Abstract
Telomerase, an enzyme essential for the synthesis and maintenance of telomeric DNA and the long-term stability of the genome, is developmentally regulated in plants. Telomerase activity is abundant in reproductive organs but low or undetectable in vegetative organs. Treatment with exogenous auxin, however, overrides this developmental control and induces telomerase in mature leaves. The Arabidopsis thaliana transcription factor TELOMERASE ACTIVATOR1 (TAC1) potentiates some responses to auxin, including the induction of telomerase activity in leaves. Here, we report that BT2, a protein with BTB, TAZ, and calmodulin binding domains, is an essential component of the TAC1-mediated telomerase activation pathway. Steady state concentration of BT2 mRNA increases in response to TAC1 expression, and TAC1 specifically binds the BT2 promoter both in vitro and in yeast one-hybrid assays. Constitutive expression of BT2 induces telomerase activity in leaves, whereas a null mutation of BT2 blocks TAC1-mediated telomerase induction, indicating that BT2 acts downstream of TAC1 to regulate telomerase activity in mature vegetative organs.
INTRODUCTION
Telomerase, the reverse transcriptase complex that synthesizes and maintains telomeric DNA repeats at the ends of chromosomes, is tightly regulated in plants. Telomerase activity is undetectable in most vegetative tissues, but it is induced upon transition from the vegetative phase of growth to the reproductive phase (Fitzgerald et al., 1996; Heller et al., 1996; Kilian et al., 1998; Riha et al., 1998; for review, see McKnight and Shippen, 2004). Although telomerase is a complex holoenzyme with multiple subunits, activity in Arabidopsis thaliana correlates closely with mRNA levels for telomerase reverse transcriptase (ATTERT), the catalytic subunit (Fitzgerald et al., 1999). A T-DNA disruption of ATTERT results in a loss of telomerase activity and a slow, but inexorable, shortening of telomeric DNA. After approximately five generations without telomerase, when telomeres shorten to a critical threshold, chromosomes begin to fuse (Riha et al., 2001) and initiate the chromosome breakage-fusion-bridge cycle initially described by McClintock (1939). After two or three subsequent generations, cells in the apical meristem lose their ability to proliferate, presumably as a consequence of rampant genome rearrangements. These late-generation telomerase mutants cannot flower and remain in a persistent vegetative phase (Riha et al., 2001).
In addition to developmental controls, the phytohormone auxin also influences telomerase expression. Exposure to exogenous auxin induces telomerase activity in leaves (Ren et al., 2004), organs in which the activity is usually absent (Fitzgerald et al., 1996; Heller et al., 1996). In synchronous tobacco (Nicotiana tabacum) cell cultures, telomerase activity peaks during late S-phase, the time of telomeric DNA replication (Tamura et al., 1999). This peak of activity can be induced to higher levels and shifted to an earlier time in S-phase by adding auxin to the culture medium (Tamura et al., 1999; Yang et al., 2002). Inhibitors of cyclin-dependent kinases required for the transition from G1- to S-phase, but not inhibitors of conventional DNA polymerases, block telomerase induction, demonstrating that the synthesis of telomeric DNA is not tightly coupled to the synthesis of bulk DNA (Tamura et al., 1999).
To identify genes that regulate telomerase in plants, we screened activation-tagged mutants of Arabidopsis (Weigel et al., 2000) for plants that ectopically expressed telomerase in their leaves (Ren et al., 2004). TELOMERASE ACTIVATOR1 (TAC1), a gene encoding a small zinc-finger protein belonging to the SUPERMAN-LIKE family of transcription factors (Bowman et al., 1992; Isernia et al., 2003), is overexpressed in the activation-tagged line tac1-1D (Ren et al., 2004). Constitutive expression of TAC1, in either tac1-1D or 35S:TAC1 transgenic plants, induces telomerase activity in leaves. This induction, however, can be blocked by reducing levels of indole acetic acid (IAA), the major endogenous auxin, with iaaL, an enzyme that conjugates free IAA to Lys (Romano et al., 1991). Other phenotypes of TAC1-overproducing plants also suggest a link between TAC1 and the response to auxin. Callus from plants constitutively producing TAC1, but not from wild-type plants, grows luxuriously in the absence of exogenous auxin. Elongation of primary roots in TAC1-overexpressing lines is more sensitive to inhibition by exogenous auxin than is that of wild-type roots, whereas root elongation in TAC1-null mutants is less sensitive. Finally, overexpression of TAC1 exacerbates the high-auxin phenotype of the yucca mutant (Zhao et al., 2001). Together, these results indicate that TAC1 potentiates some of the many responses to auxin, including the induction of telomerase (Ren et al., 2004).
Auxin signaling is mediated by the well-characterized E3 ubiquitin ligase SCFTIR1, in which the TIR1 subunit of this protein complex is a receptor for IAA (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). IAA binding to TIR1 increases the affinity of this complex for Aux/IAA proteins, which repress auxin-responsive transcription factors (ARFs) (Ulmasov et al., 1997; Tiwari et al., 2001). In the presence of IAA, the repressors are ubiquitinated by SCFTIR1 and subsequently degraded by 26S proteasomes, thereby releasing ARFs to affect changes in gene expression (for review, see Napier, 2005).
Some auxin responses also involve increased calcium concentrations in the cytosol and other calcium-signaling components. For example, treatment with exogenous auxin increases cytosolic Ca2+ concentrations in wheat protoplasts (Shishova and Lindberg, 2004). Additionally, mutation of CAX1, which encodes a Ca2+ transporter, alters expression of the auxin-responsive IAA28:β-glucuronidase reporter gene (Cheng et al., 2003). Finally, the auxin-responsive protein kinase PINOID interacts with two calcium binding proteins in a calcium-dependent manner (Benjamins et al., 2003). The presence of empirically demonstrated, calcium-dependent calmodulin binding sites on small auxin upregulated RNAs from maize (Zea mays), Arabidopsis, and soybean (Glycine max) (Yang and Poovaiah, 2000) also suggests that calcium signaling is frequently involved in auxin responses.
Here, we report that TAC1 induces the expression of BT2, a gene originally identified through yeast two-hybrid interactions as a calmodulin binding protein (Du and Poovaiah, 2004). BT2 has a BTB protein interaction domain at its N terminus, a TAZ protein interaction domain in the center, and a calcium-dependent, calmodulin binding domain at its C terminus (Du and Poovaiah, 2004). Treatment with the calcium ionophore A23187 restores the ability of TAC1 to induce telomerase in plants with low IAA concentrations, consistent with the idea that signaling through BT2 is modulated by Ca2+ concentrations. Results from gel-shift assays and yeast one-hybrid assays suggest that the BT2 promoter may be a direct target of the TAC1 transcription factor. Overexpression of BT2 alone is sufficient to activate telomerase in transgenic plants, and a T-DNA insertion into BT2 eliminates the ability of TAC1 to activate telomerase, confirming that BT2 is an essential component of the TAC1-telomerase pathway.
