little about the time course of transmuting recollection into anticipation (26). Even other rational choice models do not consider the passage of time to have utility in and of itself.
Indeed, the notion of “anticipation”can be sharpened by separately mapping neurobiological traces onto two major components (3). The consumptive element of anticipation is conceptually identical to the expected outcome term of associative learning theories but exponentially discounted in time. The defining characteristic of this process is an exponential growth up to the outcome. We found ample evidence for this process throughout the cortical pain matrix, a result consistent with previous studies of pain anticipation (6, 7, 27–31). Unlike previous studies, we are now able to identify neurobiological substrates associated specifically with a second component of anticipation: dread. Although there are potentially a wide variety of theoretical models that could explain dread, the approach described here allows for the principled comparison of one against another, as well as in brain regions outside the pain matrix. For example, comparing the dread model with a simple discounting model, we found the former to be a better fit to the fMRI data, suggesting that the dread term is necessary to account for the responses observed here (12).
In addition to suggesting a neurobiological substrate for the utility of dread, our results have implications for another assumption of utility theory: the origin of preferences. It seems likely that an individual’s relative preference for waiting for something unpleasant derives from previous experience. In our experiment, participants presumably had well-established preferences for waiting, although it is unlikely that they had previous experience with foot shocks. We thus observed the construction of waiting preference in the specific context of foot shocks without any choices being offered. That the activity patterns in the brain regions associated with the pain experience correlate with subsequent choices offers strong evidence for the existence of intrinsic preferences. Although it is not clear how malleable these preferences are, their existence may have health implications for the way in which individuals deal with events that are known to be unpleasant—for example, going to the doctor for painful procedures. The neurobiological mechanisms governing dreading behavior may hold clues for both better pain management and improvements in public health.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/312/5774/754/DC1
Materials and Methods
Figs. S1 to S6
Tables S1 to S3
References
References and Notes
- 1.Samuelson PA. Rev Econ Stud. 1937;4:155. [Google Scholar]
- 2.The opposite of utility is generally referred to as disutility, but for the sake of clarity, we refer to ‘‘utility’’ as possessing both positive and negative domains.
- 3.Loewenstein G. Econ J. 1987;97:666. [Google Scholar]
- 4.Caplin A, Leahy J. Q J Econ. 2001;116:55. [Google Scholar]
- 5.Ji RR, Kohno T, Moore KA, Woolf CJ. Trends Neurosci. 2003;26:696. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Ploghaus A, et al. Science. 1999;284:1979. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 7.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. Proc Natl Acad Sci USA. 2005;102:12950. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raij TT, Numminen J, Narvanen S, Hiltunen J, Hari R. Proc Natl Acad Sci USA. 2005;102:2147. doi: 10.1073/pnas.0409542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey I. Curr Opin Neurobiol. 2005;15:478. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. Annu Rev Neurosci. 2003;26:1. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 11.Peyron R, Laurent B, Garcia-Larrea L. Neurophysiol Clin. 2000;30:263. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 12.Science. Materials and methods are available as supporting material on. Online. [Google Scholar]
- 13.Varian HR. Intermediate Microeconomics: A Modern Approach. 6. Norton & Co; New York: 2002. [Google Scholar]
- 14.Berridge KC. Well Being. In: Kahneman D, Diener E, Schwarz N, editors. The Foundations of Hedonic Psychology. Russell Sage Foundation; New York: 1999. pp. 525–557. [Google Scholar]
- 15.Petrovic P, Petersson KM, Hansson P, Ingvar M. Neuroimage. 2002;16:1142. doi: 10.1006/nimg.2002.1069. [DOI] [PubMed] [Google Scholar]
- 16.Vogt BA. Nat Rev Neurosci. 2005;6:533. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentley DE, et al. Clin Neurophysiol. 2004;115:1846. doi: 10.1016/j.clinph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Bentley DE, Derbyshire SWG, Youell PD, Jones AKP. Pain. 2003;102:265. doi: 10.1016/S0304-3959(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 19.Phelps EA. Annu Rev Psychol. 2006;57:27. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 20.Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Pain. 1999;82:159. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 21.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Science. 2004;306:503. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 22.Kahneman D, Wakker PP, Sarin R. Q J Econ. 1997;112:375. [Google Scholar]
- 23.Camerer C, Loewenstein G, Prelec D. J Econ Lit. 2005;43:9. [Google Scholar]
- 24.Rescorla RA, Wagner AR. In: Classical Conditioning 2: Current Research and Theory. Black AH, Prokasy WF, editors. Appleton Century-Crofts; New York: 1972. pp. 64–69. [Google Scholar]
- 25.Sutton RS. Mach Learn. 1988;3:9. [Google Scholar]
- 26.Morewedge CK, Gilbert DT, Wilson TD. Psychol Sci. 2005;16:626. doi: 10.1111/j.1467-9280.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 27.Ploghaus A, et al. Proc Natl Acad Sci USA. 2000;97:9281. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ploghaus A, Becerra L, Borras C, Borsook D. Trends Cogn Sci. 2003;7:197. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 29.Salomons TV, Johnstone T, Backonja MM, Davidson RJ. J Neurosci. 2004;24:7199. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wager TD, et al. Science. 2004;303:1162. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 31.Porro CA, et al. J Neurosci. 2002;22:3206. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferretti A, et al. Neuroimage. 2004;23:1217. doi: 10.1016/j.neuroimage.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Bingel U, et al. Neuroimage. 2004;23:224. doi: 10.1016/j.neuroimage.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 34.We thank C. M. Capra, C. Noussair, A. Rangel, and A. Rustichini for comments on this paper. Supported by grants from the National Institute on Drug Abuse (DA00367 and DA016434).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


