Abstract
Posttranslational lipid modifications are important for proper localization of many proteins in eukaryotic cells. However, the functional interrelationships between lipid modification processes in plants remain unclear. Here we demonstrate that the two heterotrimeric G-protein γ-subunits from Arabidopsis (Arabidopsis thaliana), AGG1 and AGG2, are prenylated, and AGG2 is S-acylated. In wild type, enhanced yellow fluorescent protein-fused AGG1 and AGG2 are associated with plasma membranes, with AGG1 associated with internal membranes as well. Both can be prenylated by either protein geranylgeranyltransferase I (PGGT-I) or protein farnesyltransferase (PFT). Their membrane localization is intact in mutants lacking PFT activity and largely intact in mutants lacking PGGT-I activity but is disrupted in mutants lacking both PFT and PGGT-I activity. Unlike in mammals, Arabidopsis Gγs do not rely on functional Gα for membrane targeting. Mutation of the sixth to last cysteine, the putative S-acylation acceptor site, causes a dramatic change in AGG2 but not AGG1 localization pattern, suggesting S-acylation serves as an important additional signal for AGG2 to be targeted to the plasma membrane. Domain-swapping experiments suggest that a short charged sequence at the AGG2 C terminus contributes to AGG2's efficient membrane targeting compared to AGG1. Our data show the large degree to which PFT and PGGT-I can compensate for each other in plants and suggest that differential lipid modification plays an important regulatory role in plant protein localization.
Heterotrimeric guanine nucleotide-binding proteins (G proteins) are important components of signal transduction pathways and are conserved in all eukaryotic organisms examined (Jones, 2002; Jones and Assmann, 2004; Perfus-Barbeoch et al., 2004). Heterotrimeric G proteins consist of three different subunits, Gα, Gβ, and Gγ. Gβ and Gγ are tightly associated as a functional unit under physiological conditions, while Gα can signal independently or through Gβγ. In the human genome, there are a total of 17 Gα genes that encode 23 Gα-subunits, five Gβ genes, and at least 12 Gγ genes (Cabrera-Vera et al., 2003), while in the Arabidopsis (Arabidopsis thaliana) genome, only one canonical Gα (GPA1, At2G26300), one Gβ (AGB1, At4G34460), and two Gγ genes (AGG1, At3g63420 and AGG2, At3g22942) have been identified (Ma et al., 1990; Mason and Botella, 2000; Lease et al., 2001; Mason and Botella, 2001). The same number of G-protein components exists in the rice (Oryza sativa) genome (Kato et al., 2004). Studies of plant Gα and Gβ mutants have implicated G proteins in a wide range of developmental and phytohormone-mediated cellular processes (Jones, 2002; Jones and Assmann, 2004; Perfus-Barbeoch et al., 2004), including cell division (Ullah et al., 2001, 2003; Warpeha et al., 2006), leaf shape, internode elongation (Ueguchi-Tanaka et al., 2000), seed germination (Ullah et al., 2002), stomata function (Wang et al., 2001; Coursol et al., 2003; Fan et al., 2004; Mishra et al., 2006), and pathogen susceptibility (Suharsono et al., 2002; Trusov et al., 2006). Arabidopsis gpa1 and agb1 mutants share some common features, such as rounder rosette leaves, but, consistent with independent roles in particular signaling events have many distinct phenotypes. agb1 mutants produces excessive lateral roots and are more sensitive to auxin promotion of lateral roots, while gpa1 forms fewer lateral roots and is less sensitive to auxin (Ullah et al., 2003). agb1 mutants are more hypersensitive to abscisic acid (ABA) than gpa1 (Pandey et al., 2006), and GPA1 and AGB1 play opposite roles in regulating cell proliferation in roots (Chen et al., 2006).
Critical to their signaling function is the localization of G proteins to the cytoplasmic face of plasma membrane in animal cells (Casey, 1995). This subcellular localization is often facilitated by lipid modifications of the Gα- and Gγ-subunits. In mammalian and yeast (Saccharomyces cerevisiae) cells, Gα is usually modified by attachment of a 14-carbon myristate to an N-terminal Gly via an amide bond and/or by linking a 16-carbon palmitate to a nearby Cys through a thioester bond (Milligan et al., 1995). Gγ is usually modified by prenylation, attachment of a 15-carbon farnesyl or 20-carbon geranylgeranyl isoprenoid via a thioether bond to a Cys in the CaaX motif at the C terminus, where a is preferentially a small aliphatic amino acid and X significantly affects the substrate specificity (Wedegaertner et al., 1995; Lane and Beese, 2006). Extensive structural studies have been conducted to understand the molecular basis of substrate specificity and mechanisms of the mammalian protein prenyltransferase activities (Lane and Beese, 2006). Mammalian protein geranylgeranyltransferase I (PGGT-I) and protein farnesyltransferase (PFT) have distinct but overlapping substrate specificity. PGGT-I prefers a Leu and Phe residue in the X position, while PFT can recognize several other amino acids but prefers Met, Ser, Gln, or Ala (Zhang et al., 1994; Zhang and Casey, 1996; Lane and Beese, 2006). Up to 11 residues in the linker region proximal to the CaaX motif appear to affect the efficiency of prenylation (Maurer-Stroh and Eisenhaber, 2005). In general, PGGT-I substrates have a higher number of Lys residues in the proximal linker region (Maurer-Stroh and Eisenhaber, 2005). With a proximal polylysine domain, K-RasB, which has a CVIM motif, was shown to be a good substrate not only for PFT but for PGGT-I as well (James et al., 1995). Following prenylation, proteolysis of the last three amino acids and methylesterification of the prenyl Cys are closely coupled in vivo to promote the hydrophobicity of the modified protein (Hancock et al., 1991; Rodriguez-Concepcion et al., 2000; Crowell and Kennedy, 2001; Bracha et al., 2002; Narasimha Chary et al., 2002). Lipid modifications of Gγ in mammalian cells are not required for Gβγ dimerization but are essential for their plasma membrane association and interaction with Gα and effecter proteins (Wedegaertner et al., 1995; Dietrich et al., 2003), and Gβγ complex formation was shown to stabilize the Gγ-subunit (Pronin and Gautam, 1993).
Palmitoylation of a Cys adjacent to the prenyl Cys (-CCTLM) in yeast Gγ (STE18) is required for its full function in pheromone response, and its palmitoylation seemed to depend on prenylation (Hirschman and Jenness, 1999; Manahan et al., 2000). In contrast, none of the human Gγ has an adjacent Cys, or the polybasic domain proximal to the CaaX box, as in K-RasB (Takida and Wedegaertner, 2003). Consequently, Gβ1γ2 requires the presence of Gα to be targeted to the plasma membrane rather than to the endoplasmic reticulum (ER; Takida and Wedegaertner, 2003). Interestingly, when a palmitoylation site is introduced into Gγ2, the dependence on Gα for correct plasma membrane localization is abolished. No defined motifs are known that predict palmitoylation (Roth et al., 2006). A number of proteins were shown to be palmitoylated in vitro simply by incubating with palmitoyl-CoA in the absence of an S-acyltransferase (Veit, 2000, 2004; Lavy et al., 2002). However, at physiological conditions, spontaneous acylation is thought not to be a significant process, considering the inhibitory effects of acyl-binding proteins and a low concentration of acyl-CoA (Dunphy et al., 2000). A dynamic process of protein acylation and deacylation in vivo is catalyzed by S-acyltransferase and palmitoyl-protein thioesterase, and a family of Asp-His-His-Cys; Cys-rich domain (DHHC-CRD) proteins show protein S-acyltransferase activities in yeast, but the enzymology is not clearly understood (Roth et al., 2002, 2006; Mitchell et al., 2006). TIP GROWTH DEFECTIVE1 of Arabidopsis was recently reported to be a DHHC-CRD S-acyltransferase (Hemsley et al., 2005). Arabidopsis tip1 mutants show pleiotropic growth defects, including short and deformed root hairs, pale leaves, and poorer growth.