RESULTS
The TAC1-Telomerase Link Is Conserved among Angiosperms
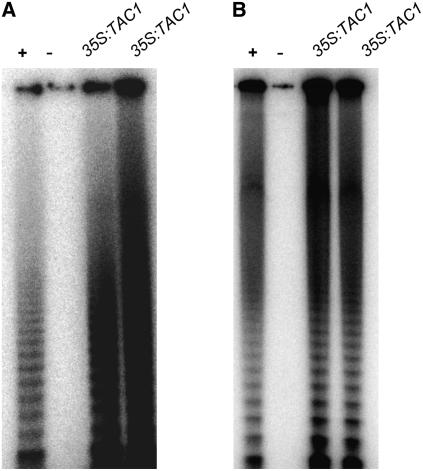
Overexpression of TAC1 in both activation-tagged lines and 35S:TAC1 transgenic lines induces telomerase expression in mature Arabidopsis leaves (Ren et al., 2004). Because ectopic expression of transcription factors can lead to nonphysiological responses, we transformed a 35S:TAC1 construct into tomato (Solanum lycopersicum) and rice (Oryza sativa) plants to determine whether the ability of TAC1 to induce telomerase was conserved in other angiosperms. As shown in Figure 1, constitutive expression of TAC1 leads to telomerase expression in both tomato and rice leaves. If induction of telomerase activity were simply an artifact of TAC1 overexpression, it is unlikely that this link would have been maintained during the 150 million years since the divergence of monocots and dicots (Chaw et al., 2004).
Figure 1.
Telomerase Assays of 35S:TAC1 Tomato and Rice Plants.
Telomerase assays of wild-type tomato (A) and rice (B) flowers and leaves were performed as positive and negative controls, respectively. The last two lanes in each part contain leaf nuclear extract from two plants independently transformed with the Arabidopsis TAC1 gene under the control of the cauliflower mosaic virus (CaMV) 35S promoter.
TAC1 Induces the Expression of BT2
Although TAC1 is a transcription factor, it does not appear to bind the ATTERT promoter in either yeast one-hybrid assays or electromobility-shift assays (data not shown). To determine how TAC1 influences telomerase expression, we used first-generation Affymetrix microarrays with probes for 8300 genes in a preliminary test to compare the transcriptional profiles of leaves from the tac1-1D activation-tagged line and the wild type. BT2 was one of the most highly overexpressed genes in the TAC1-overexpressing line, and the BT2 promoter possessed a potential binding site for plant zinc finger proteins (see below). We repeated transcriptional profiling of the tac1-1D mutant with full-genome microarrays (ATH1; Affymetrix), which confirmed that BT2 transcripts were increased compared with the wild type.
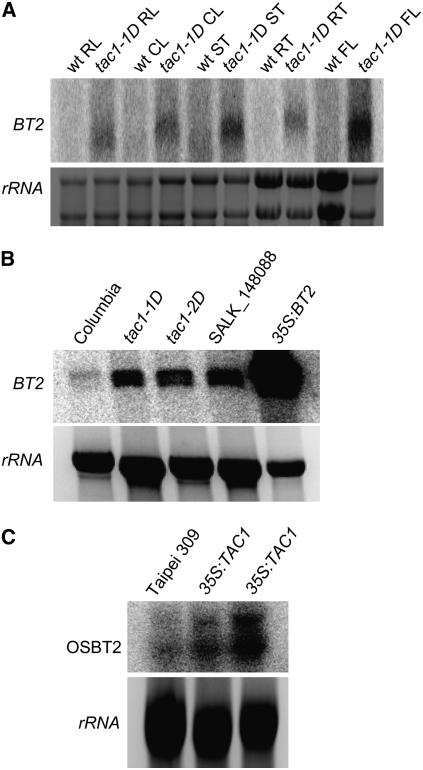
RNA gel blot analysis confirmed that BT2 expression was increased in the tac1-1D activation line in all organs examined (Figure 2A). BT2 expression was also increased in other TAC1-overexpressing lines, including tac1-2D (Figure 2B), a line from the Salk collection (Alonso et al., 2003) with an activating T-DNA at the 3′ end of the TAC1 coding region (Ren et al., 2004). Expression of TAC1 from the CaMV 35S promoter in rice results in increased expression of a BT2 homolog (Figure 2C), again indicating that TAC1 function is broadly conserved in angiosperms.
Figure 2.
Effects of TAC1 on BT2 Expression.
(A) RNA gel blot from Arabidopsis rosette leaves (RL), cauline leaves (CL), inflorescence stems (ST), roots (RT), and flowers (FL) from wild-type Columbia plants and tac1-1D mutants (Ren et al., 2004) probed with BT2 cDNA. tac1-1D is an activation-tagged line from the Weigel collection (Weigel et al., 2000).
(B) Analysis of BT2 mRNA in rosette leaves from various genetic backgrounds. tac1-2D is a Salk T-DNA insertion line that also overexpresses TAC1 (Ren et al., 2004), SALK_148088 contains an activating T-DNA insertion near the 3′ end of BT2, and 35S:BT2 is a constitutively expressing transgenic line in the Columbia background.
(C) RNA gel blot of rice leaves probed with the closest rice homolog of BT2, designated Os BT2. The first lane contains RNA from leaves of the wild-type cv Taipei 309. The last two lanes contain RNA from leaves of two independently transformed plants. Ethidium bromide–stained rRNA was used as a loading control.
BT2 belongs to a five-gene family initially characterized by Du and Poovaiah (2004), who used yeast two-hybrid screens to identify calmodulin-interacting proteins. They designated one such protein At BT1. The Arabidopsis genome encodes four additional proteins, including BT2 (designated At BT2 by Du and Poovaiah, 2004), with an identical domain structure: an N-terminal BTB protein interaction domain (Zollman et al., 1994), a central TAZ protein interaction domain (Kanai et al., 2000), and a C-terminal calcium-dependent calmodulin binding domain (Du and Poovaiah, 2004). BT1 is the closest homolog of BT2 (75% identity, 87% similarity at the amino acid level), but expression of BT1 is not affected by TAC1 (data not shown). Although previous analysis indicated that BT2 has slightly higher levels of expression in leaves than in other organs examined (Du and Poovaiah, 2004), we found relatively little expression in most wild-type organs (Figure 2A).
