Abstract
Methyl jasmonate (MeJA) elicits stomatal closing similar to abscisic acid (ABA), but whether the two compounds use similar or different signaling mechanisms in guard cells remains to be clarified. We investigated the effects of MeJA and ABA on second messenger production and ion channel activation in guard cells of wild-type Arabidopsis (Arabidopsis thaliana) and MeJA-insensitive coronatine-insensitive 1 (coi1) mutants. The coi1 mutation impaired MeJA-induced stomatal closing but not ABA-induced stomatal closing. MeJA as well as ABA induced production of reactive oxygen species (ROS) and nitric oxide (NO) in wild-type guard cells, whereas MeJA did not induce production of ROS and NO in coi1 guard cells. The experiments using an inhibitor and scavengers demonstrated that both ROS and NO are involved in MeJA-induced stomatal closing as well as ABA-induced stomatal closing. Not only ABA but also MeJA activated slow anion channels and Ca2+ permeable cation channels in the plasma membrane of wild-type guard cell protoplasts. However, in coi1 guard cell protoplasts, MeJA did not elicit either slow anion currents or Ca2+ permeable cation currents, but ABA activated both types of ion channels. Furthermore, to elucidate signaling interaction between ABA and MeJA in guard cells, we examined MeJA signaling in ABA-insensitive mutant ABA-insensitive 2 (abi2-1), whose ABA signal transduction cascade has some disruption downstream of ROS production and NO production. MeJA also did not induce stomatal closing but stimulated production of ROS and NO in abi2-1. These results suggest that MeJA triggers stomatal closing via a receptor distinct from the ABA receptor and that the coi1 mutation disrupts MeJA signaling upstream of the blanch point of ABA signaling and MeJA signaling in Arabidopsis guard cells.
Stomatal pores that are formed by a pair of guard cells respond to various stimuli, including plant hormones and elicitors. Methyl jasmonate (MeJA), which mediates various plant defense responses (Liechti and Farmer, 2002; Turner et al., 2002), has been reported to induce stomatal closing (Gehring et al., 1997; Suhita et al., 2003; Suhita et al., 2004). It has been suggested that there are several components common to both abscisic acid (ABA)-induced and MeJA-induced stomatal closing (e.g. reactive oxygen species [ROS] and cytosolic alkalization [Suhita et al., 2004]). Nitric oxide (NO) as well as ROS functions as an important second messenger in ABA-induced stomatal closing signal transduction (Desikan et al., 2002; Neill et al., 2002; Bright et al., 2006). It has been shown that NO is also involved in the defense signaling mediated by MeJA (Orozco-Cárdenas and Ryan, 2002; Huang et al., 2004). Moreover, a recent finding suggests that ABA induces NO production via hydrogen peroxide (H2O2) synthesis (Bright et al., 2006). However, the role of NO in MeJA-induced stomatal closing remains to be clarified. These second messengers have been shown to mediate ion channels in the plasma membrane of guard cells. For example, ROS and NO modulate the activation of K+ channels (Garcia-Mata et al., 2003; Köhler et al., 2003; Sokolovski and Blatt, 2004; Sokolovski et al., 2005), Cl−channels (Garcia-Mata et al., 2003; Sokolovski et al., 2005), and Ca2+ channels (Allen et al., 2000; Pei et al., 2000; Murata et al., 2001; Köhler et al., 2003; Kwak et al., 2003) in guard cells of fava bean (Vicia faba) or Arabidopsis (Arabidopsis thaliana).
The activation of several types of ion channels in the plasma membrane of guard cells is an important process for ABA-induced stomatal closing. Activation of slow (S-type) anion channels depolarizes the plasma membrane of guard cells (Schroeder and Keller, 1992; Schmidt et al., 1995; Pei et al., 1997), and the activation of S-type anion channels by ABA is impaired in many ABA-insensitive mutants (Pei et al., 1997; Li et al., 2000; Wang et al., 2001; Kwak et al., 2002). These results indicate that activation of S-type anion channels is essential for ABA-induced stomatal closing.
Ca2+ permeable cation (ICa) channels, which are activated by H2O2, contribute to elevation of cytosolic-free Ca2+ ([Ca2+]cyt) in guard cells (Pei et al., 2000). ICa channels are also activated by ABA and fungal elicitors only in the presence of cytosolic NAD(P)H (Murata et al., 2001; Klüsener et al., 2002), and activation of ICa channels by ABA is abolished in the Arabidopsis ABA-insensitive mutants gca2 (Pei et al., 2000) and ABA-insensitive 2 (abi2-1; Murata et al., 2001). In addition, both ABA-induced ROS production and ABA-induced cytosolic NAD(P)H-dependent activation of ICa channels are impaired in the Arabidopsis double mutant of catalytic subunits of plasma membrane NAD(P)H oxidases atrboh D/F (Kwak et al., 2002). These studies revealed that NAD(P)H oxidase-mediated ROS production is necessary for activation of ICa channels by ABA.
It has been demonstrated that K+ efflux across the plasma membrane of guard cells is stimulated by long-term depolarization of the plasma membrane (Schroeder et al., 1987) and that cytosolic alkalization activates outward rectifying K+ currents (Blatt and Armstrong, 1993). Recently, it was demonstrated that MeJA activates outward potassium currents in guard cell protoplasts (GCPs) of fava bean (Evans, 2003). However, to our knowledge, no study has elucidated whether MeJA regulates other ion channels in the plasma membrane of guard cells.
