Abstract
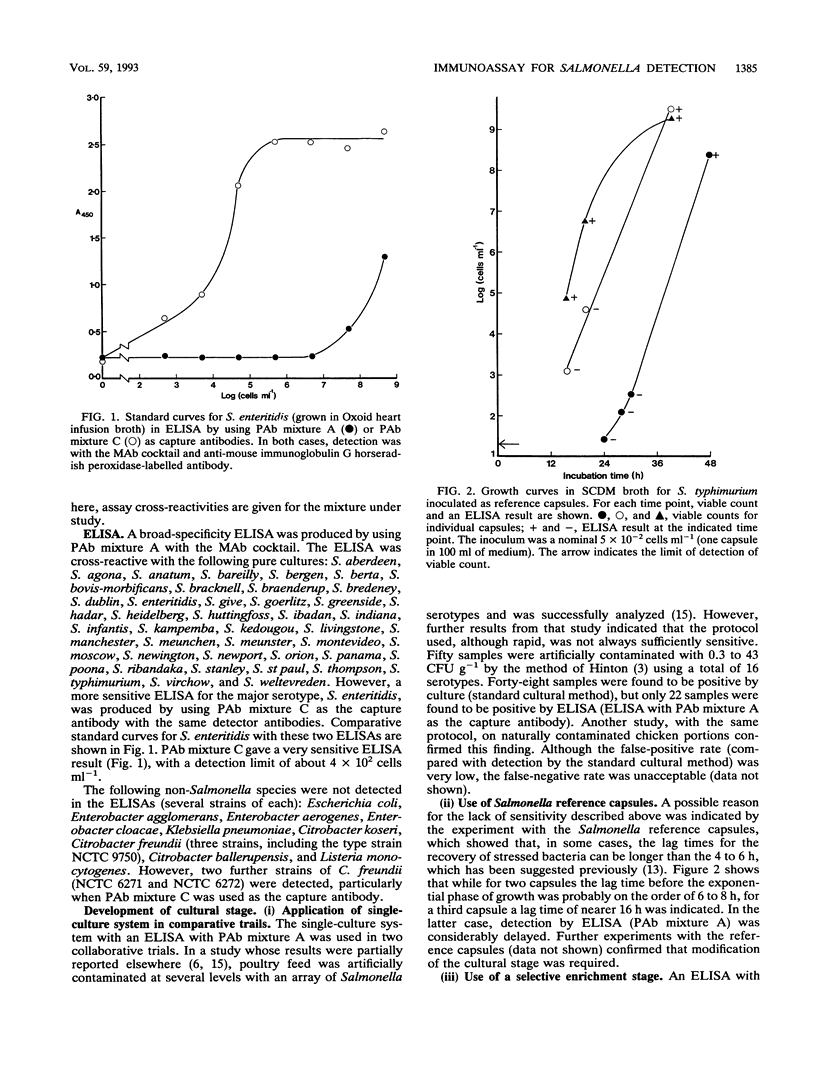
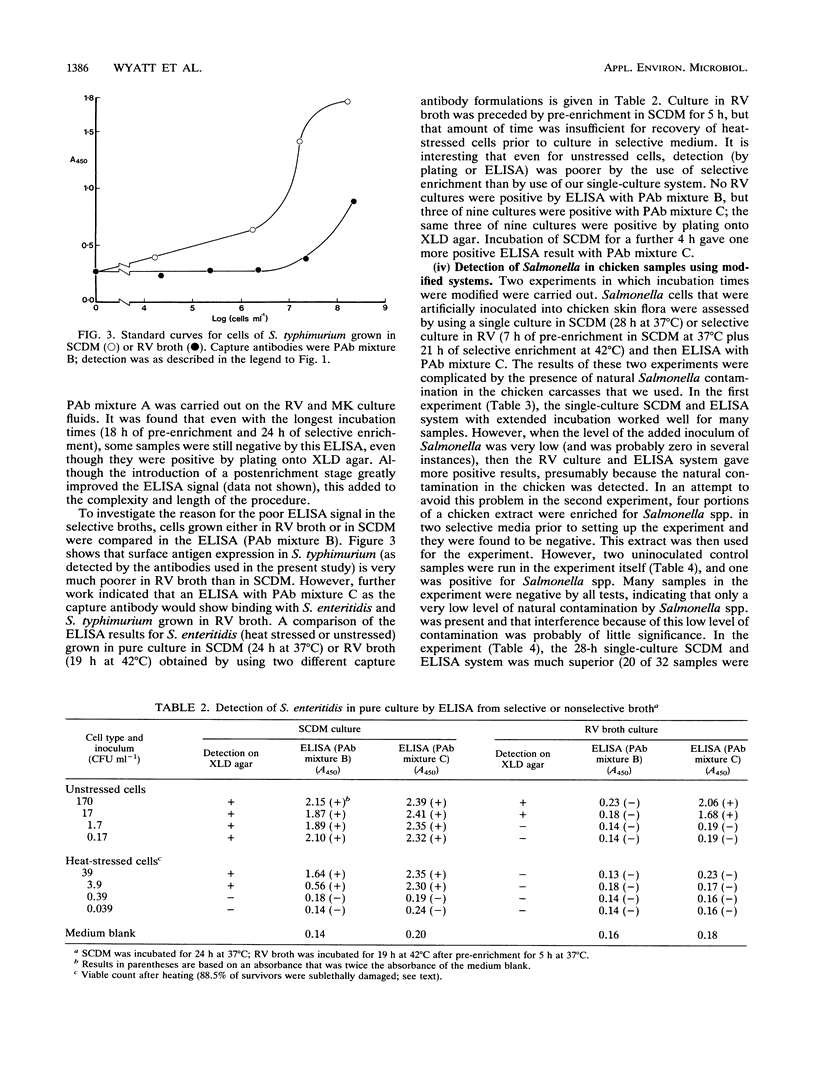
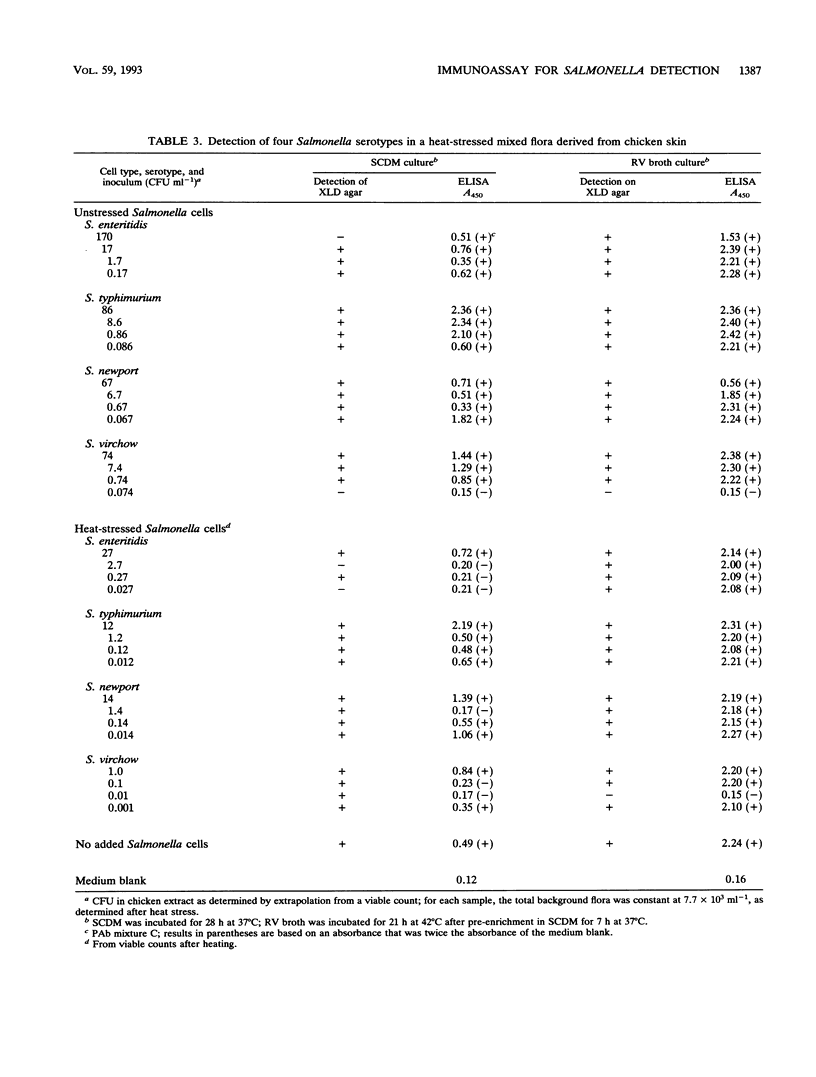
A model system previously developed for the rapid detection of Salmonella typhimurium in foods was improved and extended to many other Salmonella serotypes. The original protocol, which consisted of an overnight nonselective culture followed by a specific enzyme-linked immunosorbent assay (ELISA), was modified and improved. A sandwich ELISA which used polyclonal antibodies for the capture stage and a cocktail of monoclonal antibodies for the detector stage was developed. The assay recognized a wide range of Salmonella serotypes; S. enteritidis, the most important serotype in the United Kingdom had a detection limit in the ELISA of about 4 x 10(2) cells ml-1. The cultural stage prior to the ELISA was either a single nonselective broth (incubated for 28 h) or a preenrichment broth (incubated for 7 h) plus a selective broth (incubated for 21 h). Antibodies which bind to cells grown in the unfavorable conditions of a selective medium were selected. It was concluded that, in the future, the shortened protocols for the detection of Salmonella spp. in foods described here will be of considerable value.