Expression of BT2 Is Sufficient to Induce Telomerase Activity
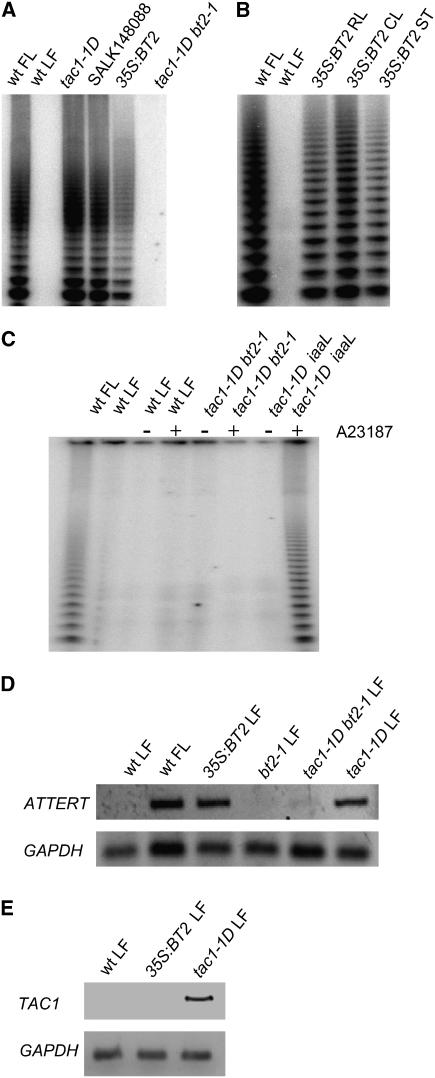
If BT2 participates in the pathway leading from TAC1 to telomerase expression, then overexpression of BT2 alone may be sufficient to activate telomerase in mature leaves. As a rapid test of this hypothesis, we examined telomerase activity in SALK_148088, a line containing a T-DNA insertion near the 3′ end of BT2. We previously noted that a T-DNA insertion in the 3′ end of TAC1 increased its expression (Ren et al., 2004), possibly through the enhancer associated with the CaMV 35S promoter in the pROK2 vector used in the Salk lines (Alonso et al., 2003). As was the case with TAC1, the T-DNA inserted 3′ of BT2 increased the expression of this gene (Figure 2B), and robust telomerase activity was detected in mature rosette leaves (Figure 3A). Conversely, a T-DNA insertion into the coding region of BT2 (SALK_002306) blocked the ability of TAC1 to induce telomerase (Figure 3A) or ATTERT message (Figure 3D) in mature leaves. For an independent examination of BT2 overexpression, we transformed plants with a 35S:BT2 construct. These transformants also exhibited telomerase activity in their rosette leaves, cauline leaves, and inflorescence stems (Figure 3B). We did not examine flowers in 35S:BT2 because flowers from wild-type plants have abundant telomerase activity. Overexpression of BT2 does not induce TAC1 expression (Figure 3E), thereby excluding the possibility that BT2 and TAC1 mutually activate each other. Together, these results indicate that BT2 is an essential component of the TAC1-telomerase pathway.
Figure 3.
Effects of TAC1 and BT2 Mutants on Telomerase Expression.
Extracts from wild-type flower (FL) and leaf (LF) were used as positive and negative controls, respectively.
(A) Effect of BT2 expression on telomerase activity in mature rosette leaves. tac1-1D is an activation-tagged line from the Weigel collection (Weigel et al., 2000) with telomerase activity in its leaves. SALK_148088 contains an activating T-DNA insertion near the 3′ end of BT2. 35S:BT2 is a constitutively expressing transgenic line. Both BT2-overexpressing lines possess telomerase activity in their leaves. The line designated bt2-1 is the SALK_002306 line, which contains an inactivating T-DNA in BT2 and blocks the ability of TAC1 to activate telomerase in the tac1-1D bt2-1 double mutant.
(B) Effect of BT2 on telomerase expression in other vegetative organs (RL, rosette leaves; CL, cauline leaves; ST, inflorescence stem).
(C) Effect of calcium ionophore treatment on telomerase induction. Mature leaves were harvested and floated on Murashige and Skoog (MS) medium with (+) or without (−) 10 μM of the Ca2+ ionophore A23187 for 23 h. Nuclei were then isolated and assayed for telomerase activity.
(D) Effect of BT2 on ATTERT expression. RNAs were isolated from mature leaves except for the positive control, which was from flowers, and analyzed by RT-PCR using primers described previously for ATTERT (Ren et al., 2004). The line designated bt2-1 is the T-DNA disruption line SALK_002306.
(E) Effect of BT2 on TAC1 message. RNA was isolated from mature leaves and analyzed by RT-PCR using primers described previously for TAC1 (Ren et al., 2004).
Effect of Ca2+ on TAC1-Mediated Induction of Telomerase
Expression of iaaL, a bacterial enzyme that conjugates free IAA to Lys (Romano et al., 1991), blocks the ability of TAC1 to induce telomerase in leaves of the tac1-1D iaaL double mutant, indicating that wild-type levels of auxin are required in the TAC1-telomerase pathway (Ren et al., 2004). The C-terminal, calcium-dependent, calmodulin binding domain of BT2 (Du and Poovaiah, 2004) suggested that calcium signaling also may be involved in the TAC1-telomerase pathway. Consistent with this prediction, exposure of mature leaves from tac1-1D iaaL plants to exogenous Ca2+ and the calcium ionophore A23187 bypassed the requirement for wild-type levels of auxin and restored telomerase induction in the double mutant (Figure 3C). The ionophore does not induce telomerase in wild-type plants or in the tac1-1D SALK_002306 double mutant, indicating that calcium does not affect telomerase activity independently of the TAC1-BT2 pathway (Figure 3C).