In this study, we used the MeJA-insensitive mutant, coronatine-insensitive 1 (coi1), which fails in various jasmonate-mediated responses, including inhibition of root growth and expression of defense genes (Feys et al., 1994; Benedetti et al., 1995; Penninckx et al., 1996; Xie et al., 1998). The coi1 mutation disrupts an F-box protein that functions in the E3 ubiquitin ligase involved in the 26S proteasome-mediated protein degradation pathway (Xie et al., 1998; Xu et al., 2002). However, the role of F-box proteins in signal transduction in guard cells remains unknown.
In this article, to elucidate the signaling interaction between ABA and MeJA in guard cells, we examined ROS production, NO production, activation of S-type anion channels, and activation of ICa channels induced by MeJA and also tested the effects of MeJA on stomatal aperture and second messenger production in the abi2-1 mutant. Based on our results, we present a new model of hormonal signaling interaction in Arabidopsis guard cells.
RESULTS
Impairment of MeJA-Induced Stomatal Closing in coi1 Mutant
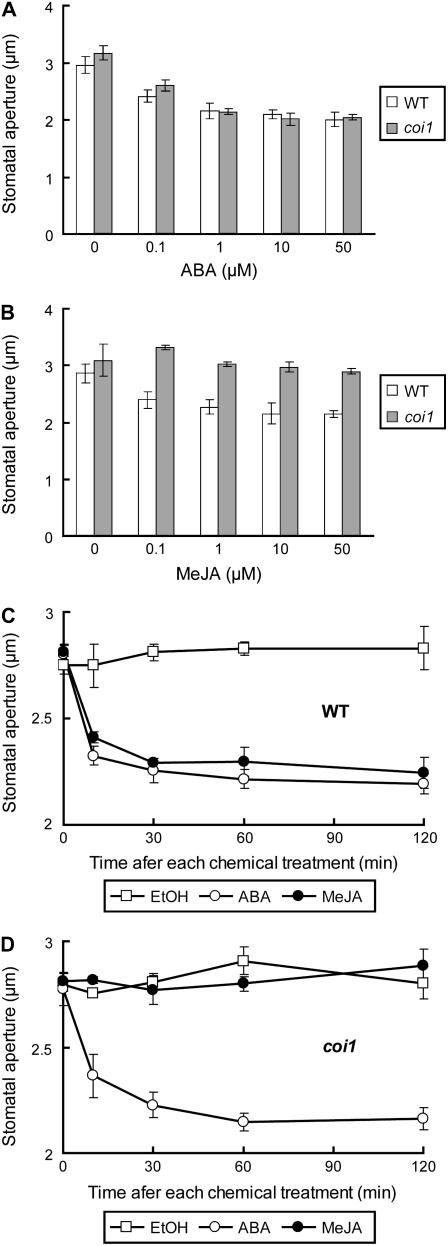
To clarify MeJA signaling in guard cells, we examined stomatal movements of the MeJA-insensitive mutant coi1. ABA induced stomatal closing in coi1 plants as well as wild-type plants (Fig. 1A). Both plants responded to ABA in a similar dose-response manner, suggesting that ABA signal cascade in the coi1 mutant could not be disrupted. However, MeJA induced stomatal closing in wild-type plants but did not induce stomatal closing in coi1 plants (Fig. 1B). Time courses of stomatal closing in wild-type plants and coi1 plants are shown in Figure 1, C and D, respectively. Application of 1 μm ABA or 1 μm MeJA reduced stomatal apertures by 18% at 30 min and by 21% at 120 min in wild-type plants (Fig. 1C). The similar time course of stomatal movements was observed in coi1 plants treated with ABA, but there was no change in stomatal aperture of coi1 plants treated with MeJA (Fig. 1D). Note that reverse transcriptional-PCR analysis with highly purified GCPs (>80%) showed that COI1 is expressed in guard cells (data not shown). This result indicates that COI1 can be involved in MeJA signaling in guard cells. Taken together, our results indicated that the coi1 mutation specifically impaired MeJA-induced stomatal closing but not ABA-induced stomatal closing.
Figure 1.
ABA-induced and MeJA-induced stomatal closing in wild-type plants and coi1 plants. A, ABA-induced stomatal closing in wild-type plants (white bars) and in coi1 plants (gray bars). B, MeJA-induced stomatal closing in wild-type plants (white bars) but not in coi1 plants (gray bars). C, The time course of stomatal apertures in wild-type plants treated with 0.1% ethanol (white squares), 1 μm ABA (white circles), and 1 μm MeJA (black circles). D, The time course of stomatal movements of coi1 plants treated with 0.1% ethanol (white squares), 1 μm ABA (white circles), and 1 μm MeJA (black circles). Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent ses.