In Arabidopsis, the two Gγ genes, AGG1 and AGG2, were identified through their interaction with AGB1 in yeast two-hybrid experiments (Mason and Botella, 2000, 2001). AGG1 has a CRCLIL and AGG2 has a CGCSIL motif at the C terminus, making them potential targets for both protein prenylation and S-acylation (Fig. 1). Arabidopsis genes encoding PFT and PGGT-I subunits have been cloned, and their respective loss-of-function mutants have been identified. pluripetala (plp-1) mutants disrupt the α-subunit shared between PFT and PGGT-I (Running et al., 2004). enhanced response to abscisic acid1 (era1) and ggb are mutants of the β-subunit of PFT and PGGT-I, respectively (Cutler et al., 1996; Ziegelhoffer et al., 2000; Johnson et al., 2005). plp-1 mutants lack detectable PFT and PGGT-I activity and show many morphological phenotypes, including extra petals, much enlarged meristems, extreme fasciation, and much slower growth, though the plants remain viable and fertile (Running et al., 2004). plp-1 mutants also exhibit G-protein mutant phenotypes such as rounder leaves and seed hypersensitivity to ABA shown in both gpa1 and agb1, and shorter siliques and bigger inflorescences in agb1 (Lease et al., 2001; Ullah et al., 2001, 2003; Pandey et al., 2006). era1 mutants have a much weaker morphological phenotype but are more hypersensitive to ABA inhibition of seed germination than plp (Running et al., 1998; Bonetta et al., 2000; Yalovsky et al., 2000a), and ggb-2 mutants have no apparent growth defects and are slightly ABA hypersensitive (Johnson et al., 2005), despite the large number of putative PGGT-I targets in plants. This is in contrast to orthologous mutants in yeast, which are lethal (He et al., 1991). era1-4 ggb-2 double mutants exactly resemble plp, indicating that PLP, ERA1, and GGB do not function independently of each other. The mild morphological phenotypes of era1 and ggb compared to plp indicate that there is likely to be great functional redundancy between plant PFT and PGGT-I, possibly much more so than in mammals and yeast (Johnson et al., 2005). This redundancy is also shown genetically by the partial rescue of era1 morphological phenotypes by overexpression of PGGT-I (Johnson et al., 2005).
Figure 1.
Comparison of AGG2 and AGG1 protein sequences. Both AGG2 and AGG1 have three Cys residues that are underscored by an asterisk. AGG2 has three putative N-glycosylation motifs, NSSG, NASA, and NATW, which are underlined. AGG1 lacks these motifs. AGG1m2 and AGG2m1 were generated to determine the effect of preceding sequence of the CAAX motif on lipid modification efficiency and protein stability.
There are approximately 700 proteins in Arabidopsis proteome that have a CaaX motif at the C terminus, with nearly 70 having a CaaL motif (Q. Zeng and M. Running, unpublished data). Only a few plant proteins have been shown to be prenylated, including a metal-binding protein ATFP3 (Dykema et al., 1999), petunia (Petunia hybrida) CaM53 (Rodriguez-Concepcion et al., 1999), Arabidopsis APETALA1 (Yalovsky et al., 2000b), the MUB family of ubiquitin-fold proteins (Downes et al., 2006), and the nucleosome assembly protein 1 homolog, AtNAP1;1 (Galichet and Gruissem, 2006). Some proteins that terminate with a CaaX motif have been shown not to be prenylated, including CAULIFLOWER (CYAA; Yalovsky et al., 2000b) and ROP10/RAC8 (CGKN; Lavy et al., 2002), highlighting the need for experimental verification of putative targets. Here, we present both biochemical and genetic evidence that the two Arabidopsis Gγ proteins AGG1 and AGG2 are indeed prenylated, not only by Arabidopsis PGGT-I but also by PFT. Enhanced yellow fluorescent protein (EYFP)-tagged AGG1 and AGG2 localization is disrupted in plp mutants, and their prenyl Cys is essential to their membrane localization. AGG1 and AGG2 localization patterns are largely normal in ggb, suggesting that PFT functionally compensates for PGGT in vivo. These results demonstrate the high degree of functional redundancy of prenylation enzymes in plants that may account for the lack of phenotype of PGGT-I mutants. We also provide evidence that AGG2 is subject to palmitoylation, which serves as an important second signal to target AGG2 to the plasma membrane. Although not a sufficient second membrane targeting signal, the short charged preceding sequences in AGG2 are important for its correct localization.
RESULTS
AGG1 and AGG2 Are Prenylated in Vitro
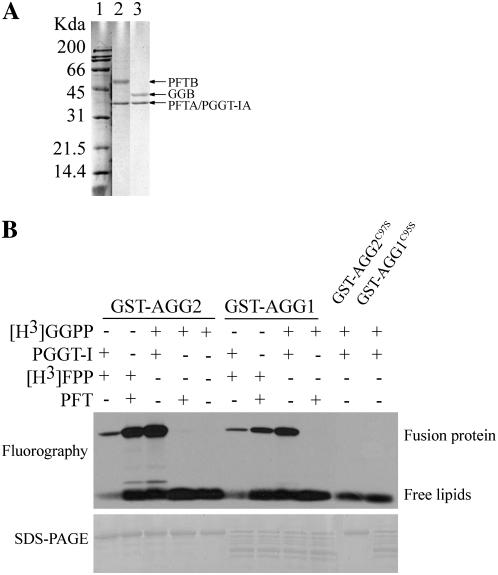
AGG1 and AGG2 contain a CaaL motif for prenylation (Fig. 1). To determine if AGG1 and AGG2 serve as prenylation targets, glutathione S-transferase (GST)-fused proteins GST-AGG2 and GST-AGG1, as well as mutant proteins GST-AGG2C97S and GST-AGG1C95S, in which the prenyl Cys was mutated to Ser, were induced and purified. Arabidopsis prenyltransferases PFT and PGGT-I were expressed and purified from yeast (Fig. 2A). The β-subunits of PFT and PGGT were copurified with the α-subunit fused in frame with FLAG epitope at the C terminus. Incubation of purified GST-AGG2 and GST-AGG1 with different combinations of enzyme and substrate showed that the two γ-subunits are not only geranylgeranylated by PGGT-I but also farnesylated by PFT, while the mutated forms are not prenylated (Fig. 2B). The results also show that Arabidopsis PGGT-I can utilize farnesyl pyrophosphate (FPP), albeit with a much weaker signal detected. That signals were not detected when no PGGT-I was added, or when GST-AGG2C97S or GST-AGG1C95S was incubated with the purified PGGT-I, confirmed that prenylation of AGG1 and AGG2 requires the conserved fourth to last Cys, and the signals from the other reactions are not due to nonspecific substrate affinity but due to enzymatic activities.
Figure 2.
In vitro prenylation of AGG1 and AGG2. A, Arabidopsis recombinant PFT (lane 2) and PGGT-I (lane 3) were induced and purified from yeast using Anti-Flag antibody (Sigma). Numbers to the left of lane 1 indicate positions of molecular marker (Bio-Rad) in kilodaltons. Positions of protein bands are indicated by the arrows to the α-subunit shared by PFT and PGGT-I, which is tagged with FLAG peptide (PFTA/PGGT-IA, 39 kD), the β-subunit of PFT (PFTB, 54 kD), and the β-subunit of PGGT-I (GGB, 41 kD). B, Purified GST-AGG1, GST-AGG2, GST-AGG1C95S, and GST-AGG2C97S (in which the fourth to last Cys are replaced by Ser), were incubated with (+) or without (−) the purified protein prenyltransferase and tritium-labeled prenyl substrate as indicated in each lane, resolved by SDS-PAGE, and visualized by fluorography.
Prenylation Is Critical for the Proper Subcellular Localization of AGG1 and AGG2
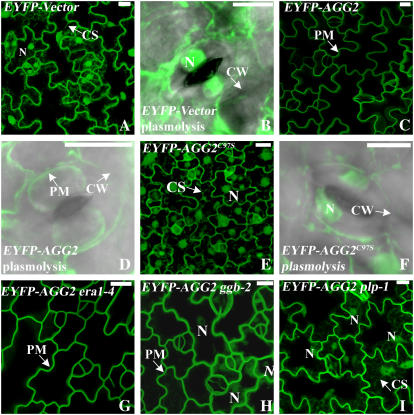
To demonstrate that the lack of prenylation affects the localization of AGG1 and AGG2, AGG1, AGG1C95S, AGG2, and AGG2C97S were fused to EYFP at the N terminus under control of the 35S promoter of Cauliflower mosaic virus. Stable transgenic Arabidopsis plants for all the constructs as well as the EYFP vector alone were assessed by fluorescence confocal microscopy. Without other constraints, nonnuclear proteins within about 70 kD have been shown to freely enter into the nucleus through the nuclear pore complex (Galy et al., 2003). EYFP (27 kD) and its variant fluorophores are cytosolic proteins that can go into the nucleus but not vacuoles (Dixit et al., 2006). For the EYFP vector alone, a bright signal was detected in the nuclei, perinuclear cytoplasm, cytoplasmic strands, and the cortical cytoplasm that was pressed by the large vacuole against the cell wall (Fig. 3A). After plasmolysis, EYFP signal was detected in the condensed cytoplasm and nucleus, as shown in a guard cell for its easily recognizable shape in an overlay of differential interference contrast (DIC) and confocal image (Fig. 3B). Fluorescent signals of EYFP-AGG2 were detected as sharp, thin lines surrounding the cell periphery, suggesting that AGG2 is associated with the plasma membrane (Fig. 3C). Plasmolysis further confirmed that signals are confined to the plasma membrane (Fig. 3D).
Figure 3.