TAC1 Interacts with the BT2 Promoter Sequence
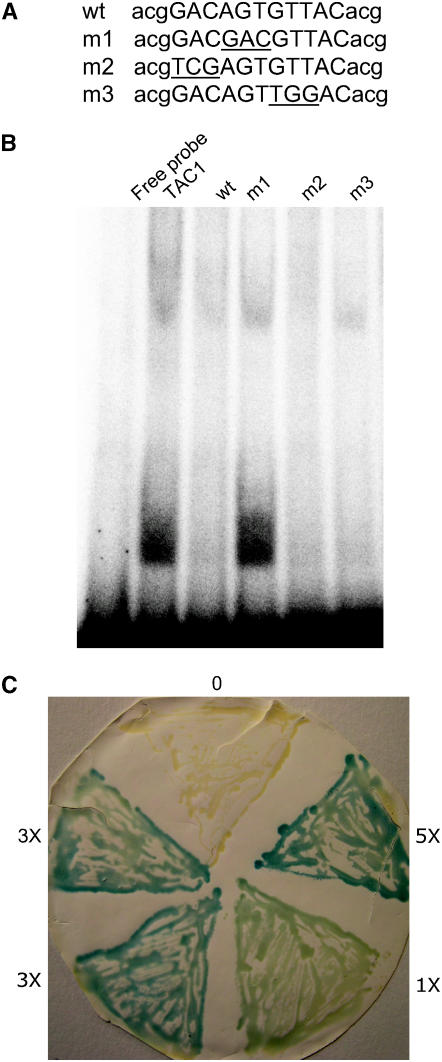
TAC1 could increase BT2 expression either directly by binding to the BT2 promoter or indirectly by affecting the expression of another regulatory factor that ultimately increases BT2 expression. One hint that TAC1 may interact directly with the BT2 promoter is the presence of a sequence similar to the binding site of other plant zinc-finger transcription factors. The C2H2 zinc-finger protein EPF2-5 from petunia (Petunia hybrida) binds directly to the sequence GACAGTGTCAC in the promoter of the gene encoding 5-enolpyruvylshikimate-3-phosphate synthase (Takatsuji and Matsumoto, 1996). The Arabidopsis C2H2 zinc finger protein SUPERMAN binds to the same sequence in vitro (Dathan et al., 2002). Both SUPERMAN and EPF2-5 require the central AGT trinucleotide (underlined above) for binding. The BT2 promoter contains a site at −1023 bp relative to the initiation codon where 10 of the 11 nucleotides in the EPF2-5 binding site are conserved (GACAGTGTTAC), including the central AGT. To examine the possibility that the BT2 promoter could be a direct target of the TAC1 transcription factor, we performed a gel mobility-shift analysis with recombinant TAC1 protein and oligonucleotides containing wild-type and mutated versions of this sequence (Figure 4A). TAC1 specifically bound to wild-type oligonucleotides (Figure 4B). Substitution of the AGT core sequence with GAC dramatically reduced TAC1 binding, whereas substitution of three nucleotides on either side of the AGT core did not affect TAC1 binding. As an independent examination of potential interaction between TAC1 and the BT2 promoter, we performed yeast one-hybrid assays. These assays confirmed the interaction, and the strength of the interaction depended on the number of binding sites upstream of the lacZ reporter gene (Figure 4C).
Figure 4.
Interaction between TAC1 and the BT2 Promoter.
(A) Sequences of oligonucleotides used for mobility-shift assays. An 11-nucleotide region from the BT2 promoter that shared 10 nucleotides with a binding site for another plant zinc-finger protein (see text) is shown in uppercase letters. Three arbitrary nucleotides flanking this sequence are shown in lowercase letters. Wild-type sequence for one strand is shown on the top. Oligonucleotides m1, m2, and m3 carry mutations at the underlined positions.
(B) Mobility-shift assays with the TAC1 protein and BT2 oligonucleotides. Radiolabeled wild-type oligonucleotide alone is designated Free Probe. TAC1 binds to this oligonucleotide, resulting in a slower mobility (TAC1). Cold wild-type oligonucleotide at 50-fold excess effectively competes for TAC1 binding to the labeled probe (lane wt). Mutation of the central AGT triplet eliminates this competition (lane m1). Oligonucleotides with mutations at three nucleotides on either side of the AGT core compete as effectively as the wild-type sequence (lanes m2 and m3).
(C) Interaction between TAC1 and the BT2 promoter sequence in yeast one-hybrid assays. Zero, one, three, or five copies of the wild-type oligonucleotide shown in (A) were cloned upstream of the lacZ gene in the pYC7 plasmid and transformed into yeast expressing TAC1. lacZ activity, judged by staining with X-Gal, correlates with the number of putative binding sites.
Auxin and BT2
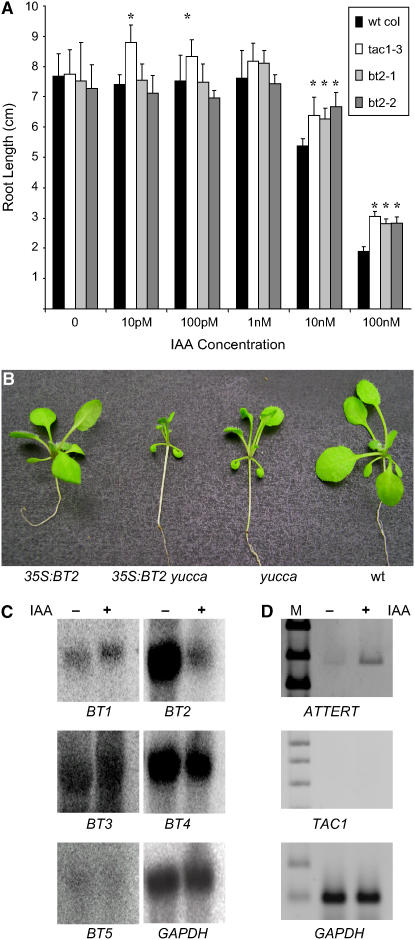
Because TAC1 potentiates several responses to auxin, including inhibition of primary root growth, exacerbation of the high-auxin phenotype of the yucca mutant, and induction of telomerase (Ren et al., 2004), and because BT2 is required for this induction (Figure 3), we analyzed the interaction between auxin and BT2. Elongation of primary roots was less sensitive to inhibition by 2,4-D in two independent BT2 T-DNA disruption lines (Figure 5A). These responses parallel those of the TAC1-null mutant (Figure 5A) (Ren et al., 2004). Furthermore, constitutive expression of BT2 exacerbates the high-auxin phenotype of the yucca mutant (Figure 5B), just as TAC1 does (Ren et al., 2004), consistent with the idea that TAC1's ability to potentiate responses to auxin is also mediated by BT2.
Figure 5.
BT2 and Response to Auxin.
(A) Sensitivity of primary root elongation to exogenous auxin. Roots from a TAC1-null line (tac1-3; Ren et al., 2004) and two independent T-DNA disruption lines of BT2 (bt2-1 = SALK_002306 and bt2-2 = SALK_088241) were more resistant to inhibition by higher concentrations of exogenous IAA than roots of wild-type plants. Bars indicate average primary root length from 10 plants. Asterisks indicate significant differences (α = 0.01). Error bars indicate sd.
(B) Overexpression of BT2 exacerbates the high-auxin phenotype in the 35S:BT2 yucca double mutant.
