Abstract
The following study describes the discovery of a new inherited metabolic disorder, dolichol kinase (DK1) deficiency. DK1 is responsible for the final step of the de novo biosynthesis of dolichol phosphate. Dolichol phosphate is involved in several glycosylation reactions, such as N-glycosylation, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, and C- and O-mannosylation. We identified four patients who were homozygous for one of two mutations (c.295T→A [99Cys→Ser] or c.1322A→C [441Tyr→Ser]) in the corresponding hDK1 gene. The residual activity of mutant DK1 was 2%–4% when compared with control cells. The mutated alleles failed to complement the temperature-sensitive phenotype of DK1-deficient yeast cells, whereas the wild-type allele restored the normal growth phenotype. Affected patients present with a very severe clinical phenotype, with death in early infancy. Two of the patients died from dilative cardiomyopathy.
The transfer of sugar units from polyisoprenyl glycosyl–carrier lipids to other biomolecules is highly conserved in prokaryotic and eukaryotic cells.1 In prokaryotes, undecaprenyl monophosphate serves as both a carrier and a donor of sugar residues involved in the synthesis of lipopolysaccharides, cell-wall peptidoglycans, and capsular polysaccharides.2,3 In eukaryotes, dolichol monophosphate is the most prevalent polyisoprenyl-glycosyl carrier involved in reactions such as the C-4 and O-mannosylation of proteins, the formation of glycosylphosphatidylinositol (GPI) anchors,5 and the N-glycosylation of proteins.6
The chain length of eukaryotic dolichol molecules differs from 14 to 17 isoprene units in unicellular organisms like the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe,7 whereas mammalian cells produce dolichol with 18–21 isoprene units.8 In contrast to bacterial undecaprenyl monophosphate, the α-isoprene unit of eukaryotic dolichol is completely saturated. Only in a few bacterial glycosylation processes are lipids from the dolichyl type, rather than from the undecaprenyl type, used.9,10
During the de novo synthesis of dolichol in eukaryotes, farnesyl pyrophosphate, a metabolite of cholesterol biosynthesis, is elongated by its successive condensation to isopentenyl pyrophosphate molecules. These reactions are catalyzed by cis-isopentenyltransferases, enzymes that appear to be closely bound to microsomes in mammalian tissues.11 After the polyisoprene pyrophosphate chain has reached its final length, both phosphate residues are released by mono- or pyrophosphatases. Both mono- and pyrophosphatase activities could be shown in microsomal fractions.2,12,13
The α-isoprene unit of the polyprenol is then reduced by a nicotinamide adenine dinucleotide phosphate (NADPH)–dependent microsomal reductase.14 The final step in this biosynthetic pathway is catalyzed by dolichol kinase (DK1), an enzyme that transfers a phosphate from choline-phosphate cytidine triphosphate (CTP) to dolichol.15,16 The same enzyme might also play a role in the “recycling” process of dolichol diphosphate (dolichol-PP), which is released after the transfer of the oligosaccharide structure to proteins in the endoplasmic reticulum (ER).1 The gene encoding DK1 was first identified in temperature-sensitive yeast cells deficient for this protein and was termed “Sec59.” Sec59 mutants stop dividing and become enlarged at the restrictive temperature of 37°C,17,18 at which temperature the cells accumulate inactive and incompletely glycosylated secretory proteins.19 The human homologue of the yeast Sec59 gene was recently cloned and characterized. The overexpression of the human gene complements the temperature-sensitive defect of the S. cerevisiae cells.20
In this work, we describe the first defect in humans that affects the biosynthesis of dolichol phosphate by the disturbance of the final phosphorylation step. Two homozygous mutations in the human homologue of the yeast DK1 cause a lethal phenotype, with death in early infancy. Four patients with this newly discovered disorder are described.
Material and Methods
Patients
Skin-biopsy samples were taken from the patients after their parents provided informed consent, and fibroblasts were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulphate, at 37°C, with 5% CO2. EDTA blood samples were taken from the parents, to extract genomic DNA to confirm their heterozygous carrier status.
Yeast
The following yeast strains were used for the experiments: the control strain was AH22 (MATa, Leu2, His4, and Cir+/−), and the mutant strain was PRY134 (MATa, Sec59, and ura3-52) (the temperature-sensitive S. cerevisiae Sec59 mutant). Yeast cells were grown in yeast extract/peptone/dextrose medium containing 1% yeast extract, 2% peptone, and 1% glucose (pH 6.5). Selection was done in a yeast nitrogen base medium (Difco Yeast Nitrogen Base [BD Biosciences]) with 2% glucose. For plates, 2% agar was added.
Labeling of Human Fibroblasts
Human fibroblasts were labeled for 30 min with 100 μCi [2-3H]-mannose or [6-3H]-glucosamine per ml labeling medium (DMEM without glucose:MEM, 9:1), were chased for 10 min in MEM, and were washed with PBS.
