Abstract
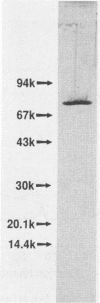
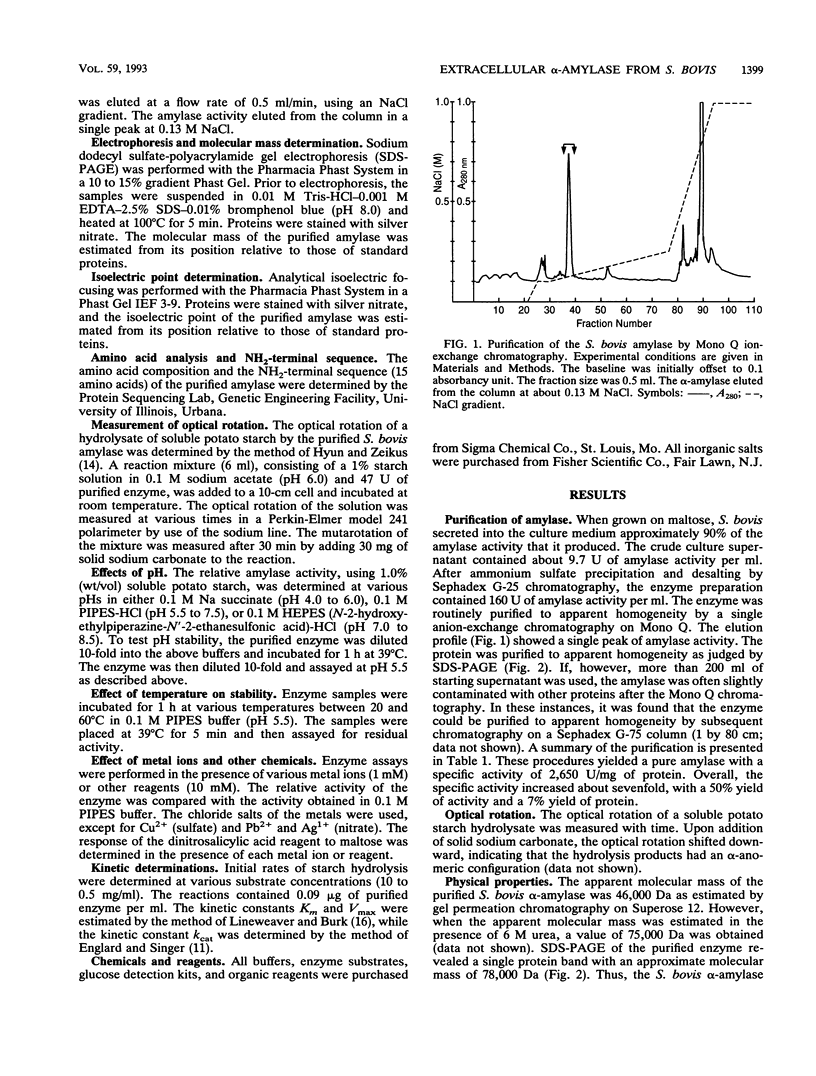
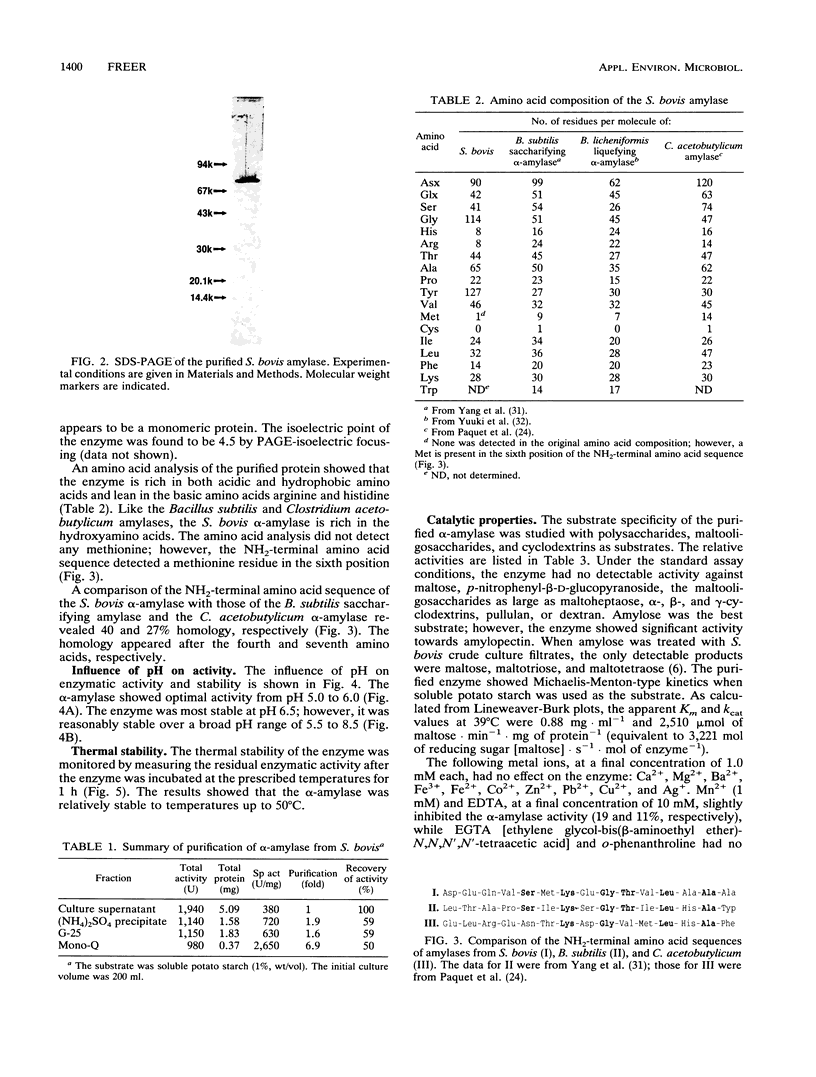
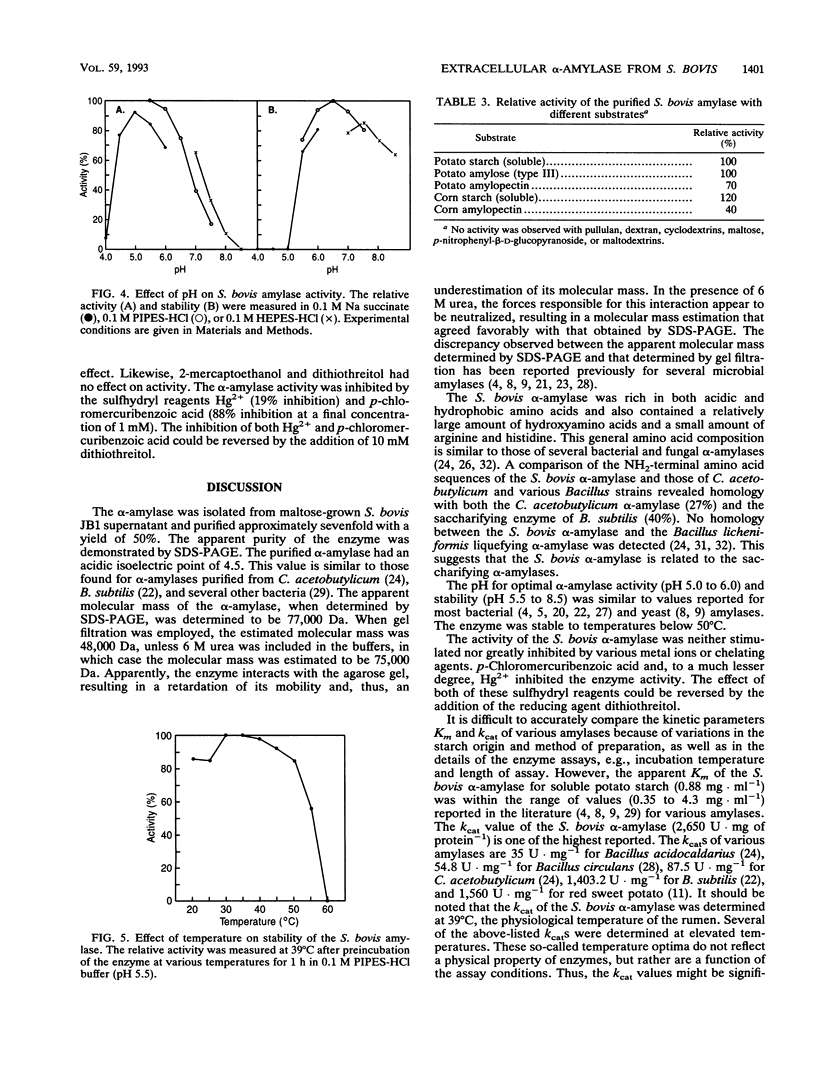
The extracellular alpha-amylase (1,4-alpha-D-glucanglucanohydrolase; EC 3.2.1.1) from maltose-grown Streptococcus bovis JB1 was purified to apparent homogeneity by ion-exchange chromatography (Mono Q). The enzyme had an isoelectric point of 4.50 and an apparent molecular mass of 77,000 Da, as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The enzyme was rich in acidic and hydrophobic amino acids. The 15-amino-acid NH2-terminal sequence was 40% homologous with the Bacillus subtilis saccharifying alpha-amylase and 27% homologous with the Clostridium acetobutylicum alpha-amylase. alpha-Amylase activity on soluble starch was optimal at pH 5.0 to 6.0. The enzyme was relatively stable between pH 5.5 and 8.5 and at temperatures below 50 degrees C. When soluble potato starch was used as the substrate, the enzyme had a Km of 0.88 mg.ml-1 and a kcat of 2,510 mumol of reducing sugar.min-1.mg of protein-1. The enzyme exhibited neither pullulanase nor dextranase activity and was 40 to 70% as active on amylopectin as on amylose. The major end products of amylose hydrolysis were maltose, maltotriose, and maltotetraose.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. R. A specific method for quantitative determination of glucose. Anal Biochem. 1966 Feb;14(2):258–260. doi: 10.1016/0003-2697(66)90134-5. [DOI] [PubMed] [Google Scholar]