Impairment of ROS Production and NO Production in coi1 Mutant
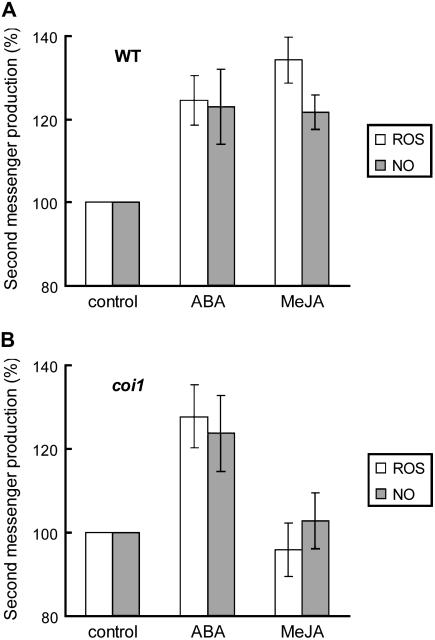
To elucidate that ROS and NO act as second messengers in MeJA signaling pathway in guard cells, we examined effects of the coi1 mutation on ROS production and NO production induced by MeJA using the ROS detection fluorescence dye 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), and the NO detection fluorescence dye 4,5-diaminofluorescein-2 diacetate (DAF-2DA). As shown in Figure 2A, ABA induced ROS production (P < 0.01) and NO production (P < 0.05) in wild-type guard cells, which is consistent with previous results (Pei et al., 2000; Murata et al., 2001; Neill et al., 2002; Garcia-Mata et al., 2003). MeJA also promoted both ROS production (P < 0.001) and NO production (P < 0.01) in wild-type guard cells. ABA stimulated ROS production (P < 0.02) and NO production (P < 0.04) in coi1 guard cells as well as wild-type guard cells, whereas MeJA did not induce either ROS production (P = 0.55) or NO production (P = 0.69) in coi1 guard cells unlike wild-type guard cells (Fig. 2B).
Figure 2.
ABA-induced and MeJA-induced production of ROS and NO in wild-type guard cells and coi1 guard cells. A, Effects of ABA (50 μm) and MeJA (50 μm) on ROS production (n = 4, white bars) and NO production (n = 4, gray bars) in wild-type guard cells. B, Effects of ABA (50 μm) and MeJA (50 μm) on ROS production (n = 4, white bars) and NO production (n = 5, gray bars) in coi1 guard cells. The vertical scale represents the percentage of H2DCF-DA fluorescence levels (ROS) and DAF-2DA fluorescence levels (NO) when fluorescent intensities of ABA- or MeJA-treated cells are normalized to control value taken as 100% for each experiment. Each data was obtained from >60 total guard cells. Error bars represent ses.
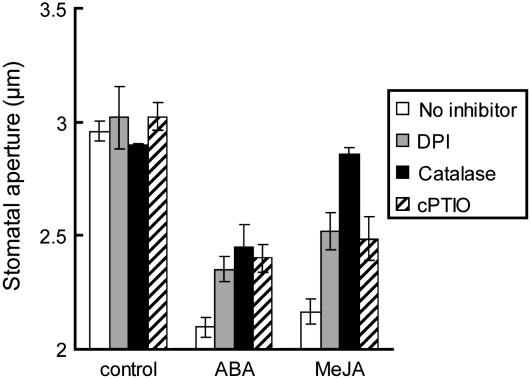
To confirm that ROS and NO function as second messengers on MeJA-induced stomatal closing, we evaluated the effects of the NAD(P)H oxidase inhibitor diphenylene iodonium chloride (DPI), the H2O2-specific scavenger catalase, and the NO-specific scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) on MeJA-induced stomatal closing (Fig. 3). Treatment with 25 μm DPI, 100 units/mL catalase, or 100 μm cPTIO significantly prevented ABA-induced stomatal closing, as shown in previous reports (Pei et al., 2000; Zhang et al., 2001; Neill et al., 2002). Treatment with each compound also inhibited MeJA-induced stomatal closing in wild-type plants. These results suggest that MeJA as well as ABA induces stomatal closing via ROS production and NO production in guard cells.
Figure 3.
ABA-induced and MeJA-induced stomatal closing inhibited by DPI, catalase, and cPTIO. Rosette leaves of wild-type plants were treated with 20 μm DPI (gray bars), 100 units/mL catalase (black bars), or 100 μm cPTIO (hatched bars). Then, rosette leaves pretreated with each inhibitor for 30 min were treated with 1 μm ABA or 1 μm MeJA. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent ses.