Extraction of Lipid-Linked Oligosaccharides (LLOs)
Cells were extracted three times with chloroform/methanol (2:1). The pellet was dried under nitrogen and was extracted several times with water. Dolichylpyrophosphate-linked oligosaccharides were predominantly recovered from the subsequent choloroform/methanol/water (10:10:3) extract and were released by mild acid hydrolysis, for 20 min at 100°C, in N-propanolol/0.1 N HCl (1:2). High-performance liquid chromatography (HPLC) was done in an acetonitril/water gradient by use of a Microsorb MV column (Varian) with a Waters Alliance system.21
Mutation Analysis
Human hDK1 mRNA was transcribed by reverse transcriptase (PowerScript Reverse Transcriptase [BD Biosciences]), and the coding sequence was amplified in four parts by PCR. Primers for amplification reactions (Sec59-1f–Sec59–Sec59Res-1f) are listed in table 1. Exact PCR protocols are available on request. All products were sequenced directly with an automated DNA sequencer (Applied Biosystems).
Table 1. .
Primers for the Amplification of the Human hDK1 Gene[Note]
| Primer Name | Primer Sequence (5′→3′) |
| Sec59-1f | AACGGAGGGAGAAGGTTG |
| Sec59-1r | TAACACCTCCAGCCAAGC |
| Sec59-2f | ATCAGTGTTGGCGCTCGG |
| Sec59-2r | CCGCTTGGCATTCTGGTAC |
| Sec59-3f | TCTTCCAGACAGACACCCG |
| Sec59-3r | AGAAATGATCTGCGCAAATATAG |
| Sec59-4f | CCCTTTTTCTGGATGAACGAG |
| Sec59-4r | CCCAAGTAGCTGTCTGCTGTG |
| Sec59Res-1f | ACAGAAGGGTAGCCTGGGAGGAGCCAGGGCCCTCGTCCGCT |
Note.— Primer Sec59Res-1f was used for restriction analysis.
To exclude common polymorphisms, 240 alleles of healthy donors of Turkish or white European background were investigated by restriction analysis. To perform restriction analysis of the c.1322A→C allele, a forward primer was designed that contained 5 of the 6 base pairs of a BsrBI restriction site at its 3′ end. If the next base pair added through the Taq polymerase during the PCR is the mutation, a complete BsrBI restriction site arises. If it is a wild-type sequence, no restriction site is generated. After the incubation of the PCR products gained from genomic DNA with BsrBI (New England Biolabs), both alleles could be separated on 2% agarose gels.
The second mutation, c.295T→A, is abrogating a BanI restriction site. After the amplification of the first part of the Sec59 gene from genomic DNA through use of the Sec59-1f and Sec59-1r primers, a BanI (New England Biolabs) restriction of the PCR product was performed according to the supplier’s protocols. Both alleles could be separated on an agarose gel.
In this investigation, the published sequences of human hDK1 at the genomic level (GenBank accession number NC_000009) and of the coding region (GenBank accession number NM_014908) were used and confirmed.
Construction of the Expression Vector
The amplicon generated by the PCR with use of the primers Sec59-1f and Sec59-4r (described above) was cloned into the pCR 2.1-TOPO vector by TOPO TA cloning (Invitrogen). After confirmation of the sequence by use of an automated DNA sequencer (Applied Biosystems), the hDK1 gene was cloned into the expression vector pYEX-BX (Clontech Laboratories) by using two EcoRI restriction sites flanking the Sec59 sequence (New England Biolabs). After amplification in bacteria (TOP10F [Invitrogen]), the vector constructs were purified, and the sequence of the coding region was verified again. Transformation of yeast cells was done using standard procedures.22
DK1 Assay
The DK1 assay was performed as a modification of the procedure described by Keller et al.23 The reaction mixtures consisted of 100 mM Tris-HCl buffer (pH 7.4), 30 mM CaCl2, 20 mM uridine 5′-triphosphate (UTP), 6 μM CTP (3,000 Ci/mmol specific activity), and 5 μg of dolichol, previously suspended in Triton X-100 (final concentration of 0.1%). The reaction was started by the addition of up to 700 μg of crude cell-extract proteins, to a final volume of 100 μl. The background was determined in samples that were incubated without the addition of dolichol.
The reaction was stopped by the addition of 1,000 μl of chloroform/methanol (2:1 v/v) and was incubated for 30 min at room temperature. After the addition of 190 μl water and mixing, the cup was centrifuged. The chloroform phase was then washed with a chloroform/methanol/water mixture (3:48:47 v/v/v) and was measured afterward by scintillation counting.
To confirm that the arising compound was dolichol phosphate, extracts were also applied to thin-layer chromatography (TLC). The retention-factor (RF) values were compared with a commercially available standard running in parallel.