Subcellular localization of EYFP-tagged AGG2. Projection of multiple stacks taken from the lower epidermis of cotyledon or leaf is presented for every section. The images for the guard cells are an overlay of DIC (gray) and EYFP (green). A, EYFP-vector is cytosolic as evidenced by the bright nuclei and cytoplasmic strands. B, A guard cell from EYFP-vector after plasmolysis. C, In wild type, AGG2 is predominantly located at the plasma membrane as evidenced by a thin fluorescing line surrounding the cell. D, A guard cell from EYFP-AGG2 after plasmolysis confirmed that the signal is confined to the plasma membrane. E, Mutation of the prenyl Cys to Ser makes EYFP-AGG2C97S cytosolic. F, A guard cell from EYFP-AGG2C97S after plasmolysis looks very similar to that from YFP-Vector. G, The localization pattern of AGG2 is not altered in era1-4. H, EYFP-AGG2 is largely detected at the plasma membrane in ggb-2 mutants, but weak signal is detected in the nuclei. I, In plp mutants, AGG2 is detected in the cytosol as nuclei and cytoplasmic strands are fluorescing. N, Nucleus; PM, plasma membrane; CS, cytoplasmic strands; CW, cell wall. All scale bars = 10 μm.
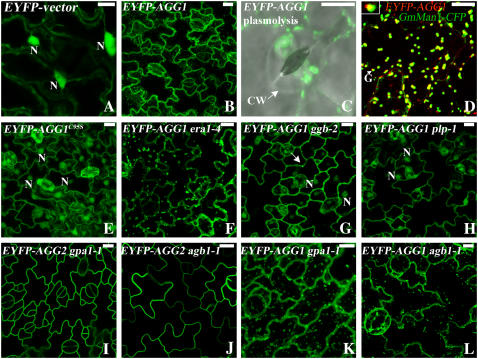
In EYFP-AGG1 plants, the fluorescence pattern is different from both AGG2 and EYFP vector alone. While most of the EYFP-AGG1 fluorescence is detected in the plasma membrane, there is considerable signal associated with more internal structures, especially some bright, small, and motile particles (Fig. 4B). The dynamic feature of these brightly fluorescing particles is visible in a supplemental movie (Supplemental Movie S1). After plasmolysis, EYFP-AGG1 displays aggregates of bright spots and endomembrane localization (Fig. 4C). To see if these bright dots are Golgi or Golgi-associated structures, we crossed EYFP-AGG1 plants with plants containing the Golgi marker GmMan1 fused to cyan fluorescent protein (CFP; Nebenfuhr et al., 1999). GmMan1-CFP plants alone exhibit weaker growth. Seeds from crosses between EYFP-AGG1 plants and GmMan1-CFP can germinate on hygromycin plates but cannot initiate true leaves. Nevertheless, using the expanded cotyledons, EYFP-AGG1 was indeed detected associated with Golgi (Fig. 4D, closeup in inset). A consistent pattern of the merged yellow color between the red (EYFP-AGG1) and green (GmMan1-CFP), indicating that EYFP-AGG1 colocalizes with GmMan1-CFP but does not overlap completely. GmMan1-CFP was shown to localize at the cis/medial side of the Golgi stacks (Nebenfuhr et al., 1999). Thus, it appears that EYFP-AGG1 is most likely associated with the trans side of the Golgi and possibly with the trans-Golgi network, suggesting AGG1 is transported to the plasma membrane through the vesicular membrane trafficking pathway like the human G proteins (Michaelson et al., 2002).
Figure 4.
Subcellular localization of EYFP tagged AGG1. Projection of multiple stacks taken from the lower epidermis of cotyledon or leaf is presented for every section except A and D. The images for the guard cells are an overlay of DIC (gray) and EYFP (green). A, EYFP-vector is cytosolic as evidenced by the bright nuclei and cytoplasmic strands. B, EYFP-AGG1 is associated with plasma membrane and internal membranes, with bright spots apparent. C, A guard cell from EYFP-AGG1 after plasmolysis displays aggregates of bright spots and endomembrane localization. D, EYFP-AGG1 (red) colocalizes with the Golgi marker GmMan1-CFP (green) in the middle of the Golgi stack as indicated by the merged yellow. The inset at the top left corner is from the arrow pointed at Golgi, showing the yellow merged color between the green and red. GmMan1 has been reported to locate at the cis/medial Golgi (Nebenfuhr et al., 1999). AGG1 is possibly located at the medial/trans Golgi. E, EYFP-AGG1C95S is cytosolic. F, The localization pattern of AGG1 is not altered in era1-4. G, In ggb-2, EYFP-AGG1 can be detected in the nuclei, but some bright spots still remain as indicated by the arrow. H, In plp-1 mutants, AGG1 is largely detected in the cytosol as evidenced by the bright nuclei. I, The localization pattern of AGG2 is not altered in gpa1-1. J, The localization pattern of AGG2 is not altered in agb1-1. K, The localization pattern of AGG1 is not altered in gpa1-1. L, The localization pattern of AGG1 is not altered in agb1-1. N, Nucleus; CW, cell wall; G, Golgi. All scale bars = 10 μm.
Both EYFP-AGG2C97S (Fig. 3, E and F) and EYFP-AGG1C95S (Fig. 4E) showed similar localization patterns to that of EYFP-vector alone (Figs. 3, A and B, and 4A), indicating that prenylation is critical for membrane localization of AGG2 and AGG1.
We crossed EYFP-AGG2 and EYFP-AGG1 plants to ggb-2, plp-1, and era1-4 mutants to see if the AGG1 and AGG2 distribution pattern is altered in prenylation mutants. Localization of AGG2 does not change in era1-4 (Fig. 3G) or AGG1 (Fig. 4F), suggesting they are primarily prenylated by PGGT-I in vivo and loss of PFT function does not affect their prenylation. In ggb-2, AGG2 (Fig. 3H) and AGG1 (Fig. 4G) still retain most of their membrane association, indicating that loss of PGGT-I function is largely compensated by PFT, in agreement with the results from the in vitro assay. However, weakly fluorescing nuclei can be detected in the projections of multiple stacks for both AGG2 and AGG1, and for AGG1, there are fewer fluorescing Golgi stacks, indicating that a portion of AGG1 and AGG2 is not prenylated and not membrane associated in the absence of PGGT-I, further suggesting that AGG1 and AGG2 are preferably prenylated by PGGT-I but not by PFT in vivo. In plp-1 mutants, cytoplasmic strands and nuclei were clearly visualized for AGG2 (Fig. 3I) and AGG1 (Fig. 4H), indicating that AGG2 and AGG1 is significantly distributed in the cytosol when both PFT and PGGT-I activities are abolished.
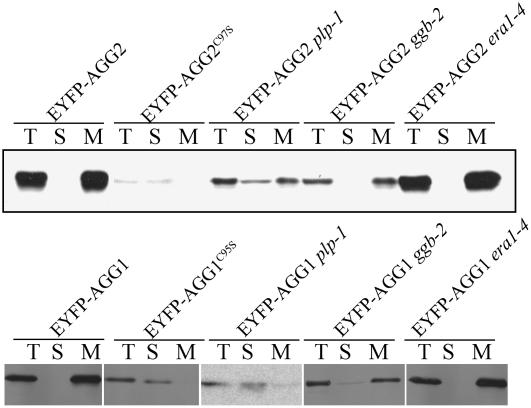
To show the membrane versus cytosol partitioning of the Arabidopsis G-protein γ-subunits, we prepared total, membrane, and soluble fractions of protein from leaves of the above-mentioned overexpression plants, resolved on SDS-PAGE and visualized by immunoblotting with antibodies specific for green fluorescent protein (GFP; Fig. 5). Consistent with confocal imaging, EYFP-AGG2 is detected in the membrane fraction in both wild-type and era1-4 mutants, and EYFP-AGG2C97S is present in the cytosolic fraction. Due to the observed lower fluorescence intensity in the ggb-2 and plp-1 mutant background, 2 times more protein was loaded for AGG2 ggb-2 and 3 times more protein was loaded for AGG2 plp-1 (see “Methods and Materials”). In ggb-2 mutants, the majority of EYFP-AGG2 remains in the membrane fraction, demonstrating that the loss of PGGT-I function does not severely alter its localization. However, in a blot with longer exposure, weak signal of EYFP-AGG2 is detected in the soluble fraction in ggb-2 but not in wild type or in era1-4 (Supplemental Fig. S1), in agreement with the faintly stained nuclei showing in the projection of the confocal image of EYFP-AGG2 ggb-2 (Fig. 3H). In plp-1 mutants, EYFP-AGG2 is significantly detected in the soluble fraction, although a portion still remains in the membrane fraction. The remaining membrane association of EYFP-AGG2 in plp-1 is resistant to 1 m of NaCl and 0.1 m of Na2CO3 wash (data not shown), suggesting that it is not due to peripheral protein-protein interaction but by stable membrane association. A similar immunoblotting pattern is observed for EYFP-AGG1, EYFP-AGG1C95S, and EYFP-AGG1 in plp-1, ggb-2, and era1-4 backgrounds (Fig. 5, bottom).
Figure 5.