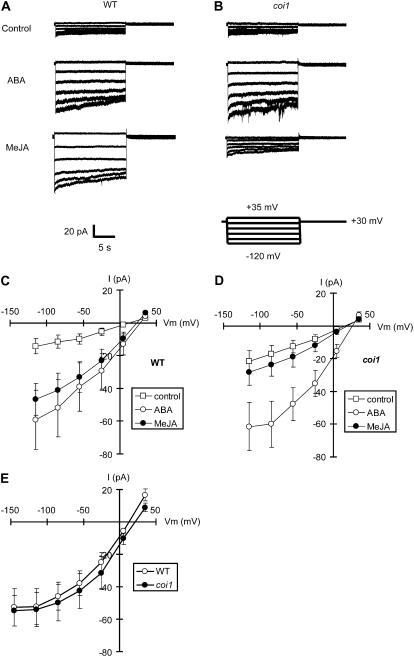
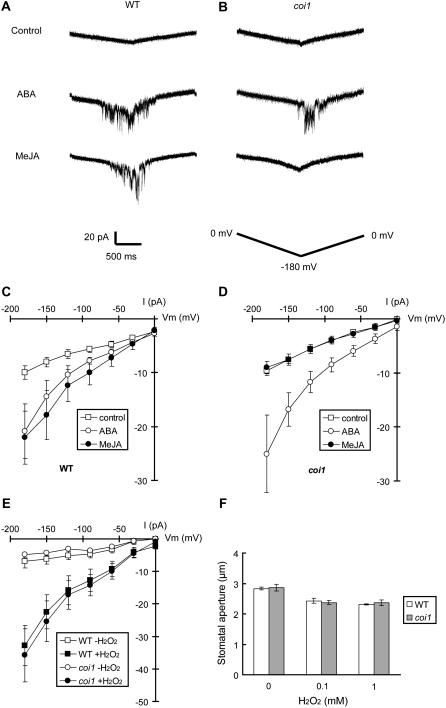
Activation of S-Type Anion Currents and ICa Currents by MeJA in Wild-Type Guard Cells and Impairment of Activation of These Ion Channels in coi1 Guard Cells
Many reports have demonstrated that the activation of S-type anion channels in the plasma membrane of guard cells is important for ABA-induced stomatal closing. However, no report demonstrates activation of S-type anion channels in the plasma membrane of guard cells induced by MeJA. Thus, we examined whether MeJA activates S-type anion channels. ABA activated S-type anion currents in wild-type GCPs (P < 0.03 at −115 mV; Fig. 4, A and C), which is consistent with previous reports (Pei et al., 1997; Wang et al., 2001; Kwak et al., 2002). Pretreatment with MeJA elicited S-type anion currents in wild-type GCPs (P < 0.01 at −115 mV; Fig. 4, A and C). These results suggest that S-type anion channels may play an important role in MeJA-induced stomatal closing as well as ABA-induced stomatal closing. ABA elicited S-type anion currents in coi1 GCPs (P < 0.04 at −115 mV; Fig. 4, B and D) as well as wild-type GCPs, whereas MeJA did not activate S-type anion currents in coi1 GCPs (P = 0.57 at −115 mV; Fig. 4, B and D) unlike wild-type GCPs. These results are consistent with the results of stomatal movements, ROS production, and NO production shown in Figures 1 and 2.
Figure 4.
S-type anion currents in wild-type GCPs and coi1 GCPs. A, Whole-cell recordings of S-type anion currents in wild-type GCPs treated with no hormones (top trace), 50 μm ABA (middle trace), or 50 μm MeJA (bottom trace). B, Whole-cell recordings of S-type anion currents in coi1 GCPs treated with no hormones (top trace), 50 μm ABA (middle trace), or 50 μm MeJA (bottom trace). C, Steady-state current-voltage relationships for ABA and MeJA activation of S-type anion currents in wild-type GCPs as recorded in A (white squares, control; white circles, 50 μm ABA; black circles, 50 μm MeJA). D, Steady-state current-voltage relationships for ABA and MeJA activation of S-type anion currents in coi1 GCPs as recorded in B (white squares, control; white circles, 50 μm ABA; black circles, 50 μm MeJA). E, Steady-state current-voltage relationships for extracellular high Ca2+ (40 mm) activation of S-type anion currents in wild-type GCPs (white circles) and in coi1 GCPs (black circles). The voltage protocol was stepped up from 35 mV to −115 mV in 30-mV decrements (holding potential, 30 mV). GCPs were treated with 50 μm ABA or 50 μm ABA MeJA for 2 h before recordings. Each datum was obtained from at least n = 6 GCPs. Error bars represent ses.
It has been suggested that signals to induce stomatal closing (i.e. ABA and extracellular high Ca2+ condition) modulate priming of Ca2+ sensors, including calcium-dependent protein kinases in guard cells, which can response elevation of [Ca2+]cyt and then activate S-type anion channels (Allen et al., 2002; Mori et al., 2006). We tested whether the coi1 mutation affects activation of S-type anion channels by Ca2+. Preincubation of GCPs with 40 mm CaCl2 evoked S-type anion currents both in wild-type GCPs and coi1 GCPs when the concentration of [Ca2+]cyt was 2 μm (Fig. 4E).
Elevation of [Ca2+]cyt in guard cells occurs during stomatal closing (McAinsh et al., 1995), and application of ABA and H2O2 elicits ICa currents and [Ca2+]cyt oscillation in guard cells (Allen et al., 1999b, 2000; Hamilton et al., 2000; Pei et al., 2000; Köhler et al., 2003). Thus, we examined whether MeJA activates ICa channels. MeJA (P < 0.04 at −180 mV) as well as ABA (P < 0.05 at −180 mV) elicited typical spiky ICa currents in wild-type GCPs (Fig. 5, A and C). However, in coi1 GCPs, MeJA (P = 0.60 at −180 mV) did not elicit ICa currents in contrast with ABA (P < 0.01 at −180 mV; Fig. 5, B and D). We also examined activation of ICa currents by H2O2 in the coi1 mutant. Application of 1 mm H2O2 to bath solution elicited ICa currents both in wild-type GCPs and in coi1 GCPs (Fig. 5E). Additionally, treatment with H2O2 induced stomatal closing both in wild-type plants and coi1 plants (Fig. 5F).