Results
Clinical Phenotype
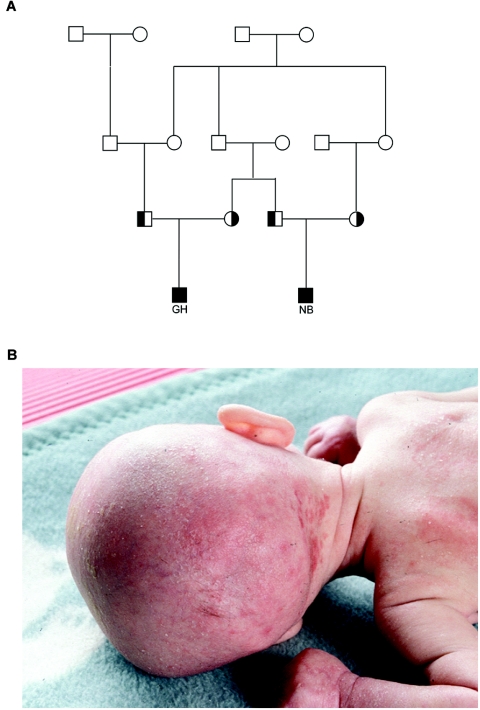
Subject GH was born in 1992 to parents of German origin. His grandparents were first cousins (fig. 1A). At birth, his weight, length, and head circumference were within the normal range. Secondary microcephaly developed within the first months of life. The skin was dry, thin, and parchmentlike. Electron microscopy of a skin biopsy sample revealed hyperkeratosis. Minimal hair growth was noticed. Seizures due to hypsarrhythmia started at age 7 wk. Muscular hypotonia and tetraplegia developed rapidly. Progressive bilateral nystagmus occurred, and reaction to external stimulation became poor. Sonography of the brain showed no abnormalities, and cardiac ultrasound was normal. The patient died at age 8.5 mo.
Figure 1. .
A, Pedigree of the family with the c.295T→A mutation, showing the high degree of consanguinity. Affected patients are indicated by blackened squares, proven heterozygotes by half-blackened symbols. B, Clinical presentation of DK1 deficiency. The 6-mo-old girl (ASB) showed profound muscular hypotonia, inflammation and ichthyosis of the skin, nearly complete secondary loss of hair, and severe DCM.
Subject NB, a first cousin of GH (fig. 1A), was carried to term in 2003. Weight, body length, and head circumference were normal at birth but were below the 3rd percentile at age 5 mo. Several episodes of hypoketotic hypoglycemia with high levels of free fatty acids and normal insulin levels were recorded in the first months of life, which necessitated continuous glucose supplementation via a gastric tube. Results of nuclear magnetic resonance imaging of the brain at age 1 mo were normal. No seizures occurred, and electroencephalogram recordings showed normal patterns. Ichthyosis of the skin was present during the first weeks of life. Death was caused by a pulmonary infection, with respiratory syncytial virus leading to massive cell lysis and cardiac failure at age 6 mo.
Subject ASB was born in 1999 to consanguineous Turkish parents. Ichthyosis congenita with inflammation of the skin was present (fig. 1B). At age 5 mo, progressive hair loss was nearly complete, with sparse eyelashes and eyebrows. Dilative cardiomyopathy (DCM) was present from birth and persisted throughout life. Severe muscular hypotonia was present (see a movie at the Dolichol Kinase Deficiency Web site). Death occurred at home at age 7 mo, most likely from aspiration.
ASB's sister, AYB, was born in 2003. Muscular hypotonia and normal creatine kinase levels were present, and progressive DCM developed shortly after birth. Dry, ichthyosiform skin occurred at the bend of the elbow, the hollow of the knee, and the scalp. At age 4 mo, while being treated in the hospital for diarrhea and vomiting, bradycardia with arterial hypotonia occurred. The girl died after 2 h of attempts to resuscitate.
Gel Electrophoresis of Serum Proteins
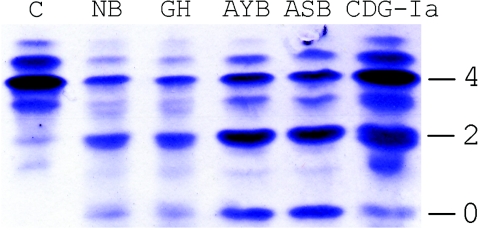
Selective screening for inherited disorders of metabolism was performed in all patients. Investigations included the isoelectric focusing (IEF) of serum transferrin, a screening test for the detection of inherited disorders affecting the N-glycosylation of proteins. Transferrin IEF is abnormal in subjects who abuse alcohol and in subjects with certain inherited metabolic disorders like untreated galactosemia (MIM 230400) or congenital disorders of glycosylation (CDG) (MIM 212065).