(C) Effect of exogenous auxin on mRNA from the BT family. Wild-type leaves were floated on MS medium with (+) or without (−) 10 μM IAA for 1 h. Total RNA was isolated, resolved by electrophoresis, blotted to nylon membranes, and probed with radiolabeled PCR fragments from each of the five BT family members. The mRNA for the glyceraldehyde phosphate dehydrogenase gene (GAPDH) was used as a loading control. Brief treatment with IAA specifically and dramatically reduces the concentration of BT2 mRNA.
(D) Effect of IAA on ATTERT and TAC1 mRNA. Leaves were treated and RNA was isolated as described for (C). mRNA for ATTERT and TAC1 was analyzed by RT-PCR using primers described previously (Ren et al., 2004).
To determine whether BT2 expression was affected by auxin, we excised leaves from wild-type plants, treated them with 10 μM IAA for 1 h, and examined mRNA levels for all five members of the BT family on RNA gel blots. This brief IAA treatment had no effect on mRNA levels for four of the five BT genes. However, mRNA for BT2 rapidly and specifically decreased upon exposure to exogenous IAA (Figure 5C). Despite this dramatic decrease in BT2 mRNA, transcript for ATTERT was rapidly induced in leaves treated with IAA (Figure 5D).
DISCUSSION
We previously described TAC1 as a member of the SUPERMAN-LIKE family of zinc-finger transcription factors whose overexpression leads to increased concentrations of ATTERT mRNA and the induction of telomerase activity in mature leaves of Arabidopsis (Ren et al., 2004). Mutants carrying a T-DNA disruption of TAC1 had no apparent defect in telomere biology. This lack of a telomere phenotype could be attributable to redundant function supplied by another of the 29 SUPERMAN-LIKE genes or a parallel pathway for telomerase regulation. It was also possible that induction of telomerase activity in leaves was a nonphysiological response to ectopic expression of the TAC1 transcription factor. However, constitutive expression of TAC1 also induces telomerase activity in leaves of transgenic tomato and rice plants (Figure 1). Conservation of a link between this transcription factor and telomerase over the 150 million years since the divergence of monocots and dicots (Chaw et al., 2004) suggests not only that this link has a physiological role but also that there is strong selective pressure to maintain it.
The fact that TAC1 apparently does not interact with the ATTERT promoter led us to use microarrays to compare transcript profiles between the wild type and the tac1-1D activation-tagged line. Initial analysis revealed that BT2 was one of the most highly induced genes in tac1-1D. The increase in BT2 message was confirmed by RNA gel blots (Figure 2). TAC1 interacts with the BT2 promoter in gel-shift and one-hybrid assays (Figure 4) at a site similar to that recognized by other members of the SUPERMAN-LIKE family, including the petunia protein EPF2-5 (Takatsuji and Matsumoto, 1996) and SUPERMAN itself (Dathan et al., 2002). The BT2 promoter, therefore, may be a direct target of TAC1, although we have not yet confirmed this in vivo. Regardless of whether BT2 is a direct or indirect target of TAC1, null mutations in BT2 block the ability of TAC1 to induce telomerase, demonstrating that it encodes a necessary component of the pathway downstream of TAC1. Induction of a BT2 homolog in transgenic 35S:TAC1 rice plants (Figure 2) further indicates that the TAC1-telomerase pathway is conserved in angiosperms.
Overexpression of TAC1 potentiates several responses to auxin, including increased sensitivity of primary root growth to inhibition by synthetic auxins, exacerbation of the high-auxin phenotype of yucca mutants, growth in culture without exogenous auxin, and induction of telomerase in mature organs (Ren et al., 2004). As mentioned previously, some auxin responses involve increased calcium concentrations in the cytosol and other calcium-signaling components (Benjamins et al., 2003; Cheng et al., 2003; Shishova and Lindberg, 2004). The presence of a calmodulin binding domain in BT2 could account for the requirement of both TAC1 and wild-type levels of IAA for the induction of telomerase: TAC1 is required for the increased transcription of BT2, and IAA is required to maintain cytosolic Ca2+ at a concentration sufficient for calmodulin to bind and regulate BT2. This hypothesis was supported by the finding that TAC1's requirement for wild-type levels of IAA could be bypassed with exposure of tac1-1D iaaL leaves to exogenous Ca2+ and the ionophore A23187 (Figure 3).
mRNA for BT2, but not for the other four members of this family, is rapidly and specifically depleted upon brief exposure to auxin (Figure 5C), possibly as a way to attenuate signaling through the TAC1-BT2 pathway. Many signal transduction systems rapidly inactivate themselves through negative feedback loops as a way to produce transient responses and prevent runaway signaling. Classic examples of negative feedback in animal cells include pathways regulated by JAK-STAT (Alexander and Hilton, 2004; Ridderstrale, 2005) and p53 (Harris and Levine, 2005). Signaling pathways in plants are also regulated by negative feedback loops. In Arabidopsis, ABI1 and ABI2 protein phosphatases are induced by abscisic acid and function to repress abscisic acid signaling (Merlot et al., 2001). Two major classes of auxin-induced genes encode proteins that attenuate auxin signaling: Aux/IAA proteins, which act to repress ARF transcription factors (Ulmasov et al., 1997; Tiwari et al., 2001), and GH3-like proteins, which conjugate free IAA to amino acids (Staswick et al., 2002, 2005). Similarly, BT2 appears to be a target of a negative feedback loop that limits auxin-mediated signaling through this pathway.
BT2 belongs to a five-gene family initially identified by Du and Poovaiah (2004) in a yeast two-hybrid screen for proteins that interact with calmodulin. Each of the five BT proteins encoded in the genome have an N-terminal BTB domain, a central TAZ domain, and a C-terminal calcium-dependent calmodulin binding domain (Du and Poovaiah, 2004). The BTB protein interaction domain, named for founding members of the class in Drosophila (Bric-a-brac, Tramtrack, and Broad complex) (Zollman et al., 1994), has been found on proteins that interact with a variety of partners, including components of an E3 ubiquitin ligase complex (Pintard et al., 2003; Gingerich et al., 2005), transcriptional regulators (Kinyamu et al., 2005), and the basal transcriptional machinery (Pointud et al., 2001). Only a few of the ∼80 BTB-containing proteins in Arabidopsis have been characterized, but several of these proteins appear to interact with cullin 3, in which they may assume functions analogous to those of both the F-box proteins and SKP-like proteins in typical cullin 1–based ubiquitin ligases such as SCFTIR1 (Pintard et al., 2004). The Caenorhabditis elegans BTB protein Mel-26 binds to cullin 3 through its BTB domain and to a protein targeted for ubiquitination through a separate protein interaction domain (Pintard et al., 2003). GST pull-down assays indicate that both BT1 and BT2 interact with cullin 3 in vitro (Figueroa et al., 2005), although yeast two-hybrid analysis failed to detect this interaction with BT2 (Dieterle et al., 2005). If BT2 participates in a ubiquitin ligase, it may direct ubiquitination and subsequent degradation of a telomerase repressor.