Figure 5.
ICa currents in wild-type GCPs and coi1 GCPs. A, Whole-cell recordings of ICa currents in wild-type GCPs treated with no hormones (top trace), 50 μm ABA (middle trace), or 50 μm MeJA (bottom trace). B, Whole-cell recordings of ICa currents in coi1 GCPs treated with no hormones (top trace), 50 μm ABA (middle trace), or 50 μm MeJA (bottom trace). C, Current-voltage relationships for ABA and MeJA activation of ICa currents in wild-type GCPs as recorded in A (white squares, control; white circles, 50 μm ABA; black circles, 50 μm MeJA). D, Current-voltage relationships for ABA and MeJA activation of ICa currents in coi1 GCPs as recorded in B (white squares, control; white circles, 50 μm ABA; black circles, 50 μm MeJA). E, Current-voltage relationships for H2O2 (1 mm) activation of ICa currents in wild-type GCPs and coi1 GCPs (white squares, wild-type − H2O2; black squares, wild-type + H2O2; white circles, coi1 − H2O2; black circles, coi1 + H2O2). A ramp voltage protocol from 20 to −180 mV (holding potential, 0 mV; ramp speed, 200 mV/s) was used. After making the whole-cell configuration, control currents were recorded 16 times for each GCP as control data. Then, 50 μm ABA, 50 μm MeJA, or 1 mm H2O2 was applied to bath solution and ICa currents were recorded another 16 times. Each datum was obtained from at least n = 6 GCPs. Error bars represent ses. F, H2O2-induced stomatal closing in wild-type plants (white bars) and in coi1 plants (gray bars). Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent ses.
Impairment of MeJA-Induced Stomatal Closing by the abi2-1 Mutation
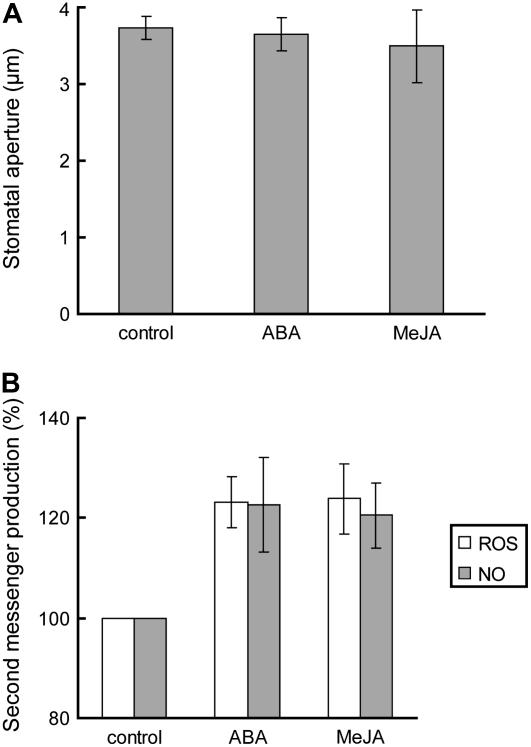
To clarify the signaling interaction between ABA and MeJA in Arabidopsis guard cells, we examined whether MeJA induces stomatal closing, ROS production, and NO production in the ABA-insensitive protein phosphatase 2C mutant, abi2-1 (Meyer et al., 1994; Leung et al., 1997), of which the mutation disrupts ABA signaling downstream of ROS production and NO production in guard cells (Murata et al., 2001; Desikan et al., 2002). Neither ABA nor MeJA induced stomatal closing in abi2-1 plants unlike in wild-type plants (Fig. 6A). MeJA as well as ABA elicited ROS production and NO production in abi2-1 guard cells (Fig. 6B).
Figure 6.
ABA-induced and MeJA-induced stomatal closing in the abi2-1 mutant. A, Stomatal closing caused by 10 μm ABA and 10 μm MeJA in abi2-1 plants. Note that the concentration of each chemical was sufficient to induce stomatal closing in wild-type plants. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent ses. B, ROS production (white bars) and NO production (gray bars) caused by 50 μm ABA and 50 μm MeJA. The vertical scale represents the percentage of H2DCF-DA fluorescence levels (ROS) and DAF-2DA fluorescence levels (NO) when fluorescent intensities of ABA- or MeJA-treated cells are normalized to control value taken as 100% for each experiment. Each datum was obtained from >60 total guard cells. Error bars represent ses.
DISCUSSION
MeJA-Induced Stomatal Closing
Similar to ABA, MeJA accumulates in planta under drought conditions (Creelman and Mullet, 1995). As shown in Figure 1, MeJA induces stomatal closing in a dose- and time-dependent manner similar to that of ABA-induced stomatal closing. Furthermore, we also found that, similar to ABA, MeJA regulates production of second messengers and activation of ion channels in Arabidopsis guard cells. Taken together, these findings suggest that MeJA could play a physiological role in inducing stomatal closing to accommodate the drought condition as ABA.
