Abstract
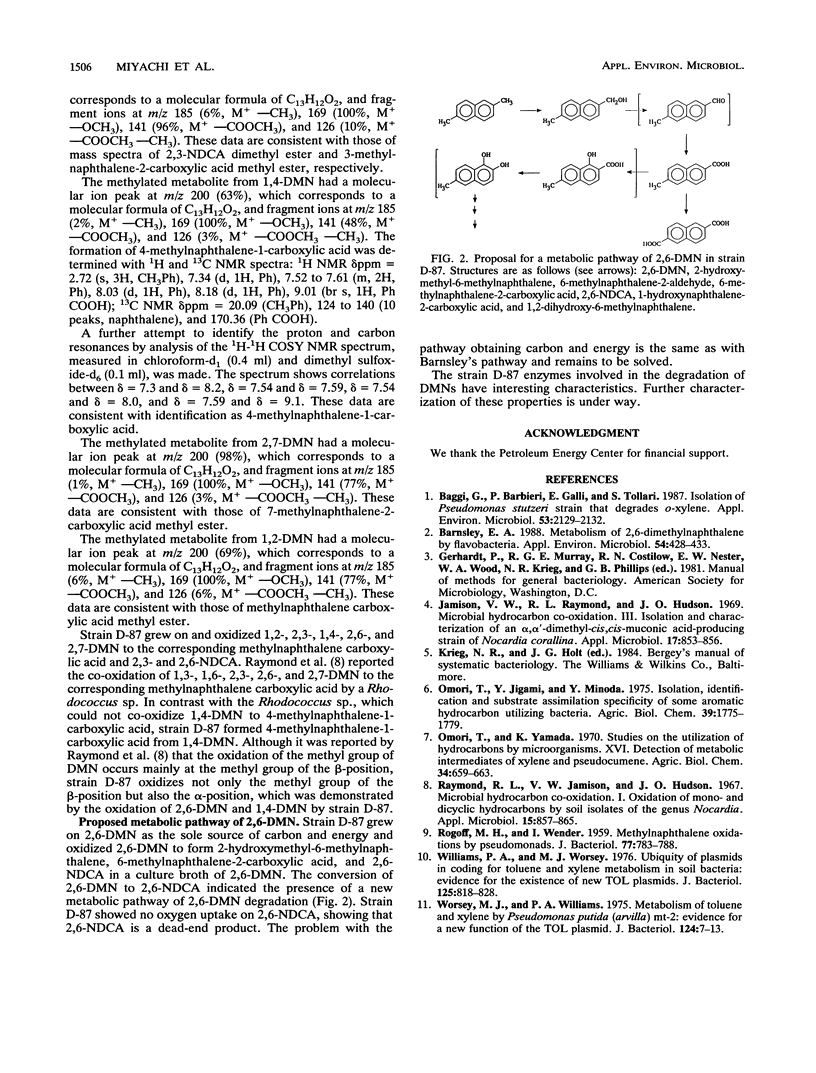
Three bacterial strains, identified as Alcaligenes sp. strain D-59 and Pseudomonas sp. strains D-87 and D-186, capable of growing on 2,6-dimethylnaphthalene (2,6-DMN) as the sole source of carbon and energy were isolated from soil samples. 2,6-Naphthalene dicarboxylic acid was formed in the culture broths of these three strains grown on 2,6-DMN. In addition, 2-hydroxymethyl-6-methylnaphthalene and 6-methylnaphthalene-2-carboxylic acid were detected in the culture broth of strain D-87. Strain D-87 grew well on 1,2-, 1,3-, 1,4-, 1,5-, 2,3-, and 2,7-DMN as the sole source of carbon and energy and accumulated 2-methylnaphthalene-3-carboxylic acid and 2,3-naphthalene dicarboxylic acid from 2,3-DMN, 4-methylnaphthalene-1-carboxylic acid from 1,4-DMN, and 7-methylnaphthalene-2-carboxylic acid from 2,7-DMN.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggi G., Barbieri P., Galli E., Tollari S. Isolation of a Pseudomonas stutzeri strain that degrades o-xylene. Appl Environ Microbiol. 1987 Sep;53(9):2129–2132. doi: 10.1128/aem.53.9.2129-2132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. Metabolism of 2,6-dimethylnaphthalene by flavobacteria. Appl Environ Microbiol. 1988 Feb;54(2):428–433. doi: 10.1128/aem.54.2.428-433.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison V. W., Raymond R. L., Hudson J. O. Microbial Hydrocarbon Co-oxidation. III. Isolation and Characterization of an alpha, alpha'-Dimethyl-cis, cis-Muconic Acid-producing Strain of Nocardia corallina. Appl Microbiol. 1969 Jun;17(6):853–856. doi: 10.1128/am.17.6.853-856.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. Methylnaphthalene oxidations by pseudomonads. J Bacteriol. 1959 Jun;77(6):783–788. doi: 10.1128/jb.77.6.783-788.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond R. L., Jamison V. W., Hudson J. O. Microbial hydrocarbon co-oxidation. I. Oxidation of mono- and dicyclic hydrocarbons by soil isolates of the genus Nocardia. Appl Microbiol. 1967 Jul;15(4):857–865. doi: 10.1128/am.15.4.857-865.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Worsey M. J. Ubiquity of plasmids in coding for toluene and xylene metabolism in soil bacteria: evidence for the existence of new TOL plasmids. J Bacteriol. 1976 Mar;125(3):818–828. doi: 10.1128/jb.125.3.818-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