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckers H. J., van Leusden F. M., Meijssen M. J., Kampelmacher E. H. Reference material for the evaluation of a standard method for the detection of salmonellas in foods and feeding stuffs. J Appl Bacteriol. 1985 Dec;59(6):507–512. doi: 10.1111/j.1365-2672.1985.tb03353.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim G. F., Fleet G. H., Lyons M. J., Walker R. A. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985 Dec;22(6):1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee H. A., Wyatt G. M., Bramham S., Morgan M. R. Enzyme-linked immunosorbent assay for Salmonella typhimurium in food: feasibility of 1-day Salmonella detection. Appl Environ Microbiol. 1990 Jun;56(6):1541–1546. doi: 10.1128/aem.56.6.1541-1546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey B. M., Derrick C. M. A comparison of solid and liquid media for measuring the sensitivity of heat-injured Salmonella typhimurium to selenite and tetrathionate media, and the time needed to recover resistance. J Appl Bacteriol. 1982 Oct;53(2):233–242. doi: 10.1111/j.1365-2672.1982.tb04682.x. [DOI] [PubMed] [Google Scholar]
- Mackey B. M., Derrick C. M. The effect of sublethal injury by heating, freezing, drying and gamma-radiation on the duration of the lag phase of Salmonella typhimurium. J Appl Bacteriol. 1982 Oct;53(2):243–251. doi: 10.1111/j.1365-2672.1982.tb04683.x. [DOI] [PubMed] [Google Scholar]