Specific Impairment of MeJA-Induced Stomatal Closing in coi1 Mutant
Suhita et al. (2004) investigated the signal interaction between ABA and MeJA using ABA-insensitive mutant ost1 and MeJA-insensitive mutant jar1 and reported that the ost1 mutation and the jar1 mutation impaired both ABA-induced stomatal closing and MeJA-induced stomatal closing. These results showed some signal interactions between ABA and MeJA but failed to clarify MeJA-specific signaling or ABA-specific signaling. In this article, the abi2-1 (Fig. 6A) and abi1-1 (data not shown) mutations impaired both ABA-induced stomatal closing and MeJA-induced stomatal closing. These data also indicated that there are some interactions between ABA signaling and MeJA signaling but could not give us any valuable information about ABA-specific signaling and MeJA-specific signaling.
The coi1 mutants exhibit male sterility, susceptibility to insect attack and pathogen infection, and insensitivity to jasmonates, which are involved in root growth and defense gene expression (Feys et al., 1994; Benedetti et al., 1995; Penninckx et al., 1996; McConn et al., 1997; Xie et al., 1998). In this article, we investigated MeJA signaling and the signaling interaction between ABA and MeJA using the coi1 mutant. The coi1 mutation impaired MeJA-induced stomatal closing but did not impair ABA-induced stomatal closing (Fig. 1). These results indicate that, in contrast with the jar1 mutation, the coi1 mutation disrupts MeJA signaling upstream of a blanch point between ABA signaling and MeJA signaling in Arabidopsis guard cells, suggesting that COI1 could be an early signaling component in MeJA signaling and that COI1 might be closely involved in perception of MeJA. In future experiments, the coi1 mutant could be more available to elucidate different roles of MeJA from ABA in guard cell response to various environmental stresses.
Involvement of ROS and NO Production in MeJA Signaling in Guard Cells
Both ROS and NO play important roles in MeJA-induced defense signal transduction (Orozco-Cárdenas et al., 2001; Orozco-Cárdenas and Ryan, 2002; Huang et al., 2004), but roles of ROS and NO in MeJA-induced stomatal closing remain unclear. In this study, we found that MeJA as well as ABA induces both ROS production and NO production in wild-type guard cells (Fig. 2) and that ROS and NO actually function as second messengers in the signal pathway of MeJA-induced stomatal closing (Fig. 3). However, in contrast with ABA, MeJA did not induce either ROS production or NO production in coi1 guard cells (Fig. 2). These results suggest that the coi1 mutation disrupts MeJA signaling upstream of production of ROS and NO, while the abi2-1 mutation disrupts MeJA signaling downstream of production of ROS and NO in Arabidopsis guard cells (Fig. 6).
DPI, catalase, and cPTIO partly inhibited ABA-induced and MeJA-induced stomatal closing (Fig. 3). Interestingly, catalase showed stronger inhibitory effects on MeJA-induced stomatal closing than ABA-induced stomatal closing (Fig. 3). This finding suggests that, in addition to the production of NAD(P)H oxidase in the guard cell plasma membrane, apoplastic production of ROS by epidermal cells and/or mesophyll cells adjacent to guard cells might be involved in MeJA-induced stomatal closing, because catalase is a cell-impermeable scavenger of H2O2 (Lee et al., 1999; Zhang et al., 2001).
Activation of Ion Channels in the Plasma Membrane of Guard Cells by MeJA
It has been shown that the activation of S-type anion channels, which triggers long-term plasma membrane depolarization that results in K+ efflux via outward K+ channels, is the primary driving force causing K+ efflux in guard cells (Schroeder et al., 1987; Schroeder and Keller, 1992; Schmidt et al., 1995; Pei et al., 1997); that is, the activation of S-type anion channels is essential for ABA-induced stomatal closing. In this study, we found that MeJA as well as ABA activates S-type anion channel in wild-type GCPs (Fig. 4, A and C).
S-type anion channels are activated via cytosolic Ca2+ dependent and phosphorylation events (Schroeder and Hagiwara, 1989; Schmidt et al., 1995; Allen et al., 1999a, 2002; Mori et al., 2006). Although activation of S-type anion currents by MeJA was impaired in coi1 GCPs (Fig. 4, B and D), pretreatment with extracellular high Ca2+ elicited S-type anion currents both in wild-type GCPs and in coi1 GCPs (Fig. 4E). This result suggested that COI1 could function upstream of Ca2+ sensor priming and [Ca2+]cyt elevation to activate S-type anion channel activation.
We also observed activation of ICa channels by MeJA in wild-type GCPs but not in coi1 GCPs (Fig. 5). Suhita et al. (2003) showed that Ca2+ channel blockers inhibit MeJA-induced stomatal closing. These findings suggested that the activation of ICa channels is necessary for Ca2+-dependent signal transduction in MeJA-induced stomatal closing. We also found the coi1 mutation had no effect on activation of ICa channels by H2O2 (Fig. 5E) and disrupted production of ROS and NO caused by MeJA (Fig. 2). These results also support the conclusion that ROS and NO function as second messengers to activate the plasma membrane ion channels on MeJA signaling in guard cells.