The transferrin IEF of all study patients revealed a severe hypoglycosylation, with the majority of the transferrin missing either two (disialo-) or four (asialotransferrin) terminal sialic acids of the carbohydrate sides chains, a pattern typically seen in patients who have CDG-I but that was much more severe in our patients than in most patients with CDG-I (fig. 2). SDS-PAGE of immunoprecipitated transferrin revealed the presence of two additional bands. These bands differed from each other in their apparent molecular weights by ∼2 kDa, suggesting the absence or severe truncation of one or both of the carbohydrate side chains (not shown).
Figure 2. .
Transferrin IEF, which shows increased amounts of disialo- and asialotransferrin in the patients. Numbers designate the number of carbohydrate side chains.
Analysis of Structural Integrity and Quantity of LLOs
N-glycan biosynthesis is a complex multistep process that begins in the ER. The stepwise addition of sugar molecules from activated-sugar nucleotide donors leads to a final oligosaccharide of two N-acetylglucosamine (GlcNAc), nine mannose (Man), and three glucose (Glc) residues residing on the polyisopren-carrier molecule dolichol. The carbohydrate portion of these LLOs is transferred to the nascent protein by a protein complex called “oligosaccharyltransferase.” After transfer to the nascent chain, the oligosaccharides are further processed during the passage through the ER and the Golgi apparatus.
The structural integrity of the LLOs was investigated by labeling the patients’ fibroblasts with [3H]-mannose. After the extraction of the LLOs by a mixture of chloroform/methanol/water (10:10:3), extracts were applied to size fractionation by HPLC. No major structural abnormalities could be detected in the patients’ fibroblasts (data not shown).
In addition, LLOs from fibroblasts labeled with glucosamine were prepared and separated on a TLC plate, to analyze the first two steps in the LLO biosynthesis. No structural differences could be detected in comparison with control cells (data not shown).
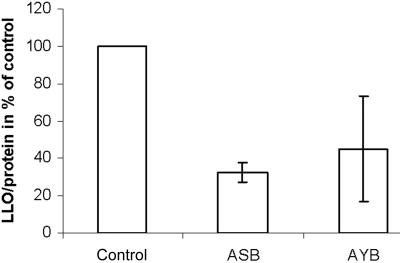
Since no structural LLO abnormalities could be detected but complete chains appeared to be missing on transferrin, the amount of LLO was determined in the patients’ fibroblasts. Severely reduced amounts of newly synthesized LLO related to total cellular protein were detected in the patients’ fibroblasts (fig. 3).
Figure 3. .
Incorporation of [3H]-glucosamine into LLOs. Fibroblasts were labeled for 30 min with 100 mCi [6-3H]glucosamine per ml labeling medium, and dolichol-linked oligosaccharides were extracted as described. Incorporation of [3H]-glucosamine was related to total protein. Controls were set to 100%.
Sequencing the hDK1 Gene
Since no apparent problem could be detected during the synthesis of the complete LLO structure or the transfer of the LLOs to protein, the biosynthesis of dolichol phosphate was characterized subsequently. We started by analyzing the last enzyme involved in dolichol phosphate biosynthesis. The mutation analysis of the hDK1 gene of our patients revealed two mutations. Both were found in a homozygous state. In patients NB and GH, the mutation c.295T→A (99Cys→Ser) was detected, whereas patients ASB and AYB were homozygous for a c.1322A→C (441Tyr→Ser) base-pair exchange. The parents were heterozygous for the mutations detected in their children. To exclude common polymorphisms, 240 alleles from members of the same ethnic groups were analyzed, without the detection of any variability at the specific positions.
DK1 Assay
The human hDK1 gene product phosphorylates dolichol in a CTP-dependent manner. The DK1 assay described by Keller et al.23 was modified to measure the activity of this enzyme in crude cell extracts of human fibroblasts, as described in the “Material and Methods” section.
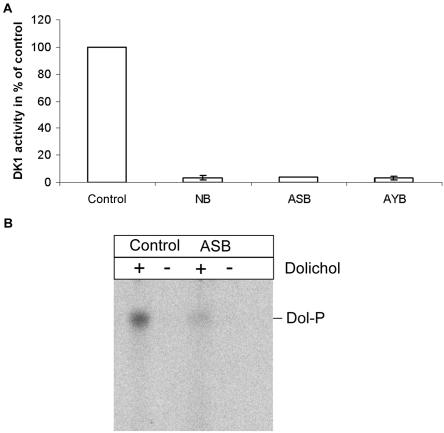
The crude extracts gained from patient fibroblasts showed a remarkably lower enzyme activity than extracts from control cells. In comparison with controls, the decrease in activity was 94.5%–98.6% for all patients (fig. 4A).
Figure 4. .
