Abstract
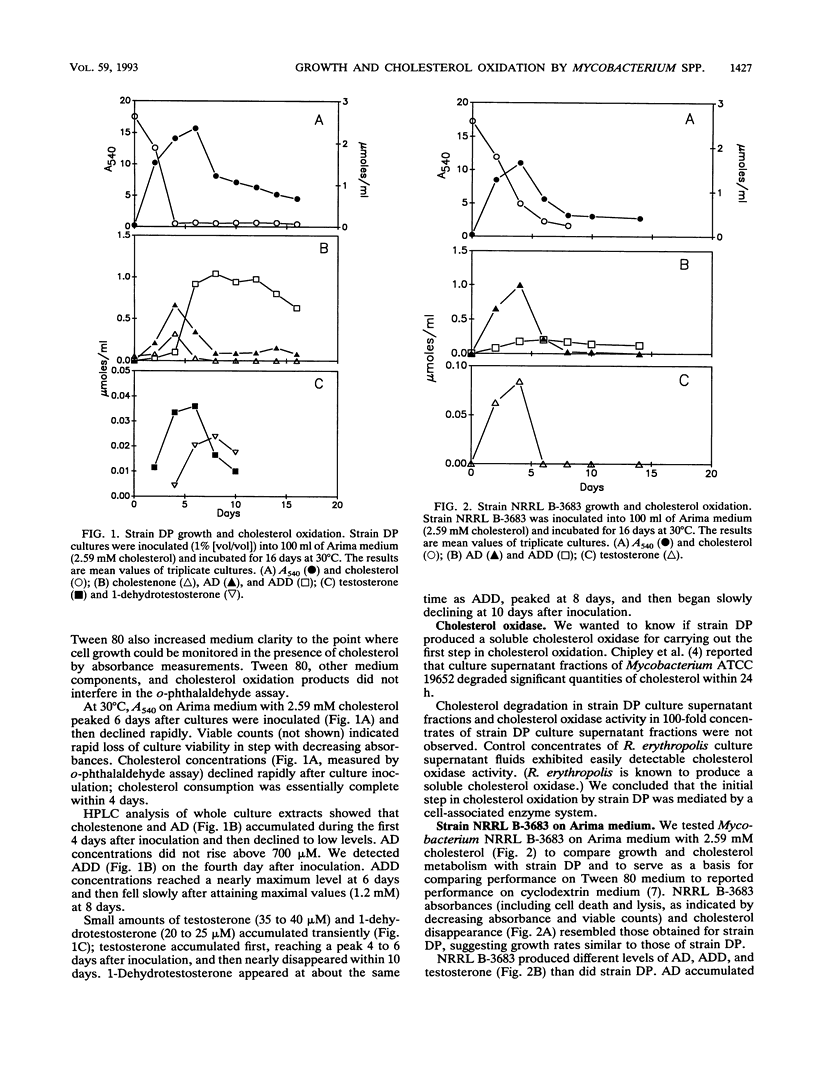
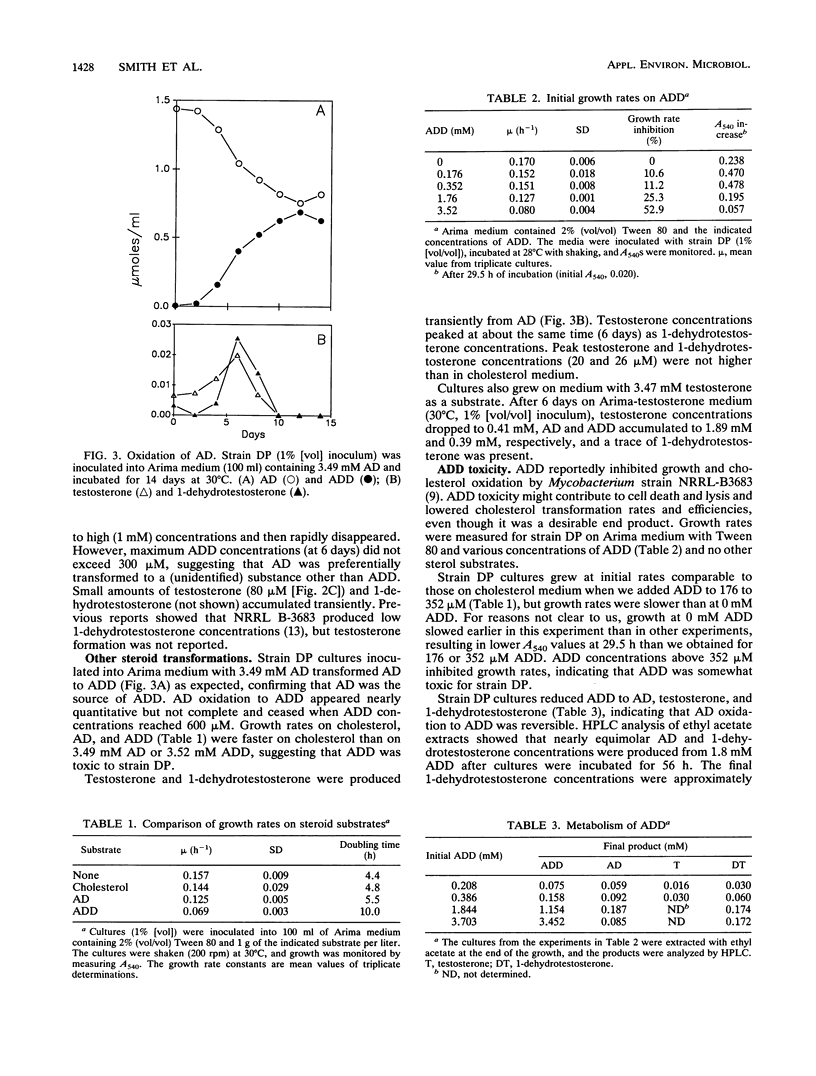
Mycobacterium strain DP was isolated from marine coastal sediment and tested for its ability to oxidize cholesterol in Tween 80-cholesterol (2.59 mM) medium. Strain DP degraded cholesterol to 4-cholesten-3-one (cholestenone), 4-androsten-3,17-dione (AD), 1,4-androstadien-3,17-dione (ADD), testosterone, and 1-dehydrotestosterone (DHT). Cholesterol disappeared in about 4 days. Cholestenone, AD, testosterone, and DHT accumulations were transient with peak concentrations of 300, 600, 30 to 40, and 21 microM. ADD production peaked after 6 days with a concentration of 1,100 microM. Peak ADD concentrations and production rates compared well with those reported for strain NRRL B3683 on cyclodextrin medium. Tween 80 medium was superior to finely dispersed cholesterol particles for both strains. In comparison, NRRL B3683 (patented for its ability to accumulate AD and ADD) on Tween 80 medium transiently accumulated more AD (approximately 1,000 microM) than did strain DP, but ADD accumulations (200 microM) were significantly lower than those for strain DP. Strain DP could be adapted to grow on ADD, which was initially inhibitory at 3.25 mM. ADD-adapted strain DP cultures produced approximately four times as much DHT from ADD than unadapted cultures did from cholesterol, showing that additional manipulation might enhance testosterone production. We believe that ADD toxicity might account for the low ADD accumulations by NRRL B3683 in Tween 80 medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chipley J. R., Dreyfuss M. S., Smucker R. A. Cholesterol metabolism by Mycobacterium. Microbios. 1975;12(50):199–207. [PubMed] [Google Scholar]