- Buonocore V., Caporale C., De Rosa M., Gambacorta A. Stable, inducible thermoacidophilic alpha-amylase from Bacillus acidocaldarius. J Bacteriol. 1976 Nov;128(2):515–521. doi: 10.1128/jb.128.2.515-521.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A. Amylolytic activity of selected species of ruminal bacteria. Appl Environ Microbiol. 1988 Mar;54(3):772–776. doi: 10.1128/aem.54.3.772-776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A. Interaction of ruminal bacteria in the production and utilization of maltooligosaccharides from starch. Appl Environ Microbiol. 1992 Jan;58(1):48–54. doi: 10.1128/aem.58.1.48-54.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and Characterization of Extracellular Amylolytic Enzymes from the Yeast Filobasidium capsuligenum. Appl Environ Microbiol. 1985 Dec;50(6):1474–1482. doi: 10.1128/aem.50.6.1474-1482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and characterization of extracellular alpha-amylase and glucoamylase from the yeast Candida antarctica CBS 6678. Eur J Biochem. 1987 May 4;164(3):643–654. doi: 10.1111/j.1432-1033.1987.tb11175.x. [DOI] [PubMed] [Google Scholar]
- ENGLARD S., SINGER T. P. Physicochemical studies on beta-amylase. J Biol Chem. 1950 Nov;187(1):213–219. [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackie R. I., Gilchrist F. M. Changes in Lactate-Producing and Lactate-Utilizing Bacteria in Relation to pH in the Rumen of Sheep During Stepwise Adaptation to a High-Concentrate Diet. Appl Environ Microbiol. 1979 Sep;38(3):422–430. doi: 10.1128/aem.38.3.422-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. H., Keay L. Purification and characterization of the amylase of B. subtilis NRRL B3411. Biotechnol Bioeng. 1970 Mar;12(2):251–271. doi: 10.1002/bit.260120207. [DOI] [PubMed] [Google Scholar]
- Paquet V., Croux C., Goma G., Soucaille P. Purification and characterization of the extracellular alpha-amylase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1991 Jan;57(1):212–218. doi: 10.1128/aem.57.1.212-218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Vihinen M., Mäntsälä P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol. 1989;24(4):329–418. doi: 10.3109/10409238909082556. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuuki T., Nomura T., Tezuka H., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a gene coding for heat- and pH-stable alpha-amylase of Bacillus licheniformis: comparison of the amino acid sequences of three bacterial liquefying alpha-amylases deduced from the DNA sequences. J Biochem. 1985 Nov;98(5):1147–1156. doi: 10.1093/oxfordjournals.jbchem.a135381. [DOI] [PubMed] [Google Scholar]