DK1 assay of patient fibroblasts. The reaction mixtures (A) consisted of 100 mM Tris-HCl buffer (pH 7.4), 30 mM CaCl2, 20 mM UTP, 6 μM CTP (3,000 Ci/mmol–specific activity), and 5 μg of dolichol, previously suspended in Triton X-100 (0.1% final concentration). The reaction was started by the addition of up to 700 μg of crude cell extracts, to a final volume of 100 μl, and was stopped after 20 min by the addition of 800 μl of chloroform/methanol (2:1 v/v). After dolichol phosphate (Dol-P) was extracted, the samples were dried under nitrogen and were measured in a scintillation counter. The activity of the patients' crude extracts were severely reduced (residual activity [±SD]: NB 3.2%±1.8%; ASB 4%; AYB 3.9%±1.6%) compared with control cell lines. B, Assay samples, separated by TLC. Pig Dol-P was used as standard.
To make sure that the arising compound was dolichol phosphate, extracts were also applied to thin-layer chromatography. The RF values were compared with a commercially available standard running in parallel (fig. 4B).
Complementation of Sec59-Deficient Yeast Strain PRY134
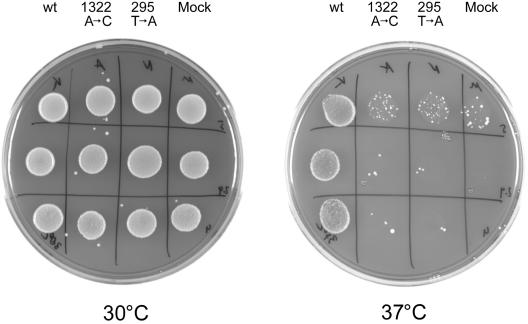
The temperature-sensitive yeast strain PRY134 is deficient for the Sec59 gene product at the restrictive temperature. As a result, the cells show a temperature-sensitive phenotype. PRY134 cells stop dividing and become enlarged at the restrictive temperature of 37°C, whereas the growth at 30°C is comparable to that of wild-type strains. It was shown recently that the temperature-sensitive growth phenotype of those yeast cells could be complemented by the overexpression of the human hDK1 gene.20
To analyze the functional relevance of the mutations found in our patients, both mutated alleles and the human wild-type sequence were cloned into a yeast expression vector and were transformed into PRY134 cells. Mock transformation was performed, with the expression vector bearing no insert.
At the permissive temperature (30°C), all cells showed similar growth characteristics. After the incubation of the cells at the restrictive temperature (37°C), the mock-transfected cells showed a severely reduced growth.
As shown before, the human wild-type allele of the hDK1 gene was able to complement the temperature-sensitive growth phenotype. Both mutated alleles (c.295T→A [99Cys→Ser] and c.1322A→C [441Tyr→Ser]) showed only a slight complementation in yeast, demonstrating the functional relevance of both mutations (fig. 5).
Figure 5. .
Growth characteristics of transformed Sec59 yeast cells, showing 10-fold dilutions from top to bottom. Pictures were taken after 1 wk at the permissive temperature (30°C) (left) or at the restrictive temperature (37°C) (right). From left to right in both panels: cells transformed with human wild-type allele (wt), cells transformed with the c.1322A→C allele (for subjects ASB and AYB), cells transformed with the c.295T→A allele (for subjects NB and GH), and mock-transformed cells.
Discussion
We describe the first known human disorder affecting the synthesis of dolichol phosphate. Four patients from two unrelated families with a defect in the hDK1 gene presented with a severe phenotype, with death in early infancy.
All patients showed a remarkable loss of oligosaccharide structures on serum transferrin, as shown by IEF and immunoprecipitation of the protein, implicating a disorder affecting N-glycosylation. The analysis of LLOs showed no structural abnormalities of the N-glycans assembled on dolichol but did show a severely reduced amount of total LLOs. Therefore, the biosynthetic pathway of dolichol phosphate was analyzed.
At least eight dolichol phosphate molecules are required for the assembly of one N-glycan, one as the carrier of the N-glycan and seven more for the import of mannose and glucose into the lumen of the ER.24 It is well established that the availability of dolichol phosphate is one of the rate-limiting factors in controlling the synthesis of LLOs in eukarytic organisms.25,26 Dolichol phosphate is the substrate for several enzymes in the glycosylation pathway. GlcNAc-1-P transferase—which transfers the first GlcNAc residue to dolichol phosphate—as well as mannose-P-dolichol synthase and glucose-P-dolichol synthase is dependent on a sufficient supply of free dolichol phosphate.27
The arising metabolites like mannose-P-dolichol are themselves substrates for a number of downstream glycosylation reactions, such as N-glycosylation, GPI-anchor biosynthesis, and C- and O-mannosylation. Therefore, it is not surprising that a defect of DK1 in humans has severe effects on the affected individuals.
The final step during the de novo synthesis of dolichol phosphate is the phosphorylation of dolichol by DK1. Sequencing of the corresponding gene revealed homozygosity for either one of the two detected mutations (c.295T→A [99Cys→Ser] or c.1322A→C [441Tyr→Ser]) in the affected individuals.