Immunoblotting using GFP-specific monoclonal antibody. Total (T), soluble (S), and total membrane (M) fraction of protein was prepared from equivalent amounts of Arabidopsis leaf tissue within each sample expressing EYFP-AGG2, AGG2C97S, AGG1, and AGG1C95S as well as EYFP-AGG2 and AGG1 in the background of plp-1, ggb-2, and era1-4 mutants. Intact EYFP-AGG2 in the wild type, as well as in era1-4, was detected in the membrane fraction. EYFP-AGG2C97S was in the cytosol. In the plp-1 mutant, EYFP-AGG2 was detected in both soluble and membrane fractions. In the ggb-2 mutant, the majority of EYFP-AGG2 was detected in the membrane fraction, and a weaker band in the cytosol was shown with longer exposure (as shown in Supplemental Fig. S2). A similar pattern was observed for AGG1.
AGG1 and AGG2 Do Not Depend on GPA1 or AGB1 for Their Membrane Localization
The farnesylation of mammalian Gγ2 was shown to be critical but not sufficient to target Gβγ2 to the plasma membrane. Farnesylation is sufficient to target Gβγ2 to the ER, but it requires the presence of Gα to target to the plasma membrane (Takida and Wedegaertner, 2003). To see if Gα is necessary for Arabidopsis Gβγ to localize to the plasma membrane, we crossed Arabidopsis plants expressing EYFP-AGG1 and EYFP-AGG2 with both gpa1-1 and agb1-1. gpa1-1 is a T-DNA insertion that results in no detectable protein in western blots, and agb1-1 is a splice site mutation that introduces a stop codon early in the transcript, suggesting both are null alleles (Lease et al., 2001; Ullah et al., 2001). No alteration of localization pattern was observed for either EYFP-AGG2 or EYFP-AGG1 in gpa1-1 or agb1-1 mutants (Fig. 4, I–L), suggesting that AGG1 and AGG2 can localize to the membrane solely through their lipid modification.
AGG2 Localization Is Disrupted by Mutation of a Potential Acylation Acceptor Cys Two Residues Preceding the Prenyl Cys
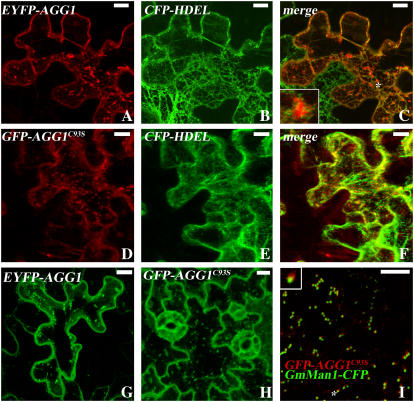
In yeast Gγ, an adjacent Cys to the prenyl Cys has been shown to be palmitoylated, and palmitoylation occurs after prenylation (Hirschman and Jenness, 1999; Manahan et al., 2000). In Arabidopsis, there are two additional Cys to the prenyl Cys in AGG1 and AGG2, and one of them is separated by one amino acid upstream of the prenyl Cys (Fig. 1). To see if this Cys is subject to acylation, we conducted point mutation analysis by mutating the Cys at the 93rd position for AGG1 (AGG1C93S) and at the 95th position for AGG2 (AGG2C95S) to Ser. Because farnesylated but nonacylated human Gγ2 was only sufficiently targeted to the ER (Takida and Wedegaertner, 2003), binary vectors containing EYFP-AGG1, EYFP-AGG2, GFP-AGG1C93S, and GFP-AGG2C95S were either infiltrated alone or coinfiltrated with an ER localization marker CFP-HDEL into Nicotiana benthamiana leaf epidermal cells. As also shown in stable transgenic plants, wild-type EYFP-AGG1 in transient assays shows many bright spots (Fig. 6G). These bright spots were embedded within the ER network but do not colocalize with the ER marker (Fig. 6, A–C). The weakly fluorescing signal in the background suggests that, unlike farnesylated human Gγ2, geranylgeranylated EYFP-AGG1 does not significantly accumulate in the ER, and its delivery from ER to trans-Golgi is a rapid process. GFP-AGG1C93S showed a similar localization pattern to that of wild-type EYFP-AGG1 (Fig. 6, D–F). Stable transformed Arabidopsis plants with GFP-AGG1C93S (Fig. 6H) and the one that was crossed with GmMan1-CFP (Fig. 6I) also showed a similar pattern to that of EYFP-AGG1 (Fig. 4, B and D), suggesting the mutated Cys is not required for AGG1's proper targeting.
Figure 6.
Mutation of the putative S-acylation acceptor Cys of AGG1 does not change AGG1's subcellular localization pattern. Agrobacterium C58 containing binary vector EYFP-AGG1 and GFP-AGG1C93S were coinfiltrated with ER localization marker CFP-HDEL into N. benthamiana. Projection of multiple stacks taken from the lower epidermal of cotyledon or leaf cells is presented for every section except I. A to C, Similar to that shown in the stable transformants, transient assays of AGG1 show bright spots and weak signals with other internal membranes. The inset in C was from the spot designated by an asterisk. D to F, Transient assay of GFP-AGG1C93S, with a mutation of the putative S-acylation acceptor Cys, shows a similar pattern to that of wild-type EYFP-AGG1. G, Transient expression pattern of EYFP-AGG1 alone is similar to that of A, suggesting that coinfiltration of CFP-HDEL does not alter EYFP-AGG1's localization pattern. H, GFP-AGG1C93S in stable Arabidopsis transformants has a similar fluorescing pattern to wild-type EYFP-AGG1. I, Colocalization pattern of GFP-AGG1C93S and GmMan1-CFP. All scale bars = 10 μm.
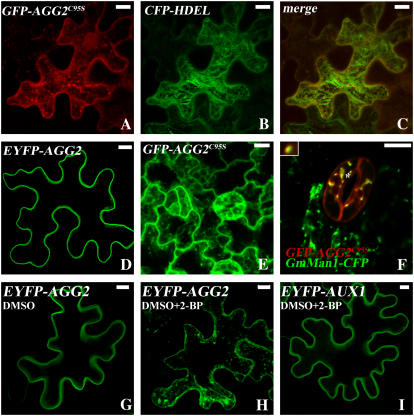
In contrast, AGG2C95S displayed a distinct pattern from that of wild-type AGG2. GFP-AGG2C95S is associated with internal membranes in both transient assays (Fig. 7, A–C) and stable transformed Arabidopsis plants (Fig. 7E), while wild-type EYFP-AGG2 presents only sharp lines of signal circumventing the plasma membrane (Figs. 3C and 7D). Like EYFP-AGG1 and GFP-AGG1C93S, the GFP-AGG2C95S Arabidopsis plants with GmMan1-CFP are extremely unhealthy when grown on hygromycin plates and not every cell showed both signals. Within those cells that do detect both signals, the bright spots in the GFP-AGG2C95S were also shown to be associated with Golgi (Fig. 7F) in the same manner as that observed in EYFP-AGG1 (Fig. 4D) and GFP-AGG1C93S (Fig. 6I).
Figure 7.
Mutation of the putative S-acylation acceptor Cys of AGG2 and S-acylation inhibitor treatment dramatically disrupt AGG2's localization pattern. Projection of multiple stacks is presented for every section except F. A to C, GFP-AGG2C95S was coinfiltrated with ER localization marker CFP-HDEL into N. benthamiana. With a mutation of the putative S-acylation acceptor Cys, GFP-AGG2C95S shows a distinct pattern from that of the wild-type EYFP-AGG2 (D), suggesting that AGG2 is S-acylated. E, GFP-AGG2C95S in stable Arabidopsis transformants is also very different from what was shown for the wild-type EYFP-AGG2 with more signals associated with internal membranes. F, Colocalization pattern of GFP-AGG2C95S and GmMan1-CFP. G, EYFP-AGG2's localization pattern is altered by coinfiltration with 10% DMSO, a solvent used to dissolve 2-BP. H, 2-BP disrupts EYFP-AGG2's localization pattern. I, 2-BP does not change the localization pattern of AUX1, a protein with transmembrane domains.
The fatty acid analog 2-bromo-palmitate (2-BP) is known as a palmitoylation inhibitor both in vivo and in vitro. 2-BP blocks the synthesis of acyl-CoA and also interferes with the transfer of fatty acids to the thiol group (Resh, 2006b). When EYFP-AGG2 were infiltrated into N. benthamiana along with 2-BP, a similarly disrupted pattern to that of GFP-AGG2C95S was displayed (Fig. 7H), while plasma membrane localization of EYFP-AUX1 that contains transmembrane domains (Yang et al., 2006) was not altered by the same treatment (Fig. 7I), nor was the EYFP-AGG2 mock treatment with 10% dimethyl sulfoxide (DMSO) alone (Fig. 7G). It appears that 2-BP treatment does not alter AGG1's localization, consistent with the results from the point mutation (data not shown). The disruption of the plasma membrane localization of AGG2 by both mutation of the putative S-acylated Cys and S-acylation inhibitor suggests that wild-type AGG2 is S-acylated, and S-acylation serves as an important second signal for plasma membrane targeting of the prenylated protein.