There is a second possible mechanism for BT2 function. When BT1, the closest homolog of BT2, was used as bait in a yeast two-hybrid screen of a cDNA library, it bound to two bromodomain proteins, GTE9 and GTE11, but apparently not to cullin 3 (Du and Poovaiah, 2004). GST pull-down experiments and a second round of yeast two-hybrid analysis using only BT1 and GTE11 confirmed the interaction. In this second round of two-hybrid assays, BT2 interacted as strongly as BT1 with GTE11. Analysis of deletion mutants of BT1 showed that the first 192 amino acids, including the BTB domain (residues 26 to 128), but not the TAZ or calmodulin binding domains, were required to bind GTE11 (Du and Poovaiah, 2004). Bromodomain transcription regulators such as GTE11 interact with other proteins through acetylated Lys residues and may affect chromatin structure by binding to acetylated histones (Zeng and Zhou, 2002). If BT2 interacts with bromodomain proteins in vivo, this interaction may alter their ability to bind chromatin and control the expression of other telomerase regulators or telomerase genes. The potential roles for BT2 as a component of a ubiquitin ligase and as a modifier of transcriptional regulators are not necessarily mutually exclusive, as ubiquitination of these regulators (Sun and Chen, 2004) and even histones (Kim et al., 2005) can stimulate transcription. Characterization of proteins that interact with BT2 in vivo should help resolve the mechanism by which it induces telomerase activity.
METHODS
RNA Isolation and Analysis
Total RNA was isolated from leaves of 3-week-old Arabidopsis thaliana and rice (Oryza sativa) wild-type plants and 35S:TAC1 transformants at flowering stage using Tri Reagent according to the manufacturer's instructions (Sigma-Aldrich). To determine the effect of auxin on BT2 mRNA levels, leaves were treated with IAA before RNA isolation as described previously (Ren et al., 2004).
For initial microarray analyses, 15 μg of total RNA from tac1-1D and wild-type plants was labeled and used to probe first-generation Arabidopsis microarrays according to the manufacturer's instructions (Affymetrix). Data were analyzed with the Affymetrix Microarray Suite 5.0 software package. Microarray analysis was later repeated through a commercial service (Asuragen) with full-genome Affymetrix chips. Data from the full-genome analysis are available through the Gene Expression Omnibus database (accession number GSE5896).
For RNA gel blots, unless specified otherwise, 15 μg of total RNA from each sample was separated on 1.2% formaldehyde denaturing gels and transferred to Hybond N+ membranes (Amersham). Blots were probed with 32P-labled PCR products from individual BT genes with the following primers: for BT1, 5′-GCAATATAAGAATAGAGGTGAAAAG-3′ and 5′-CAAAAACCCTAATACCATTTTATAACC-3′; for BT2, 5′-ACATGGTCACCCAGCTGAAG-3′ and 5′-CAGACACAACCCTTGTCACC-3′; for BT3, 5′-TCATCGAATCCGACAGTAGG-3′ and 5′-ACGAATCACGACTCGGCATG-3′; for BT4, 5′-ATCAGAGGATATATTCGCAGC-3′ and 5′-CTTGGCCGACCAAAATATGTG-3′; and for BT5, 5′-ACAGCTCTATGAAGCAATGG-3′ and 5′-GGCCAAAGATTGGATCTTAGG-3′.
The rice RNA gel blot was probed with 32P-labeled PCR product of the most closely related rice BT2 homolog, represented by two alternatively spliced cDNAs and designated in Figure 2C as Os BT2. Primers used to amplify this gene from rice genomic DNA were 5′-AGGCAAGAGAGAGCAAGAAGG-3′ and 5′-TCCACTTGGCCTCCTCCTTTC-3′. Ethidium bromide–stained rRNA was used as a loading control.
Plasmid Construction, Plant Transformation, and Growth Conditions
Full-length BT2 coding sequence was cloned behind the CaMV 35S promoter in pBI101, and the construct was transformed first into Agrobacterium tumefaciens GV3101 and then into Arabidopsis by the floral dip method (Clough and Bent, 1998).
Tomato (Solanum lycopersicum) plants were transformed with Agrobacterium containing binary vector with the 35S:TAC1 construct using the method described by Park et al. (2003) and grown in a greenhouse with natural lighting.
Mature seeds of rice (cv Taipei 309) were used for the production of embryogenic callus cultures on N6-2D medium (N6 medium [Chu et al., 1975; Chu, 1978], 0.5 g/L casamino acids, 3% sucrose, 2 mg/L 2,4-D, and 0.2% phytagel, pH 5.8).
Two- to 3-mm pieces of callus from 6- to 8-week-old embryogenic cultures were transformed with preinduced (Sunilkumar and Rathore, 2001) Agrobacterium EHA105 harboring a binary vector with the 35S:TAC1 construct (Ren et al., 2004). After cocultivation for 3 d, cultures were transferred to N6-2D medium containing 250 mg/L carbenicillin and 100 mg/L cefotaxime (N6CC) for 1 week. Transformed tissue was selected by two additional subcultures on N6CC medium supplemented with 50 mg/L hygromycin. Callus induction, maintenance, cocultivation, and selection were performed in the dark at 25°C. After two rounds of 3-week-long selections on hygromycin, surviving callus cells were transferred to MSR medium (MS medium, 2% sucrose, 3% sorbitol, 0.1 mg/L naphthylacetic acid, 2.5 mg/L kinetin, and 0.2% phytagel, pH 5.8) and cultured under light for 4 weeks to induce regeneration. Regenerating shoots were selected and grown in jars on MSR medium supplemented with 50 mg/L Trp and 30 mg/L hygromycin. Plants with well-developed root systems were transferred to soil and grown to maturity in a greenhouse.
Telomerase Assays
Plants were analyzed for telomerase activity by the Telomere Repeat Amplification Protocol (TRAP) (Kim et al., 1994), as modified by Fitzgerald et al. (1996). For ionophore treatment experiments, before TRAP assay, mature rosette leaves were surface-sterilized and incubated with shaking at 125 rpm in 0.2× MS liquid medium containing 10 mM Ca2+ with or without 10 μM ionophore A23178 (Sigma-Aldrich) for 23 h.
Auxin Effects
Root elongation assays were performed as described previously (Ren et al., 2004), except that IAA, rather than 2,4-D, was used as the exogenous auxin. To examine the effects of auxin on gene expression, mature leaves from wild-type plants were harvested and floated on MS medium with or without 10 μM IAA for 1 h before isolating RNA. To examine the effect of BT2 expression on the high-auxin phenotype of yucca (Zhao et al., 2001), we examined the F1 progeny of a 35S:BT2 × yucca cross.