Subsequently, the dolichol-kinase activity measured in crude fibroblast extracts from patient fibroblasts showed a decrease in activity to 2%–5%, in comparison with extracts from control cells. None of the patients' DK1 alleles was able to complement the temperature-dependent growth phenotype of DK1-deficient yeast cells, whereas the wild-type human gene completely restored the yeast growth at 37°C. Both the enzyme and the complementation assay demonstrate the functional relevance of both mutations.
Although eight dolichol phosphate molecules are needed for the synthesis of one N-glycan, no truncated LLO structures were detected. It has been suggested that two pools of dolichol phosphate exist in the membrane of the ER of eukaryotic cells: one limited pool of dolichol phosphate that is directly involved in glycosylation and a second pool that does not seem to participate in processes mentioned previously.28,29 It has been speculated that a limited pool of dolichol phosphate exists in “rafts” within the membrane. In this model, dolichol is bound by the so-called potential dolichol-recognition sequences that can be found in various glycosyltransferases. The limited pool of dolichol phosphate seems to be replenished by recycling reactions. Dolichol phosphate used in the LLO pool is released after the transfer of the carbohydrate portion to protein as dolichol-PP. Dolichol phosphate is regenerated by the pyrophosphatase CWH8/Dolpp1.30 In addition, all glycosylation reactions involving dolichol-P-mannose or dolichol-P-glucose release dolichol phosphate. It may well be that the residual activity of DK1 in fibroblasts is sufficient to fill this highly recycled and limited pool of dolichol phosphate initially and therefore guarantees a normal LLO pattern. Another reason for the structural integrity of the LLO might be differences in the Km values of the involved enzymes. If the GlcNAc-1-phosphotransferase reaction—the first enzymatic reaction with use of dolichol phosphate as a substrate—is the rate-limiting step for the completion of the LLO or if the enzyme would have a considerably higher Km for dolichol phosphate than the dolichol-P-mannose and dolichol-P-glucose synthases, no structural deficiencies would be detectable.
Severe defects of N-glycosylation (e.g., CDG) are not compatible with life,31 and the mechanism by which different molecular defects result in the very heterogeneous pattern of biochemical and clinical problems is poorly understood.24 In the patients with DK1 deficiency, another pathogenetic mechanism might be of interest. The theory of two widely independent dolichol phosphate pools suggests the possibility that some problems are not due to glycosylation defects but are directly caused by a reduction of the secondary dolichol phosphate pool. It has been shown that dolichol phosphate has a remarkable influence on membrane fluidity and structure, and it has been speculated that dolichol phosphate might influence the formation of nonbilayer structures and that it therefore facilitates membrane-fusion processes.32,33
DK1 deficiency is characterized by a very severe clinical phenotype. All affected children in our study died within the 1st year of life. DCM was the life-limiting factor in two of the DK1-deficient patients. DCM is a rare symptom of metabolic diseases in infancy, with a cumulative incidence of 4–5 in 100,000 children of this age group.34
Autosomal dominant familial DCM has been found to be caused by mutations in the genes for beta-myosin heavy chain (MIM *160760), cardiac troponin T (MIM *191045), or α-tropomyosin (MIM *191010). In these disorders, DCM has a variable time frame of manifestation, but infants have been detected with a cardiac disease on family investigations.35 Of inherited forms of DCM, 90% are reported to follow an autosomal dominant inheritance, and 5%–10% are X linked.35 Autosomal recessive inheritance, as reported here, has hardly been recognized as a cause of DCM so far. Further insights into the pathology of DCM might be gained by investigating the roles of dolichol phosphate and glycosylation in the onset of DK1 deficiency.
DK1 deficiency is the first member of a newly discovered group of metabolic disorders caused by defects in dolichol phosphate biosynthesis. Although some aspects of the clinical phenotype of these patients might be caused by something other than glycosylation-related deficiencies, most known affected pathways are involved in glycosylation processes of different kinds. The patients are of consanguineous origin, so the possibility of multiple recessive conditions coexisting in one or more of the patients cannot be completely excluded and could account for some of the clinical disparities observed. Since this metabolic disorder has been detected by the IEF of serum transferrin, the disorder could be included in the group of CDG with the name “CDG-Im” until a more satisfying nomenclature is available. When the complexity of the dolichol phosphate biosynthesis pathway is considered, the discovery in the near future of other disorders in the same pathway can be expected.