Charged Residues Preceding the CSIL Motif of AGG2 Are Indispensable for Its Correct Localization
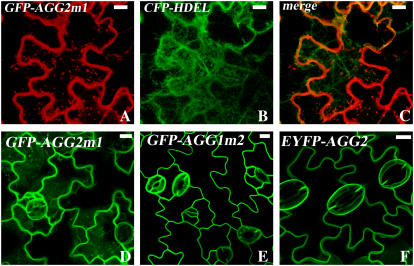
Many prenylation targets, including CaM53 (Caldelari et al., 2001) and K-RasB (Hancock et al., 1989), have a polybasic proximal domain that either promotes prenylation efficiency or serves as a second plasma membrane targeting signal. A polybasic region with a net positive charge of four or more is required to function as a plasma membrane targeting motif (Hancock et al., 1990; Michaelson et al., 2001). A polybasic sequence with six Lys was reported to be able to facilitate palmitoylation without requiring previous prenylation (Booden et al., 1999), suggesting the polybasic domain indeed serves as a membrane-targeting signal. A polybasic domain with 11 Arg and three Lys residues in CaM53 has been shown to increase PGGT-I prenylation efficiency by an order of magnitude in vitro (Caldelari et al., 2001). Neither AGG1 nor AGG2 has a long proximal polybasic domain as does CaM53 (Fig. 1). However, AGG2 does have three more charged amino acid residues in the preceding hypervariable region than AGG1 (KEAKRCG versus NGGEGCR). We suspected this might be responsible for the observed different localization patterns between AGG1 and AGG2. We swapped the hypervariable regions of AGG1 and AGG2 proximal to the CaaX boxes (Fig. 1) and observed subcellular localization patterns of the resulting GFP-tagged proteins, AGG2m1 and AGG1m2, in N. benthamiana leaf epidermal cells and Arabidopsis transformants. AGG2m1 was associated with Golgi and other internal membranes as seen in S-acylation-diminished GFP-AGG2C95S (Fig. 8, A–C), while wild-type AGG2 was only detected on the plasma membrane, strongly suggesting that the basic residues contribute to the efficient targeting of AGG2. Stable transformation of GFP-AGG2m1 (Fig. 8D) also displays more fluorescing internal signals than AGG2 (Fig. 8F). In contrast, the localization pattern of AGG1m2 in stable transformants (Fig. 8E) is very different from that of wild-type AGG1 (Fig. 4B) but very similar to that of AGG2 (Fig. 8F), with GFP signal mainly detected in the plasma membrane, suggesting that the added short basic sequence possibly promotes both prenylation and S-acylation process.
Figure 8.
The proximal basic residues of AGG2 are important for AGG2's efficient plasma membrane targeting. A to C, GFP-AGG2m1, swapped with AGG1's proximal sequence, was coinfiltrated with ER localization marker CFP-HDEL into N. benthamiana. Internal membrane association of GFP-AGG2m1 is detected. D, Cells from stable transformants of GFP-AGG2m1 also show internal fluorescing signals. E, GFP-AGG1m2, AGG1 with AGG2's basic proximal sequence, is primarily detected associated with the plasma membrane as shown in stable Arabidopsis transformants, with a similar pattern to that of EYFP-AGG2 (F). All scale bars = 10 μm. [See online article for color version of this figure.]
DISCUSSION
The importance of G protein in signal transduction processes and some phenotypic similarities between the protein prenyltransferase mutant plp-1 and the Gα mutant gpa1 and Gβ mutant agb1 led us to choose the potential dual lipid modification targets AGG1 and AGG2 as models for the study of lipid modification mechanisms in Arabidopsis and to delineate PLP's physiological function. AGG1 and AGG2 share a CaaX box with Gγs from mammals and yeast but show unique proximal sequence domains. We showed that both AGG1 and AGG2 are prenylated, and prenylation is essential but not sufficient for their proper plasma membrane association. S-acylation functions as an important second signal to efficiently target AGG2 to the plasma membrane.
Arabidopsis PFT can largely compensate for loss of function of PGGT-I, as shown both biochemically and genetically. This may help explain the lack of visible phenotypes of ggb mutants. The result that PFT can efficiently farnesylate the ideal PGGT-I targets AGG1 and AGG2 is largely consistent with earlier reports that the recombinant tomato (Lycopersicon esculentum) farnesyltransferase can farnesylate mutated APETALA1 with a CaaL box, GST-ap1mL, with [3H]FPP (Yalovsky et al., 1997, 2000b). The change of localization patterns of wild-type AGG1 and AGG2 in the plp-1 background provides strong evidence that AGG1 and AGG2 are prenylated in vivo.
Although prenylation is critical for the membrane association of many target proteins, there is always a question if prenylation is sufficient for plasma membrane association. The membrane affinity provided by prenylation alone, particularly farnesylation, is thought not enough to stably anchor the associated protein to the plasma membrane as a sticky finger, but rather provides a greasy handle to interact with the other membrane-bound proteins (Magee and Seabra, 2003). Prenyl groups are also thought to be too bulky and too rigid to associate with the lipid rafts, where sphingolipid and cholesterol is enriched and receptor-mediated signaling events are organized (Dunphy et al., 2001). In contrast, protein palmitoylation is well suited to this role, and mammalian Gαi palmitoyltransferase activity was determined to be enriched in the lipid rafts (Dunphy et al., 2001). Dual lipid modification was required for the yeast Gγ-subunit Ste18p (-CCTLM) for a fully functional pheromone response, and its palmitoylation seemed to depend on its farnesylation (Manahan et al., 2000). Unlike Ste18, which has two adjacent Cys, both AGG1 and AGG2 have two Cys interspersed by another amino acid residue at their C terminus (Fig. 1). Similar CxCxxx structures exist in H-Ras and R-Ras, both of which have been shown to be prenylated and palmitoylated (Hancock et al., 1989). K-RasB does not have an extra Cys for palmitoylation, but it has a long stretch of basic amino acids that offers an alternative second signal to facilitate plasma membrane targeting (Hancock et al., 1989). Although AGG2 has a Lys and Arg right next to the -CGCSIL motif, neither AGG1 nor AGG2 has a long stretch of polybasic domain like in K-RasB. The disrupted fluorescence pattern resulting from mutation of the potential S-acyl acceptor Cys indicates that AGG2 is likely subject to S-acylation in vivo and also suggests that the proximal basic residues of AGG2 do not serve as a sufficient second plasma membrane targeting signal. Unlike AGG2, AGG1 is possibly either not S-acylated or at least not efficiently S-acylated based on the fact that no dramatic change was observed among wild-type AGG1 and the putative S-acylation acceptor mutant form in both transient assay and stable transformants. However, we cannot exclude other factors that might prevent AGG1 from efficient plasma membrane targeting or promote retrograding from plasma membrane to the trans-Golgi network. Recent studies from yeast on the role of Gα on pheromone signaling revealed that a new branch of G-protein signaling occurs at the endosome rather than plasma membrane (Slessareva et al., 2006). AGG1's internal membrane association implies that Arabidopsis Gβγ1 might activate effectors at these domains as well.
The complete cytosolic distribution of the prenyl Cys mutants AGG2C97S and AGG1C95S suggests that initial prenylation is required for the S-acylation of their adjacent Cys. S-acyltransferase is found to be associated with ER (Lobo et al., 2002), Golgi (Resh, 2006a), plasma membrane (Dunphy et al., 2001), or vacuolar membranes (Veit et al., 2003). Thus for S-acylation to occur under physiological conditions, a mechanism is required to bring the target proteins close to the membranes where the S-acyltransferase resides. In contrast, protein prenyltransferases are cytosolic, and, thus, protein prenylation can occur simultaneously once the protein is translated. The reported dependence of palmitoylation of the second Cys on the farnesylation of the prenyl Cys in Ste18 and Ras2P from yeast cells agrees with this primary requirement of protein membrane association prior to S-acylation (Manahan et al., 2000; Lobo et al., 2002; Dong et al., 2003). Alternatively, the membrane attachment can be facilitated by a polybasic domain that interacts electrostatically with the head groups of negative-charged phospholipids in the cytoplasmic leaflet of the membrane (Magee and Marshall, 1999). It has been shown that requirement of farnesylation for palmitoylation of H-Ras can be replaced by a polybasic domain with six Lys (Booden et al., 1999). A nonprenylated but palmitoylated type II RAC, AtRAC10 (Lavy et al., 2002), also has a long stretch of charged residues with many Lys residues that is required for palmitoylation (Lavy and Yalovsky, 2006). Unsurprisingly, proteins with one or more intrinsic transmembrane domains have been identified to be palmitoylated through a global analysis approach (Roth et al., 2006). Proteins with none of the membrane association mechanisms mentioned above have also been identified to be palmitoylated in yeast, and possibly these types of targets were brought to the peripheral of membranes through interacting with membrane-associated proteins.