Production of Recombinant TAC1 and Gel-Shift Assays
TAC1 cDNA was subcloned into the BamHI-SalI sites of pET28(a) to place six His codons at the 5′ end of the coding region. Recombinant TAC1 was expressed in Escherichia coli BL21(DE3) by induction with 1 mM isopropylthio-β-galactoside overnight at 25°C. Total proteins were extracted using BugBuster protein extraction reagent (Novagen) and dialyzed against 20 mM Tris, pH 8.0, for 3 h. Five micrograms of total protein was used for gel-shift analysis as described (Dathan et al., 2002). The wild-type duplex oligonucleotide 5′-ACGGACAGTGTTACACG-3′ (−1040 to −1024 bp relative to the BT2 start codon) was radiolabeled and used as probe. The 50-fold excess cold wild-type duplex oligonucleotide or 50-fold excess mutated duplex oligonucleotides m1 (5′-ACGGACGACGTTACACG-3′), m2 (5′-ACGTCAAGTGTTACACG-3′), and m3 (5′-ACGGACAGTTGGACACG-3′) were used as competitors.
Yeast One-Hybrid Assay
One, three, and five copies of the putative binding element from the BT2 promoter, described above, were cloned between the SmaI and XhoI sites of the pYC7 vector. The TAC1 coding region was fused with the GAL4 activation domain by inserting it between the BamHI and SalI sites of pGAD424.
Plasmids pGAD424-TAC1 and pYC7-BT2 (BDS) with various copies of the binding element were cotransformed into yeast strain Y4741 and selected on medium lacking uracil and Leu. As a negative control, pGAD424 and pYC7 vectors alone were also cotransformed. Colonies were grown for 3 d and assayed for β-galactosidase activity. Cells were lifted onto a nylon membrane, immersed in liquid nitrogen for 30 s, thawed at room temperature for several minutes, and placed on Whatman 3M paper prewetted with Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol) containing 0.34 mg/mL X-Gal.
Accession Numbers
The Arabidopsis Genome Initiative locus numbers for the genes analyzed in this article are as follows: TAC1, At3g09290; BT2, At3g48360; ATTERT, At5g16850; BT1, At5g63160; BT3, At1g05690; BT4, At5g67480; BT5, At4g37610; and GAPDH, At3g04120. The rice homolog of BT2, Os BT2, is represented by GenBank accessions AK071112 and AK061917, which are alternatively spliced cDNAs from genomic accession NT_079927. Data from full-genome microarray analyses comparing transcriptional profiles of tac1-1D–overexpressing lines with the wild type are available through the Gene Expression Omnibus database (accession GSE5896).
Acknowledgments
We thank Dorothy Shippen and Wayne Versaw for insightful discussions and comments on the manuscript. This work was funded by grants from the National Science Foundation (MCB 0244159) and the Texas Advanced Technology Program (010366-74) to T.D.M.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Thomas D. McKnight (mcknight@bio.tamu.edu).
Open Access articles can be viewed online without a subscription.
References
- Alexander, W.S., and Hilton, D.J. (2004). The role of Suppressors of Cytokine Signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22 503–529. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Benjamins, R., Ampudia, C.S., Hooykaas, P.J., and Offringa, R. (2003). PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 132 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Sakai, H., Jack, T., Weigel, D., Mayer, U., and Meyerowitz, E.M. (1992). SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development 114 599–615. [DOI] [PubMed] [Google Scholar]
- Chaw, S.M., Chang, C.C., Chen, H.L., and Li, W.H. (2004). Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J. Mol. Evol. 58 424–441. [DOI] [PubMed] [Google Scholar]
- Cheng, N.H., Pittman, J.K., Barkla, B.J., Shigaki, T., and Hirschi, K.D. (2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C.C. (1978). The N6 medium and its application to anther culture of cereal crops. In Proceedings of Symposium on Plant Tissue Culture. (Beijing, China: Science Press), pp. 43–50.
- Chu, C.C., Wang, C.C., Sun, C.S., Hsu, C., Yin, K.C., Chu, C.Y., and Bi, F.Y. (1975). Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 18 659–668. [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dathan, N., Zaccaro, L., Esposito, S., Isernia, C., Omichinski, J.G., Riccio, A., Pedone, C., Di Blasio, B., Fattorusso, R., and Pedone, P.V. (2002). The Arabidopsis SUPERMAN protein is able to specifically bind DNA through its single Cys2-His2 zinc finger motif. Nucleic Acids Res. 30 4945–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., and Estelle, M. (2005). The F-box protein TIR1 is an auxin receptor. Nature 435 441–445. [DOI] [PubMed] [Google Scholar]
- Dieterle, M., Thomann, A., Renou, J.P., Parmentier, Y., Cognat, V., Lemonnier, G., Muller, R., Shen, W.H., Kretsch, T., and Genschik, P. (2005). Molecular and functional characterization of Arabidopsis Cullin 3A. Plant J. 41 386–399. [DOI] [PubMed] [Google Scholar]
- Du, L., and Poovaiah, B.W. (2004). A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: Interaction with fsh/Ring3 class transcription activators. Plant Mol. Biol. 54 549–569. [DOI] [PubMed] [Google Scholar]
- Figueroa, P., Gusmaroli, G., Serino, G., Habashi, J., Ma, L., Shen, Y., Feng, S., Bostick, M., Callis, J., Hellmann, H., and Deng, X.W. (2005). Arabidopsis has two redundant cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 17 1180–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, M.S., McKnight, T.D., and Shippen, D.E. (1996). Characterization and developmental patterns of telomerase expression in plants. Proc. Natl. Acad. Sci. USA 93 14422–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, M.S., Riha, K., Gao, F., Ren, S., McKnight, T.D., and Shippen, D.E. (1999). Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc. Natl. Acad. Sci. USA 96 14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich, D.J., Gagne, J.M., Salter, D.W., Hellmann, H., Estelle, M., Ma, L., and Vierstra, R.D. (2005). Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 280 18810–18821. [DOI] [PubMed] [Google Scholar]
- Harris, S.L., and Levine, A.J. (2005). The p53 pathway: Positive and negative feedback loops. Oncogene 24 2899–2908. [DOI] [PubMed] [Google Scholar]
- Heller, K., Kilian, A., Piatyszek, M.A., and Kleinhofs, A. (1996). Telomerase activity in plant extracts. Mol. Gen. Genet. 252 342–345. [DOI] [PubMed] [Google Scholar]