- Lee C. Y., Liu W. H. Production of androsta-1,4-diene-3,17-dione from cholesterol using immobilized growing cells of Mycobacterium sp. NRRL B-3683 adsorbed on solid carriers. Appl Microbiol Biotechnol. 1992 Feb;36(5):598–603. doi: 10.1007/BF00183235. [DOI] [PubMed] [Google Scholar]
- Marsheck W. J., Kraychy S., Muir R. D. Microbial degradation of sterols. Appl Microbiol. 1972 Jan;23(1):72–77. doi: 10.1128/am.23.1.72-77.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. W., Mason A. N., Bilton R. F. The degradation of cholesterol by Pseudomonas sp. NCIB 10590 under aerobic conditions. J Lipid Res. 1983 Nov;24(11):1500–1511. [PubMed] [Google Scholar]
- Prome D., Lacave C., Monsarrat B., David H., Prome J. C. Conversion of sterols and triterpenes by mycobacteria. I Formation of progesterone and 1-dehydroprogesterone from Mycobacterium aurum, strain A+. Biochim Biophys Acta. 1983 Aug 29;753(1):60–64. doi: 10.1016/0005-2760(83)90098-x. [DOI] [PubMed] [Google Scholar]
- Sih C. J., Wang K. C., Tai H. H. C-22 acid intermediates in the microbiological cleavage of the cholesterol side chain. J Am Chem Soc. 1967 Apr 12;89(8):1956–1957. doi: 10.1021/ja00984a038. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Brooks C. J. Cholesterol oxidases: properties and applications. J Steroid Biochem. 1976 Sep;7(9):705–713. doi: 10.1016/0022-4731(76)90071-6. [DOI] [PubMed] [Google Scholar]