Interestingly, no human Gγ has an adjacent Cys, as in the yeast Gγ, or the preceding polybasic domains to the -CaaX box, as in the K-RasB (Takida and Wedegaertner, 2003). Gβ1γ2 requires the presence of Gα to be targeted to the plasma membrane rather than to the ER (Takida and Wedegaertner, 2003). It was also shown that when an S-acylation site was introduced into the Gγ2, the dependence on Gα for correct plasma membrane localization is not required. Using gpa1, we showed that AGG1 and AGG2 do not rely on Gα for their correct targeting. On the other hand, the cytosolic distribution of the prenylation-depleted AGG1C95S and AGG2C97S indicates that, as in humans, Arabidopsis Gα is not sufficient to bring Gβγ to the membrane.
In our EYFP tagging experiments, AGG2 appears to be more abundant and better targeted to the plasma membrane than AGG1. Although we found AGG2 has extra putative N-glycosylation motifs that AGG1 lacks using the Motif Scan program (http://myhits.isb-sib.ch/cgi-bin/motif_scan; Falquet et al., 2002; Fig. 1), AGG2 does not exhibit the smear pattern on SDS-PAGE as glycosylated proteins usually do. Thus, glycosylation probably does not account for the observed localization pattern and signal intensity difference between AGG2 and AGG1. AGG1 and AGG2 have very similar but not the same CaaX motif (CSIL versus CLIL) and the same location of a potential palmitoylation Cys (CGCSIL versus CRCLIL). However, among the five preceding amino acids of AGG2, there are four charged residues, three of which are basic, while AGG1 has only one charged amino acid, an acidic amino acid. Results from our swapping experiment demonstrate that the hypervariable sequence in AGG2 facilitates the prenylation process and makes AGG2 target better to the plasma membrane. AGG1 containing the proximal domain from AGG2 changes the localization of AGG1 to be more similar to AGG2. While the differences between AGG1 and AGG2 localization is clear, we cannot rule out the possibility that overexpression of these lines may cause localization patterns that differ from the native proteins. We obtained one T-DNA insertion mutant line in the intron region (FLAG-208A03) for AGG1 from the Institut National de la Recherche Agronomique Biological Resource Centre in Versailles and all of the T-DNA insertion lines for AGG2 from Salk (Alonso et al., 2003), but we have not observed a developmental phenotype in these lines (data not shown). It will be interesting to see the degree to which various prenylation- and palmitoylation-deficient mutant forms rescue phenotypes that may be seen in the double mutant agg1 agg2.
In the plp-1 background, where both PFT and PGGT-I functions are lost, we still detected some AGG2 remaining in the membrane fraction, although the total signal in plp-1 is already much less than that in the wild type. We are not certain what accounts for the remaining membrane association, though there are several possibilities. One is that the third type of prenyltransferase, PGGT-II or Rab geranylgeranyltransferase, known to exclusively prenylate Rab GTPases in mammalian cells (Seabra et al., 1992), may compensate to some degree for loss of both PFT and PGGT-I in plants. However, prenylation studies using either PFT or PGGT-I targets and the crude extract from plp-1 or ggb mutant did not show extra prenylation activity (Running et al., 2004; Johnson et al., 2005), indicating either there is no cross specificity between PGGT-I and PGGT-II or the activity under physiological conditions is too low to detect using the crude extract under a condition not favored by PGGT-II. Further experiments using the purified PGGT-II enzyme are needed to see if AGG1 and AGG2 are possibly prenylated by PGGT-II at low efficiency. A second possibility is that dual acylation can compensate for the loss of prenylation. It is possible that the charged residues unstably but briefly allow AGG2 access to the internal membrane where the S-acyltransferase likely resides.
MATERIALS AND METHODS
Expression of GST-Fusion Proteins in Escherichia coli
AGG1 (At3g63420) full-length cDNA was amplified with forward primer QZI1 (TTCGAATTCATGCGAGAGGAAACTGTGGTT) and reverse primer QZI2 (AGCTATGACCTCGAGTCAAAGTATTAAGCATCTGC), and AGG1C95S was amplified with QZI1 and QZI3 (AGCTATGACCTCGAGTCAAAGTATTAATGATCTGC). AGG2 (At3g22942) was amplified with QZJ1 (TTCGAATTCATGGAAGCGGGTAGCTCCAAT) and QZJ2 (AGCTATGACCTCGAGTCAAAGAATGGAGCAGCCA), and AGG2C97S was amplified with QZJ1 and QZJ3 (AGCTATGACCTCGAGTCAAAGAATGGAAGAGCCA). The PCR products were digested with EcoRI and XhoI and subcloned into pGEX-4T-1 (Pharmacia) in frame with GST and verified by sequencing. The corresponding fusion proteins (GST-AGG1, GST-AGG1C95S, GST-AGG2, and GST-AGG2C97S) were induced in E. coli XL-1 Blue and purified with glutathione-Sepharose 4B beads (Pharmacia) according to the manufacturer's instructions.
Expression of Recombinant Arabidopsis PFT and PGGT-I in Yeast
The full-length c-DNA of PLP (At3g59380) was amplified with QZT5 (CCGGAATTCTCGACTTTGGATCGGAGTC) and QZT6 (CCATCGATACAATTGCTGCCACTGTAATCTTG), linked as an EcoRI/ClaI fragment downstream to the GAL10 promoter of the yeast (Saccharomyces cerevisiae) expression vector pESC-HIS (Stratagene), and verified by sequencing in frame with the FLAG epitope at the C terminus. The consequent plasmid was named pEHP. The full-length c-DNA of ERA1 (At5g40280), the β-subunit of PFT, was amplified with QZU4 (TTCCCCTCGAGTCATGCTGCTTTAAAGAA) and QZU5 (TACGGGATCCATGCCAGTAGTAACCCG), digested with BamHI/XhoI, and ligated with BamHI/SalI digested pEHP. The full-length c-DNA of GGB (At2g39550), the β-subunit of PGGT-I, was amplified with QZR1 (TTAGGATCCGAACGACGCTATGTCAGAGA) and QZR3 (TCGTAAGTCGACGACTTTGAGACAATCTAAACAT). The PCR product was directly subcloned into pCR-Blunt II-TOPO (Invitrogen/Life Technologies), digested with XbaI/EcoRV, filled in by Klenow fragment (New England Biolabs), and ligated to SmaI-digested pEHP. ERA1 and GGB were downstream of the GAL10 promoter. The resulting plasmids pFT and pGGT-I were introduced into yeast YPH499 cells by lithium acetate-mediated transformation (Gietz and Woods, 2002). Transformed cells were selected for their ability to grow on medium lacking His.
Induction of protein expression was performed as described (Cahoon and Kinney, 2004) except for the following modifications. For medium scale protein induction, single colonies from the transformed cells were grown for 3 d with shaking (300 rpm) at 30°C in 5 mL medium consisting of 0.08% (w/v) complete supplement mixture without His (CSM-HIS, BIO101), 0.17% (w/v) yeast nitrogen base without amino acids (Difco), 0.5% (w/v) ammonium sulfate, and 2% (w/v) raffinose. Then the 5-mL culture was added into 50 mL of the same medium and continued growing for 2 d at 30°C. Cells were then collected, washed, and resuspended in 250 mL of the same medium except that the raffinose was replaced with 2% (w/v) Gal for induction of protein expression for 24 h. Spheroplast and crude protein were prepared using CelLytic Y Plus (product no. PCYP-1, Sigma). PFT and PGGT-I were purified using EZviewRed ANTI-FLAG M2 Affinity gel (product no. F2426, Sigma) and eluted with 3× FLAG peptide (product no. F4799, Sigma) according to the manufacturer's instructions. The eluted enzyme was stored at −80°C for further use.
In Vitro Protein Prenylation Assays
In vitro prenylation assays were performed as described (Randall et al., 1993; Zhang et al., 1994; Yalovsky et al., 2000b; Johnson et al., 2005). The reactions consisted of 2 μg of purified GST-AGG2, GST-AGG2C97S, GST-AGG1, or GST-AGG1C95S, 10 ng of purified recombinant Arabidopsis (Arabidopsis thaliana) PFT or PGGT-I, 50 mm Tris, pH 8.0, 5 mm MgCl2, 50 μm ZnCl2, 0.2% (w/v) glucopyranoside, 5 mm dithiothreitol, and 1 μL of either [1-3H]FPP (1 mCi mL−1) or [1-3H]GGPP (1 mCi mL−1, both from American Radiological Company) in a total volume of 20 μL. The reactions were preequilibrated at 30°C for 5 min before adding prenyl substrates and incubated for 90 min afterward, resolved by 10% SDS-PAGE, and analyzed by fluorography using Autofluor fluorographic reagent (National Diagnostics) and Kodak XAR film (Eastman Kodak).