Acknowledgments
Sec59-deficient yeast were a kind gift provided by C. J. Waechter, University of Kentucky, College of Medicine, Lexington. We are very grateful to Dr. William B. Rizzo, Department of Pediatrics, University of Nebraska Medical Center, Omaha, for taking good care of fibroblasts from one of the patients, GH, for so many years, enabling us to investigate them and to establish the diagnosis 12 years after the death of the patient. We are deeply moved by the parents of ASB and AYB, who took care of their children with incredible love and affection, and we are deeply indebted to the mothers of NB and GH, who provided pictures and many details about their children. T. Stölting is acknowledged for help with the sequencing of healthy control individuals. We are thankful to Bobby Ng for proofreading the manuscript. C.K. was supported by Deutsche Forschungs-Gemeinschaft grant KR 2916/1-1, and T.M. was supported by grants from Innovative Medizinische Forschung.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- Dolichol Kinase Deficiency, http://cdg.klinikum.uni-muenster.de/dolicholkinase.html
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for hDK1 at the genomic level [accession number NC_000009] and the coding region [accession number NM_014908)])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for galactosemia, CDG, beta-myosin heavy chain, cardiac troponin T, and α-tropomyosin)
References
- 1.Schenk B, Fernandez F, Waechter CJ (2001) The ins(ide) and out(side) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology 11:61R–70R 10.1093/glycob/11.5.61R [DOI] [PubMed] [Google Scholar]
- 2.Scher MG, Waechter CJ (1984) Brain dolichyl pyrophosphate phosphatase: solubilization, characterization, and differentiation from dolichyl monophosphate phosphatase activity. J Biol Chem 259:14580–14585 [PubMed] [Google Scholar]
- 3.Troy FA 2nd (1979) The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol 33:519–560 10.1146/annurev.mi.33.100179.002511 [DOI] [PubMed] [Google Scholar]
- 4.Doucey MA, Hess D, Cacan R, Hofsteenge J (1998) Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol Biol Cell 9:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda J, Kinoshita T (1995) GPI-anchor biosynthesis. Trends Biochem Sci 20:367–371 10.1016/S0968-0004(00)89078-7 [DOI] [PubMed] [Google Scholar]
- 6.Kornfeld R, Kornfeld S (1985) Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 54:631–664 10.1146/annurev.bi.54.070185.003215 [DOI] [PubMed] [Google Scholar]
- 7.Quellhorst GJ Jr, Piotrowski JS, Steffen SE, Krag SS (1998) Identification of Schizosaccharomyces pombe prenol as dolichol-16,17. Biochem Biophys Res Commun 244:546–550 10.1006/bbrc.1998.8098 [DOI] [PubMed] [Google Scholar]
- 8.Rip JW, Rupar CA, Ravi K, Carroll KK (1985) Distribution, metabolism and function of dolichol and polyprenols. Prog Lipid Res 24:269–309 10.1016/0163-7827(85)90008-6 [DOI] [PubMed] [Google Scholar]
- 9.Lechner J, Wieland F, Sumper M (1985) Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose: purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem 260:860–866 [PubMed] [Google Scholar]
- 10.Lechner J, Wieland F (1989) Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem 58:173–194 10.1146/annurev.bi.58.070189.001133 [DOI] [PubMed] [Google Scholar]
- 11.Crick DC, Rush JS, Waechter CJ (1991) Characterization and localization of a long-chain isoprenyltransferase activity in porcine brain: proposed role in the biosynthesis of dolichyl phosphate. J Neurochem 57:1354–1362 10.1111/j.1471-4159.1991.tb08301.x [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Tsuji M, Nakanishi Y, Suzuki S (1980) Enzymatic dephosphorylation of dolichyl pyrophosphate—the bacitracin-sensitive, rate-limiting step for dolichyl mannosyl phosphate synthesis in rat liver microsomes. Biochem Biophys Res Commun 95:770–776 10.1016/0006-291X(80)90853-0 [DOI] [PubMed] [Google Scholar]
- 13.Wolf MJ, Rush JS, Waechter CJ (1991) Golgi-enriched membrane fractions from rat brain and liver contain long-chain polyisoprenyl pyrophosphate phosphatase activity. Glycobiology 1:405–410 10.1093/glycob/1.4.405 [DOI] [PubMed] [Google Scholar]
- 14.Sagami H, Kurisaki A, Ogura K (1993) Formation of dolichol from dehydrodolichol is catalyzed by NADPH-dependent reductase localized in microsomes of rat liver. J Biol Chem 268:10109–10113 [PubMed] [Google Scholar]
- 15.Allen CM Jr, Kalin JR, Sack J, Verizzo D (1978) CTP-dependent dolichol phosphorylation by mammalian cell homogenates. Biochemistry 17:5020–5026 10.1021/bi00616a025 [DOI] [PubMed] [Google Scholar]