- Isernia, C., Bucci, E., Leone, M., Zaccaro, L., Di Lello, P., Digilio, G., Esposito, S., Saviano, M., Di Blasio, B., Pedone, C., Pedone, P.V., and Fattorusso, R. (2003). NMR structure of the single QALGGH zinc finger domain from the Arabidopsis thaliana SUPERMAN protein. ChemBioChem 4 171–180. [DOI] [PubMed] [Google Scholar]
- Kanai, F., Marignani, P.A., Sarbassova, D., Yagi, R., Hall, R.A., Donowitz, M., Hisaminato, A., Fujiwara, T., Ito, Y., Cantley, L.C., and Yaffe, M.B. (2000). TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19 6778–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski, S., and Leyser, O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451. [DOI] [PubMed] [Google Scholar]
- Kilian, A., Heller, K., and Kleinhofs, A. (1998). Development patterns of telomerase activity in barley and maize. Plant Mol. Biol. 37 621–628. [DOI] [PubMed] [Google Scholar]
- Kim, J., Hake, S.B., and Roeder, R.G. (2005). The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol. Cell 20 759–770. [DOI] [PubMed] [Google Scholar]
- Kim, N.W., Piatyszek, M.A., Prowse, K.R., Harley, C.B., West, M.D., Ho, P.L., Coviello, G.M., Wright, W.E., Weinrich, S.L., and Shay, J.W. (1994). Specific association of human telomerase activity with immortal cells and cancer. Science 266 2011–2015. [DOI] [PubMed] [Google Scholar]
- Kinyamu, H.K., Chen, J., and Archer, T.K. (2005). Linking the ubiquitin-proteasome pathway to chromatin remodeling/modification by nuclear receptors. J. Mol. Endocrinol. 34 281–297. [DOI] [PubMed] [Google Scholar]
- McClintock, B. (1939). The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc. Natl. Acad. Sci. USA 25 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight, T.D., and Shippen, D.E. (2004). Plant telomere biology. Plant Cell 16 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot, S., Gosti, F., Guerrie, D., Vavasseur, A., and Giraudat, J. (2001). The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 25 295–303. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Napier, R.M. (2005). TIRs of joy: New receptors for auxin. Bioessays 27 1213–1217. [DOI] [PubMed] [Google Scholar]
- Park, S.H., Morris, J.L., Park, J.E., Hirschi, K.D., and Smith, R.H. (2003). Efficient and genotype-independent Agrobacterium–mediated tomato transformation. J. Plant Physiol. 160 1253–1257. [DOI] [PubMed] [Google Scholar]
- Pintard, L., Willems, A., and Peter, M. (2004). Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J. 23 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintard, L., Willis, J.H., Willems, A., Johnson, J.L., Srayko, M., Kurz, T., Glaser, S., Mains, P.E., Tyers, M., Bowerman, B., and Peter, M. (2003). The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 425 311–316. [DOI] [PubMed] [Google Scholar]
- Pointud, J.C., Larsson, J., Dastugue, B., and Couderc, J.L. (2001). The BTB/POZ domain of the regulatory proteins Bric a brac 1 (BAB1) and Bric a brac 2 (BAB2) interacts with the novel Drosophila TAF(II) factor BIP2/dTAF(II)155. Dev. Biol. 237 368–380. [DOI] [PubMed] [Google Scholar]
- Ren, S., Johnston, J.S., Shippen, D.E., and McKnight, T.D. (2004). TELOMERASE ACTIVATOR1 induces telomerase activity and potentiates responses to auxin in Arabidopsis. Plant Cell 16 2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstrale, M. (2005). Signaling mechanism for the insulin-like effect of growth hormone—Another example of a classical hormonal negative feedback loop. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5 79–92. [DOI] [PubMed] [Google Scholar]
- Riha, K., Fajkus, J., Siroky, J., and Vyskot, B. (1998). Developmental control of telomere lengths and telomerase activity in plants. Plant Cell 10 1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha, K., McKnight, T.D., Griffing, L.R., and Shippen, D.E. (2001). Living with genome instability: Plant responses to telomerase dysfunction. Science 291 1797–1800. [DOI] [PubMed] [Google Scholar]
- Romano, C.P., Hein, M.B., and Klee, H.J. (1991). Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 5 438–446. [DOI] [PubMed] [Google Scholar]
- Shishova, M., and Lindberg, S. (2004). Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. J. Plant Physiol. 161 937–945. [DOI] [PubMed] [Google Scholar]
- Staswick, P.E., Serban, B., Rowe, M., Tiryaki, I., Maldonado, M.T., Maldonado, M.C., and Suza, W. (2005). Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick, P.E., Tiryaki, I., and Rowe, M.L. (2002). Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L., and Chen, Z.J. (2004). The novel functions of ubiquitination in signaling. Curr. Opin. Cell Biol. 16 119–126. [DOI] [PubMed] [Google Scholar]
- Sunilkumar, G., and Rathore, K.S. (2001). Transgenic cotton: Factors influencing Agrobacterium-mediated transformation and regeneration. Mol. Breed. 8 37–52. [Google Scholar]
- Takatsuji, H., and Matsumoto, T. (1996). Target-sequence recognition by separate-type Cys2/His2 zinc finger proteins in plants. J. Biol. Chem. 271 23368–23373. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Liu, H., and Takahashi, H. (1999). Auxin induction of cell cycle regulated activity of tobacco telomerase. J. Biol. Chem. 274 20997–21002. [DOI] [PubMed] [Google Scholar]
- Tiwari, S.B., Wang, X.J., Hagen, G., and Guilfoyle, T.J. (2001). AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13 2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, D., et al. (2000). Activation tagging in Arabidopsis. Plant Physiol. 122 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.W., Jin, E., Chung, I.K., and Kim, W.T. (2002). Cell cycle-dependent regulation of telomerase activity by auxin, abscisic acid and protein phosphorylation in tobacco BY-2 suspension culture cells. Plant J. 29 617–626. [DOI] [PubMed] [Google Scholar]
- Yang, T., and Poovaiah, B.W. (2000). Molecular and biochemical evidence for the involvement of calcium/calmodulin in auxin action. J. Biol. Chem. 275 3137–3143. [DOI] [PubMed] [Google Scholar]
- Zeng, L., and Zhou, M.M. (2002). Bromodomain: An acetyl-lysine binding domain. FEBS Lett. 513 124–128. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Christensen, S.K., Fankhauser, C., Cashman, J.R., Cohen, J.D., Weigel, D., and Chory, J. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291 306–309. [DOI] [PubMed] [Google Scholar]
- Zollman, S., Godt, D., Prive, G.G., Couderc, J.L., and Laski, F.A. (1994). The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc. Natl. Acad. Sci. USA 91 10717–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]