Construction of Plant Expression Vectors and Transformation
The AGG1 cDNA was amplified using primer sets QZI4 (ATACATAAGCTTTCATGCGAGAGGAAACTGT) and QZI5 (CGTTGCTCTAGATCAAAGTATTAAGCATCTGCA), and reverse primer QZI6 (CGTTGCTCTAGATCAAAGTATTAAAGATCTGCA) was used to change the fourth to last Cys in AGG1 to Ser. The AGG2 cDNA was amplified using QZJ4 (ATACATAAGCTTTCATGGAAGCGGGTAGCT) and QZJ5 (CGTTGCTCTAGATCAAAGAATGGAGCAGC), and reverse primer QZJ6 (CGTTGCTCTAGATCAAAGAATGGAAGAGC) was used to mutate the fourth to last Cys in AGG2 to Ser. The PCR products were subcloned into pCR-Blunt II-TOPO (Invitrogen/Life Technologies) and ligated as a HindIII/XbaI fragment into pCAM-EYFP-C1 (Preuss et al., 2004), a pCAMBIA-(CAMBIA) based plant expression vector. The cDNAs are in frame with EYFP at the N terminus under the control of the 35S promoter of Cauliflower mosaic virus. The corresponding constructs pEYFP-AGG1, pEYFP-AGG1C95S, pEYFP-AGG2, and pEYFP-AGG2C97S were transformed into Agrobacterium tumefaciens C58 by electroporation.
AGG1C93S was amplified by QZY41 (CACCATGCGAGAGGAAACTGTGGTTTACGA) and QZY42 (TCAAAGTATTAAGCATCTAGAGCCTTCTCCTCCA). AGG1m2 was amplified by QZY41 and QZY47 (TCAAAGTATTAAGCAGCCACATCGTTTTGCTTCTTTTGGTCCTTCAAACCA). AGG2C95S was amplified by QZY43 (CACCATGGAAGCGGGTAGCTCCAATTCGTC) and QZY44 (CACCATGGAAGCGGGTAGCTCCAATTCGTC). AGG2m1 was amplified by QZY43 and QZY48 (TCAAAGAATGGAGCATCTGCAGCCTTCTCCTCCATTAGGGCCTTCGAACCA). The AGG1C93S, AGG1m2, AGG2C95S, and AGG2m1 were introduced into pENTR/d-TOPO (Invitrogen/Life Technologies) and then into destination vector pMDC43 (Curtis and Grossniklaus, 2003). The corresponding binary vector GFP-AGG1C93S, GFP-AGG1m2, GFP-AGG2C95S, and GFP-AGG2m1 was transformed into C58. Arabidopsis plants were transformed by the floral dip method (Clough and Bent, 1998). Transient assay in Nicotiana benthamiana is through leaf infiltration.
Inhibition of S-Acylation
This experiment essentially follows that of Lavy et al. (2002). Lower epidermal layers of N. benthamiana leaves were infiltrated with the induced Agrobacteria C58 harboring EYFP-AGG1, EYFP-AGG2, and EYFP-AUX1 (Yang et al., 2006) along with 1 mm of palmitoylation inhibitor 2-BP (Arcros) dissolved in 10% DMSO or 10% DMSO alone. Fluorescence signals were observed 24 h later.
Plant Materials and Growth Condition
plp-1 (Running et al., 2004), era1-4 (previously known as wig-1; Running et al., 1998), ggb-2 (Johnson et al., 2005), gpa1-1(Ullah et al., 2001), and agb1-1 (Lease et al., 2001) mutants were previously described. EYFP-AGG2 plp-1, EYFP-AGG2 ggb-2, EYFP-AGG2 era1-4, EYFP-AGG1 gpa1-1, EYFP-AGG1 agb1-1, EYFP-AGG2 gpa1-1, and EYFP-AGG2 agb1-1 were generated by crossing EYFP-AGG2 transgenic plants with their respective single mutant, allowing the hygromycin-resistant F1 plants to self fertilize and screening F2 plants on one-fourth strength Murashige and Skoog plates with 30 mg/L of hygromycin and further selected by confocal imaging. Then EYFP-containing mutants were screened either by their obvious phenotypes or PCR screening if there were no apparent phenotypes, as in EYFP-AGG2 ggb-2. Arabidopsis seeds expressing GmMan1-CFP (Nebenfuhr et al., 1999) were crossed with EYFP-AGG1, GFP-AGG1C93S, and GFP-AGG2C95S and F1 seeds were used for observation of colocalization.
Arabidopsis plants were grown on the farfat soil in the greenhouse at 22°C under long-day conditions (15 h 150 μE light and 9 h dark) and 50% humidity. N. benthamiana was grown at 27°C under long-day conditions.
Microscopy Imaging
Cotyledons or leaf lower epidermal cells of transgenic plants or transiently infiltrated N. benthamiana with EYFP, GFP, or CFP signal were imaged using a Zeiss LSM 510 confocal/multiphoton microscope equipped with a 40× DIC lens that allows simultaneous acquisition of confocal, multiphoton, and transmitted light images. Colocalization of EYFP or GFP with CFP was taken under multitrack options with one channel set up for EYFP or GFP and another channel set up for CFP. To observe the signal distribution after plasmolysis, leaf sections were immersed in 30% Suc for 2 h. Images were finalized by Adobe Photoshop 7.0.
Protein Preparation and Immunoblotting
Proteins were prepared according to Preuss et al. (2004). A total of 0.2 g of leaf tissues was ground in 0.6 mL of cold buffer containing 20 mm HEPES, pH 7.5, 100 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, and 1× complete protease inhibitors (Roche Applied Science). The homogenate was transferred to a 2-mL tube and spun at 6,000g for 5 min at 4°C, and 0.4 mL of postnuclear supernatant (PNS) was collected as total protein and 0.2 mL of PNS was fractionated at 100,000g at 4°C for 1 h. The supernatant (soluble fraction) was collected. The pellet was resuspended in 200 μL of 1× loading buffer. An equal volume of 2× loading buffer was added to both the saved PNS (total protein) and soluble fraction. Samples were boiled for 10 min and spun for 2 min. Five microliters of the above sample from YFP-vector control, 10 μL from YFP-AGG2, EYFP-AGG2C97S, and YFP-AGG2 era1-4, 15 μL from EYFP-AGG2 ggb-2, and 30 μL from EYFP-AGG2 plp-1 was loaded onto 4% to 15% SDS-PAGE gradient gel (Bio-Rad) and transferred to polyvinylidene difluoride membrane (Bio-Rad) for immunodetection with monoclonal anti-GFP (mouse IgG2a isotype, cat no. 8371, CLONTECH) and horseradish peroxidase-conjugated anti-mouse secondary antibody (NA931-1ML, Amersham Biosciences). For AGG1, 1 μL of YFP-vector control, 10 μL from YFP-AGG1 and YFP-AGG1 era1-4, 30 μL from EYFP-AGG1C95S, EYFP-AGG1 ggb-2, and EYFP-AGG1 plp-1 was loaded onto 4% to 15% SDS-PAGE gradient gel. Signals were detected by exposing Kodak XAR film (Eastman Kodak) to the membrane after applying ECL western Blotting Substrate (Pierce).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Immunoblotting using GFP-specific monoclonal antibody.
Supplemental Movie S1. EYFP-AGG1 is detected associated with motile subcellular organelles.
Supplementary Material
Acknowledgments
We thank Dr. Mary Preuss for her help with membrane fractionation, Dr. Ed Cahoon for his technical advice in expressing recombinant PFT and PGGT-I in yeast, and Dr. Eric Nielsen for critical reading of the manuscript. We thank Dr. Dring Crowell for the ggb-2 mutant, Dr. Andreas Nebenfüer for the Golgi localization marker CFP-GmMan, Dr. Ming Chen for the ER localization marker CFP-HDEL, and Dr. Yaodong Yang for EYFP-AUX1. We thank Dr. Howard Berg for his help with confocal imaging and Drs. Tom Juehne and Keming Song for helpful materials.