Involvement of ABI2 Protein Phosphatase 2C in MeJA-Induced Stomatal Closing
ABI2 is a protein phosphatase 2C (Meyer et al., 1994; Leung et al., 1997) and the abi2-1 mutation disrupts ABA signaling downstream of ROS production and NO production in guard cells (Murata et al., 2001; Desikan et al., 2002). MeJA does not induce stomatal closing in abi2-1 plants (Fig. 6A) but does induce production of ROS and NO in abi2-1 guard cells (Fig. 6B). These results indicate that MeJA induces stomatal closing via ABI2-dependent signal pathway and suggest that the abi2-1 mutation could disrupt MeJA signaling downstream of production of ROS and NO in Arabidopsis guard cells. It has been shown that the protein phosphatase activity of ABI2 is sensitive to redox status (Meinhard et al., 2002). Therefore, the perception of redox signaling through redox sensors such as ABI2 could be indispensable for the stomatal closing caused by MeJA.
The Physiological Significance of COI1 in MeJA Signaling in Arabidopsis Guard Cells
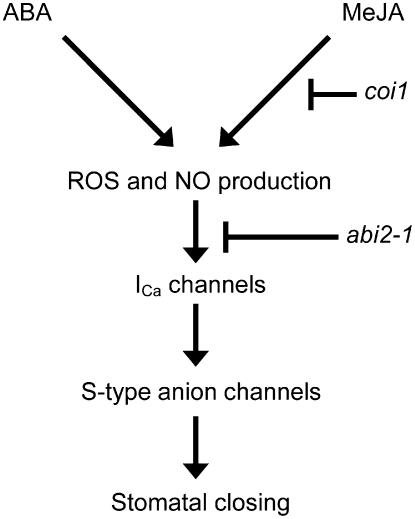
Based on our results, we presented a simple model of the signaling interaction between ABA and MeJA in Arabidopsis guard cells in Figure 7. This model positions COI1, production of ROS and NO, ABI2, ICa channels, and S-type anion channels in this hormone signal cascade. COI1 functions upstream of the blanch point of ABA signaling and MeJA signaling and ABI2 functions downstream of the blanch point.
Figure 7.
A simple model of the signaling interaction between ABA and MeJA in Arabidopsis guard cells. Similar to ABA, MeJA also induces ROS production and NO production and activates ICa channels and S-type anion channels. The coi1 mutation disrupts MeJA signaling upstream of the blanch point of ABA signaling and MeJA signaling. The abi2-1 mutation disrupts both ABA signaling and MeJA signaling downstream of production of ROS and NO.
COI1 encodes one of the F-box proteins that function in E3 ubiquitin-ligase complexes, which are involved in the 26S proteasome-mediated protein degradation pathway (Xie et al., 1998; Xu et al., 2002). In this article, we found that the coi1 mutation impaired production of second messengers and activation of ion channels induced by MeJA in guard cells, suggesting that the ubiquitin/proteasome pathway could regulate production of second messengers and activation of ion channels in plants.
MATERIALS AND METHODS
Plant Material and Growth
Throughout this study, we used the Arabidopsis (Arabidopsis thaliana) ecotype Columbia as the wild-type plant. Columbia, the coi1 mutant (Columbia accession), and the abi2-1 mutant (Landsberg erecta accession) were grown in growth chambers (22°C, 8,000 Lux under a 16-h-light/8-h-dark regime). Both ecotypes showed similar stomatal response to MeJA and ABA (data not shown). The coi1 mutant, which was used in all parts of this article, has an amber substitution in the Trp-44 of COI1 (TGG to TAG). Because coi1 mutants have the recessive sterile phenotype, this mutation was kept heterozygous. Additionally, the coi1 mutant has the JA-responsive VSP1∷luciferase reporter system, as previously described (Ellis and Turner, 2001). F2 seeds were sown on standard Murashige and Skoog plates. Plates were subjected to vernalization treatment at 4°C for 4 d and then transferred to growth chambers in a vertical orientation. Five-day-old seedlings were transferred to Murashige and Skoog plates containing 50 μm MeJA. Plates were further incubated in growth chambers for another 5 d. After that, homozygous coi1 seedlings were screened by root length, anthocyanin accumulation in cotyledons (Feys et al., 1994), and luciferase activities (Ellis and Turner, 2001). For measurements of luciferase activities, the solution containing 3 mm luciferin (Promega) and 0.01% Triton X-100 was sprayed on detached leaves. Homozygous coi1 seedlings did not show inhibition of root elongation, anthocyanin accumulation in cotyledons, and luciferase expression induced by MeJA. The screened homozygous coi1 seedlings were transferred to the soil condition as wild-type plants.
Stomatal Aperture Measurements
Stomatal aperture measurements were performed as described previously (Pei et al., 1997; Murata et al., 2001). Excised rosette leaves were floated on medium containing 5 mm KOH, 50 μm CaCl2, and 10 mm MES-Tris, pH 6.15, for 2 h in the light (8,000 Lux) to induce stomatal opening followed by the addition of MeJA or ABA. Then, stomatal apertures were measured after 2-h incubation. Leaves were blended for 30 s and epidermal peels were collected. Twenty stomatal apertures were measured on each epidermal peel. For time course experiments, stomatal apertures were measured at each pointed time after application of ethanol, ABA, or MeJA.