- 16.Burton WA, Scher MG, Waechter CJ (1979) Enzymatic phosphorylation of dolichol in central nervous tissue. J Biol Chem 254:7129–7136 [PubMed] [Google Scholar]
- 17.Bernstein M, Kepes F, Schekman R (1989) Sec59 encodes a membrane protein required for core glycosylation in Saccharomyces cerevisiae. Mol Cell Biol 9:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heller L, Orlean P, Adair WL Jr (1992) Saccharomyces cerevisiae sec59 cells are deficient in dolichol kinase activity. Proc Natl Acad Sci USA 89:7013–7016 10.1073/pnas.89.15.7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferro-Novick S, Novick P, Field C, Schekman R (1984) Yeast secretory mutants that block the formation of active cell surface enzymes. J Cell Biol 98:35–43 10.1083/jcb.98.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez F, Shridas P, Jiang S, Aebi M, Waechter CJ (2002) Expression and characterization of a human cDNA that complements the temperature-sensitive defect in dolichol kinase activity in the yeast sec59-1 mutant: the enzymatic phosphorylation of dolichol and diacylglycerol are catalyzed by separate CTP-mediated kinase activities in Saccharomyces cerevisiae. Glycobiology 12:555–562 10.1093/glycob/cwf068 [DOI] [PubMed] [Google Scholar]
- 21.Kranz C, Denecke J, Lehrman MA, Ray S, Kienz P, Kreissel G, Sagi D, Peter-Katalinic J, Freeze HH, Schmid T, et al (2001) A mutation in the human MPDU1 gene causes congenital disorder of glycosylation type If (CDG-If). J Clin Invest 108:1613–1619 10.1172/JCI200113635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96 [DOI] [PubMed] [Google Scholar]
- 23.Keller RK, Rottler GD, Cafmeyer N, Adair WL Jr (1982) Subcellular localization and substrate specificity of dolichol kinase from rat liver. Biochim Biophys Acta 719:118–125 [DOI] [PubMed] [Google Scholar]
- 24.Marquardt T, Denecke J (2003) Congenital disorders of glycosylation: review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr 162:359–379 [DOI] [PubMed] [Google Scholar]
- 25.Rosenwald AG, Stoll J, Krag SS (1990) Regulation of glycosylation: three enzymes compete for a common pool of dolichyl phosphate in vivo. J Biol Chem 265:14544–14553 [PubMed] [Google Scholar]
- 26.Spiro MJ, Spiro RG (1986) Control of N-linked carbohydrate unit synthesis in thyroid endoplasmic reticulum by membrane organization and dolichyl phosphate availability. J Biol Chem 261:14725–14732 [PubMed] [Google Scholar]
- 27.Burda P, Aebi M (1998) The ALG10 locus of Saccharomyces cerevisiae encodes the alpha-1,2 glucosyltransferase of the endoplasmic reticulum: the terminal glucose of the lipid-linked oligosaccharide is required for efficient N-linked glycosylation. Glycobiology 8:455–462 10.1093/glycob/8.5.455 [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Lehrman MA (2002) Coupling of the dolichol-P-P-oligosaccharide pathway to translation by perturbation-sensitive regulation of the initiating enzyme, GlcNAc-1-P transferase. J Biol Chem 277:39425–39435 10.1074/jbc.M205195200 [DOI] [PubMed] [Google Scholar]
- 29.Hubbard SC, Robbins PW (1980) Synthesis of the N-linked oligosaccharides of glycoproteins: assembly of the lipid-linked precursor oligosaccharide and its relation to protein synthesis in vivo. J Biol Chem 255:11782–11793 [PubMed] [Google Scholar]
- 30.Fernandez F, Rush JS, Toke DA, Han GS, Quinn JE, Carman GM, Choi JY, Voelker DR, Aebi M, Waechter CJ (2001) The CWH8 gene encodes a dolichyl pyrophosphate phosphatase with a luminally oriented active site in the endoplasmic reticulum of Saccharomyces cerevisiae. J Biol Chem 276:41455–41464 10.1074/jbc.M105544200 [DOI] [PubMed] [Google Scholar]
- 31.Marek KW, Vijay IK, Marth JD (1999) A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology 9:1263–1271 10.1093/glycob/9.11.1263 [DOI] [PubMed] [Google Scholar]
- 32.Valtersson C, van Duyn G, Verkleij AJ, Chojnacki T, de Kruijff B, Dallner G (1985) The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem 260:2742–2751 [PubMed] [Google Scholar]
- 33.van Duijn G, Valtersson C, Chojnacki T, Verkleij AJ, Dallner G, de Kruijff B (1986) Dolichyl phosphate induces non-bilayer structures, vesicle fusion and transbilayer movement of lipids: a model membrane study. Biochim Biophys Acta 861:211–223 10.1016/0005-2736(86)90423-2 [DOI] [PubMed] [Google Scholar]
- 34.Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, Wilkinson LC, Davis AM, Kahler SG, Chow CW, Wilkinson JL, et al (2003) The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 348:1639–1646 10.1056/NEJMoa021737 [DOI] [PubMed] [Google Scholar]
- 35.Burkett EL, Hershberger RE (2005) Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 45:969–981 10.1016/j.jacc.2004.11.066 [DOI] [PubMed] [Google Scholar]