This work was supported by the National Science Foundation (grant no. IOB–0344261 to M.P.R.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mark P. Running (mrunning@danforthcenter.org).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Bonetta D, Bayliss P, Sun S, Sage T, McCourt P (2000) Farnesylation is involved in meristem organization in Arabidopsis. Planta 211 182–190 [DOI] [PubMed] [Google Scholar]
- Booden MA, Baker TL, Solski PA, Der CJ, Punke SG, Buss JE (1999) A non-farnesylated Ha-Ras protein can be palmitoylated and trigger potent differentiation and transformation. J Biol Chem 274 1423–1431 [DOI] [PubMed] [Google Scholar]
- Bracha K, Lavy M, Yalovsky S (2002) The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J Biol Chem 277 29856–29864 [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE (2003) Insights into G protein structure, function, and regulation. Endocr Rev 24 765–781 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Kinney AJ (2004) Dimorphecolic acid is synthesized by the coordinate activities of two divergent 12-oleic acid desaturases. J Biol Chem 279 12495–12502 [DOI] [PubMed] [Google Scholar]
- Caldelari D, Sternberg H, Rodriguez-Concepcion M, Gruissem W, Yalovsky S (2001) Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol 126 1416–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey PJ (1995) Protein lipidation in cell signaling. Science 268 221–225 [DOI] [PubMed] [Google Scholar]
- Chen J-G, Gao Y, Jones AM (2006) Differential roles of Arabidopsis heterotrimeric G-protein subunits in modulating cell division in roots. Plant Physiol 141 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM (2003) Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423 651–654 [DOI] [PubMed] [Google Scholar]
- Crowell DN, Kennedy M (2001) Identification and functional expression in yeast of a prenylcysteine alpha-carboxyl methyltransferase gene from Arabidopsis thaliana. Plant Mol Biol 45 469–476 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273 1239–1241 [DOI] [PubMed] [Google Scholar]
- Dietrich A, Schee A, Illenberger D, Kloog Y, Henis Y, Gierschik P (2003) Studies on G-protein alpha.betagamma heterotrimer formation reveal a putative S-prenyl-binding site in the alpha subunit. Biochem J 376 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Cyr R, Gilroy S (2006) Using intrinsically fluorescent proteins for plant cell imaging. Plant J 45 599–615 [DOI] [PubMed] [Google Scholar]
- Dong X, Mitchell DA, Lobo S, Zhao L, Bartels DJ, Deschenes RJ (2003) Palmitoylation and plasma membrane localization of Ras2p by a nonclassical trafficking pathway in Saccharomyces cerevisiae. Mol Cell Biol 23 6574–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes BP, Saracco SA, Lee SS, Crowell DN, Vierstra RD (2006) MUBs, a family of ubiquitin-fold proteins that are plasma membrane-anchored by prenylation. J Biol Chem 281 27145–27157 [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Linder ME (2001) Enrichment of G-protein palmitoyltransferase activity in low density membranes: in vitro reconstitution of Gαi to these domains requires palmitoyltransferase activity. J Biol Chem 276 43300–43304 [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Schroeder H, Leventis R, Greentree WK, Knudsen JK, Silvius JR, Linder ME (2000) Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates. Biochim Biophys Acta 1485 185–198 [DOI] [PubMed] [Google Scholar]
- Dykema P, Sipes P, Marie A, Biermann B, Crowell D, Randall S (1999) A new class of proteins capable of binding transition metals. Plant Mol Biol 41 139–150 [DOI] [PubMed] [Google Scholar]
- Falquet L, Pagni M, Bucher P, Hulo N, Sigrist CJA, Hofmann K, Bairoch A (2002) The PROSITE database, its status in 2002. Nucleic Acids Res 30 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Zhao Z, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7 537–546 [DOI] [PubMed] [Google Scholar]
- Galichet A, Gruissem W (2006) Developmentally controlled farnesylation modulates AtNAP1;1 function in cell proliferation and cell expansion during Arabidopsis leaf development. Plant Physiol 142 1412–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Mattaj IW, Askjaer P (2003) Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell 14 5104–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA (2002) Transformation of yeast by the LiAc/SS carrier DNA/PEG method. Methods Enzymol 350 87–96 [DOI] [PubMed] [Google Scholar]
- Hancock J, Cadwallader K, Marshall C (1991) Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J 10 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Magee AI, Childs JE, Marshall CJ (1989) All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57 1167–1177 [DOI] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ (1990) A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63 133–139 [DOI] [PubMed] [Google Scholar]
- He B, Chen P, Chen S, Vancura K, Michaelis S, Powers S (1991) RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci USA 88 11373–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA, Kemp AC, Grierson CS (2005) The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Plant Cell 17 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman JE, Jenness DD (1999) Dual lipid modification of the yeast Gγ subunit Ste18p determines membrane localization of Gβγ. Mol Cell Biol 19 7705–7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GL, Goldstein JL, Brown MS (1995) Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J Biol Chem 270 6221–6226 [DOI] [PubMed] [Google Scholar]
- Johnson CD, Chary SN, Chernoff EN, Zeng Q, Running MP, Crowell DN (2005) Protein geranylgeranyltransferase I is involved in specific aspects of abscisic acid and auxin signaling in Arabidopsis. Plant Physiol 139 722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM (2002) G-protein-coupled signaling in Arabidopsis. Curr Opin Plant Biol 5 402–407 [DOI] [PubMed] [Google Scholar]
- Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y (2004) Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J 38 320–331 [DOI] [PubMed] [Google Scholar]
- Lane K, Beese L (2006) Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res 47 681–699 [DOI] [PubMed] [Google Scholar]
- Lavy M, Bracha-Drori K, Sternberg H, Yalovsky S (2002) A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 14 2431–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Yalovsky S (2006) Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J 46 934–947 [DOI] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC (2001) A mutant Arabidopsis heterotrimeric G-protein β-subunit affects leaf, flower, and fruit development. Plant Cell 13 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem 277 41268–41273 [DOI] [PubMed] [Google Scholar]
- Ma H, Yanofsky M, Meyerowitz E (1990) Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA 87 3821–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee AI, Seabra MC (2003) Are prenyl groups on proteins sticky fingers or greasy handles? Biochem J 376 e3–e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Marshall C (1999) New insights into the interaction of Ras with the plasma membrane. Cell 98 9–12 [DOI] [PubMed] [Google Scholar]
- Manahan CL, Patnana M, Blumer KJ, Linder ME (2000) Dual lipid modification motifs in Galpha and Ggamma subunits are required for full activity of the pheromone response pathway in Saccharomyces cerevisiae. Mol Biol Cell 11 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein gamma-subunit cDNA. Proc Natl Acad Sci USA 97 14784–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2001) Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim Biophys Acta 1520 147–153 [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Eisenhaber F (2005) Refinement and prediction of protein prenylation motifs. Genome Biol 6 r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Ahearn I, Bergo M, Young S, Philips M (2002) Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell 13 3294–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR (2001) Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 152 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI (1995) The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci 20 181–186 [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312 264–266 [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ (2006) Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res 47 1118–1127 [DOI] [PubMed] [Google Scholar]
- Narasimha Chary S, Bultema RL, Packard CE, Crowell DN (2002) Prenylcysteine alpha-carboxyl methyltransferase expression and function in Arabidopsis thaliana. Plant J 32 735–747 [DOI] [PubMed] [Google Scholar]
- Nebenfuhr A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Chen J-G, Jones AM, Assmann SM (2006) G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 141 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Perfus-Barbeoch L, Jones AM, Assmann SM (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7 719–731 [DOI] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin AN, Gautam N (1993) Proper processing of a G protein γ subunit depends on complex formation with a β subunit. FEBS Lett 328 89–93 [DOI] [PubMed] [Google Scholar]
- Randall SK, Marshall MS, Crowell DN (1993) Protein isoprenylation in suspension-cultured tobacco cells. Plant Cell 5 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD (2006. a) Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE 2006. re14. [DOI] [PubMed]
- Resh MD (2006. b) Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods 40 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Toledo-Ortiz G, Yalovsky S, Caldelari D, Gruissem W (2000) Carboxyl-methylation of prenylated calmodulin CaM53 is required for efficient plasma membrane targeting of the protein. Plant J 24 775–784 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Yalovsky S, Zik M, Fromm H, Gruissem W (1999) The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J 18 1996–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG (2002) The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 159 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR III, Davis NG (2006) Global analysis of protein palmitoylation in yeast. Cell 125 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running MP, Fletcher JC, Meyerowitz EM (1998) The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125 2545–2553 [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Or N, Yalovsky S (2004) Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA 101 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra M, Goldstein J, Sudhof T, Brown M (1992) Rab geranylgeranyl transferase: a multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J Biol Chem 267 14497–14503 [PubMed] [Google Scholar]
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG (2006) Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell 126 191–203 [DOI] [PubMed] [Google Scholar]
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99 13307–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takida S, Wedegaertner PB (2003) Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gbeta gamma. J Biol Chem 278 17284–17290 [DOI] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR (2006) Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M (2000) Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci USA 97 11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292 2066–2069 [DOI] [PubMed] [Google Scholar]
- Veit B (2004) The human SNARE protein Ykt6 mediates its own palmitoylation at C-terminal cysteine residues. Biochem J 384 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B, Dietrich LEP, Ungermann C (2003) Biochemical characterization of the vacuolar palmitoyl acyltransferase. FEBS Lett 540 101–105 [DOI] [PubMed] [Google Scholar]
- Veit M (2000) Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem J 345 145–151 [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2022–2023 [DOI] [PubMed] [Google Scholar]
- Warpeha KM, Lateef SS, Lapik Y, Anderson M, Lee B-S, Kaufman LS (2006) G-protein-coupled receptor 1, G-protein Gα-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol 140 844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR (1995) Lipid modifications of trimeric G proteins. J Biol Chem 270 503–506 [DOI] [PubMed] [Google Scholar]
- Yalovsky S, Kulukian A, Rodriguez-Concepcion M, Young CA, Gruissem W (2000. a) Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Bracha K, Toledo-Ortiz G, Gruissem W (2000. b) Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 12 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Trueblood CE, Callan KL, Narita JO, Jenkins SM, Rine J, Gruissem W (1997) Plant farnesyltransferase can restore yeast Ras signaling and mating. Mol Cell Biol 17 1986–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16 1123–1127 [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65 241–269 [DOI] [PubMed] [Google Scholar]
- Zhang FL, Moomaw JF, Casey PJ (1994) Properties and kinetic mechanism of recombinant mammalian protein geranylgeranyltransferase type I. J Biol Chem 269 23465–23470 [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM (2000) Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc Natl Acad Sci USA 97 7633–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.