Detection of ROS and NO
ROS and NO production in guard cells was analyzed by using H2DCF-DA (Lee et al., 1999; Murata et al., 2001; Suhita et al., 2004) and DAF-2DA (Foissner et al., 2000; Neill et al., 2002; Huang et al., 2004), respectively. In the case of the ROS detection, epidermal peels were incubated for 3 h in medium containing 5 mm KOH, 50 μm CaCl2, and 10 mm MES-Tris, pH 6.15, and then 50 μm H2DCF-DA was added to this medium. The epidermal tissues were incubated for 30 min at room temperature, and then the excess dye was washed out with distilled deionized water. The dye-loaded tissues were treated with 50 μm ABA or 50 μm MeJA for 20 min, and then fluorescence of guard cells was imaged and analyzed using AQUA COSMOS software (Hamamatsu Photonics). For NO detection, 10 μm DAF-2DA was added instead of 50 μm H2DCF-DA.
Electrophysiology
For whole-cell patch-clamp recordings of S-type anion and ICa currents, Arabidopsis GCPs were prepared from rosette leaves of 4- to 6-week-old plants with the digestion solution containing 1.0% cellulase R10, 0.5% macerozyme R10, 0.5% bovine serum albumin, 0.1% kanamycin, 10 mm ascorbic acid, 0.1 mm KCl, 0.1 mm CaCl2, and 500 mm d-mannitol, pH 5.5, with KOH, as previously described (Pei et al., 1997). Whole-cell currents were recorded using a CEZ-2200 patch clamp amplifier (Nihon Kohden). The resulting values were corrected for liquid junction potential, and leak currents were not subtracted. For data analysis, pCLAMP 8.1 software (Molecular Devices) was used. For S-type anion current measurements, the patch-clamp solutions contained 150 mm CsCl, 2 mm MgCl2, 6.7 mm EGTA, 5.58 mm CaCl2 (free Ca2+ concentration, 2 μm), 5 mm ATP, and 10 mm HEPES-Tris, pH 7.1, in the pipette and 30 mm CsCl, 2 mm MgCl2, 1 mm CaCl2 (40 mm CaCl2 in Fig. 4E only), and 10 mm MES-Tris, pH 5.6, in the bath (Pei et al., 1997). For ICa current measurements, the pipette solution contained 10 mm BaCl2, 0.1 mm dithiothreitol, 3 mm NADPH, 4 mm EGTA, and 10 mm HEPES-Tris, pH 7.1. The bath solution contained 100 mm BaCl2, 0.1 mm dithiothreitol, and 10 mm MES-Tris, pH 5.6 (Pei et al., 2000; Murata et al., 2001). In both cases, osmolarity was adjusted to 500 mmol/kg (pipette solutions) and 485 mmol/kg (bath solutions) with d-sorbitol.
Statistical Analysis
Significance of differences between data sets was assessed by Student's t-test analysis in all parts of this article. We regarded differences at the level of P < 0.05 as significant.
Accession Numbers
Arabidopsis Genome Initiative numbers for the genes discussed in this article are as follows: COI1, At2g39940; and ABI2, At5g57050.
Acknowledgments
We thank Koji Shimizu for his help in obtaining patch-clamp data and Masanori Nishiyama for his assistance in obtaining fluorescence data. We also thank Dr. Shigeko Utsugi for providing the abi2-1 and abi1-1 seeds.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (grants for Scientific Research on Priority Areas to Y.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Yoshiyuki Murata (muta@cc.okayama-u.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI (1999. a) Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999. b) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19 735–747 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI (2002) Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti CE, Xie D, Turner JG (1995) COI1-dependent expression of an Arabidopsis vegetative storage protein in flowers and siliques and in response to coronatine or methyl jasmonate. Plant Physiol 109 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F (1993) K+ channels of stomatal guard cells: abscisic- acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191 330–341 [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45 113–122 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92 4114–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99 16314–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NH (2003) Modulation of guard cell plasma membrane potassium currents by methyl jasmonate. Plant Physiol 131 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23 817–824 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, McConchie R, Parish RW (1997) Jasmonates induce intracellular alkalinization and closure of Paphiopedilum guard cells. Ann Bot (Lond) 80 485–489 [Google Scholar]
- Hamilton DWA, Hills A, Köhler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J (2004) Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 218 938–946 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Hills A, Blatt MR (2003) Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol 131 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI (2002) Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell 14 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, Choi EJ, Taylor SAT, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reaction oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287 300–303 [DOI] [PubMed] [Google Scholar]
- Liechti R, Farmer EE (2002) The jasmonate pathway. Science 296 1649–1650 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelmann RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard M, Rodriguez PL, Grill E (2002) The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214 775–782 [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264 1452–1455 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4 e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder JI (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128 13–16 [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA (2002) Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol 130 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734 [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux JP, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao YJ, Schroeder JI (1995) Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA 92 9535–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430 [Google Scholar]
- Schroeder JI, Keller BU (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA 89 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovski S, Blatt MR (2004) Nitric oxide block of outward-rectifying K+ channels indicates direct control by protein nitrosylation in guard cells. Plant Physiol 136 4275–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR (2005) Protein phosphorylation is prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 43 520–529 [DOI] [PubMed] [Google Scholar]
- Suhita D, Kolla VA, Vavasseur A, Raghavendra AS (2003) Different signaling pathways involved during the suppression of stomatal opening by methyl jasmonate or abscisic acid. Plant Sci 164 481–488 [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14 S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2070–2072 [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14 1919–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]