Abstract
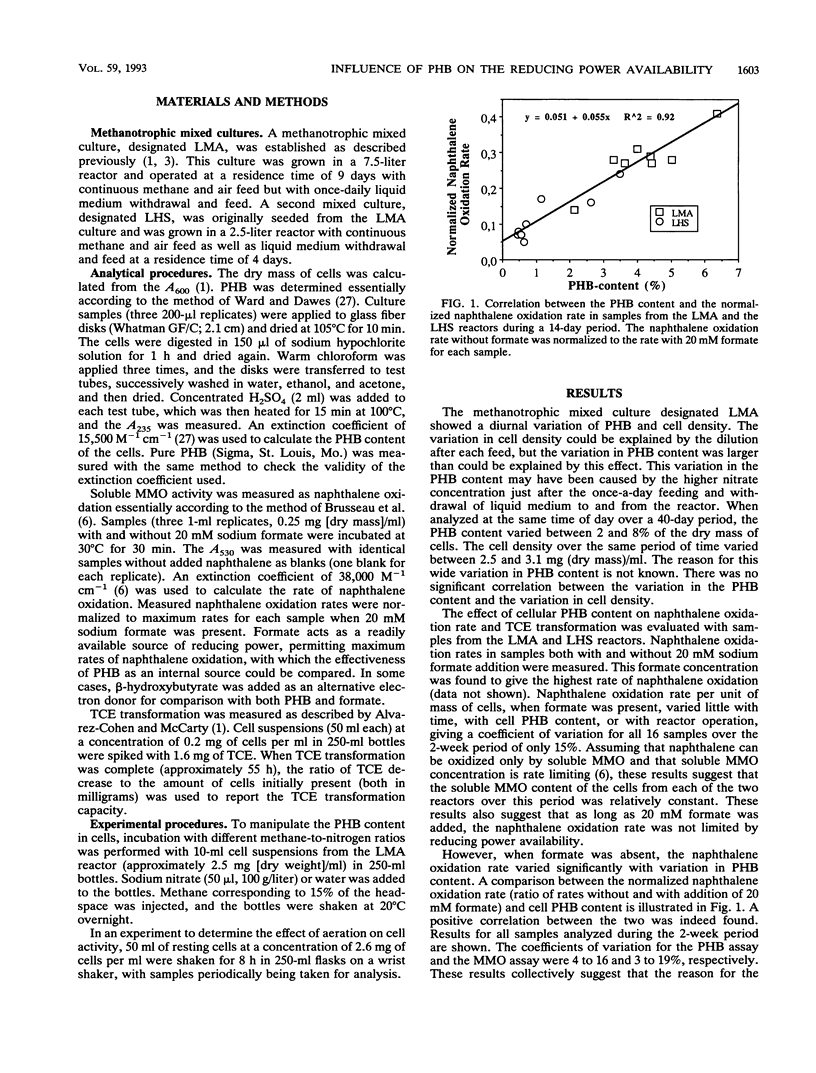
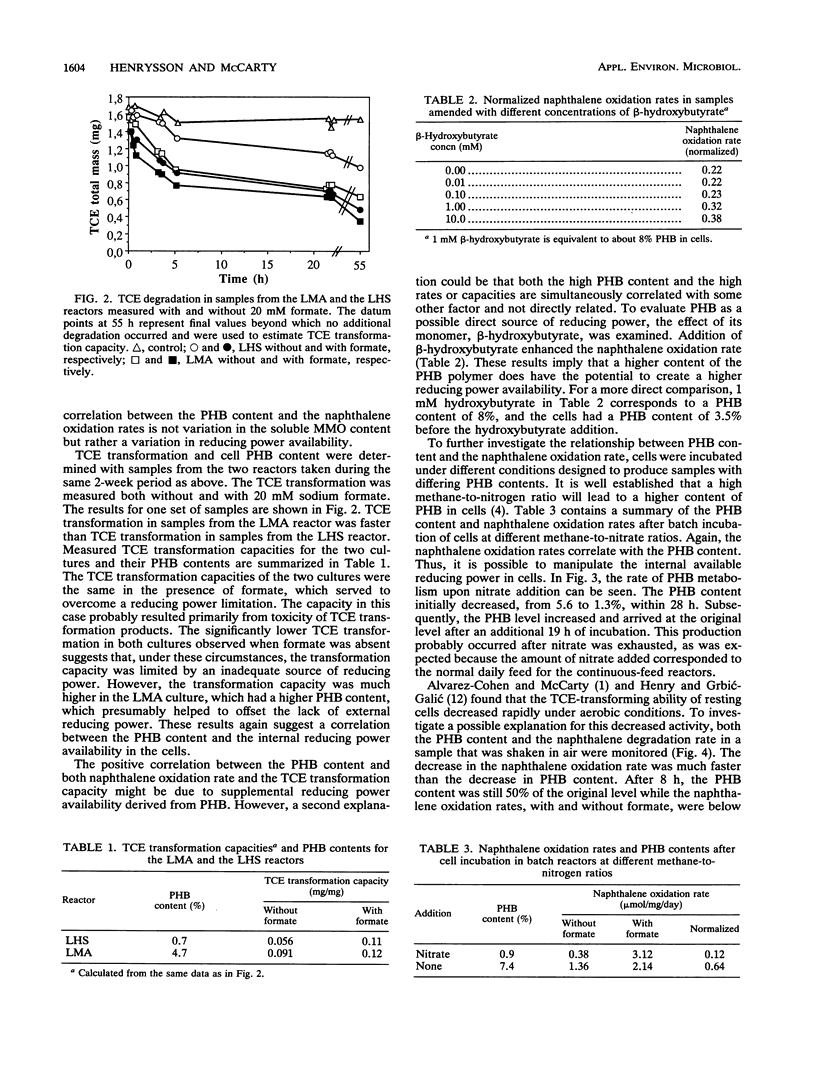
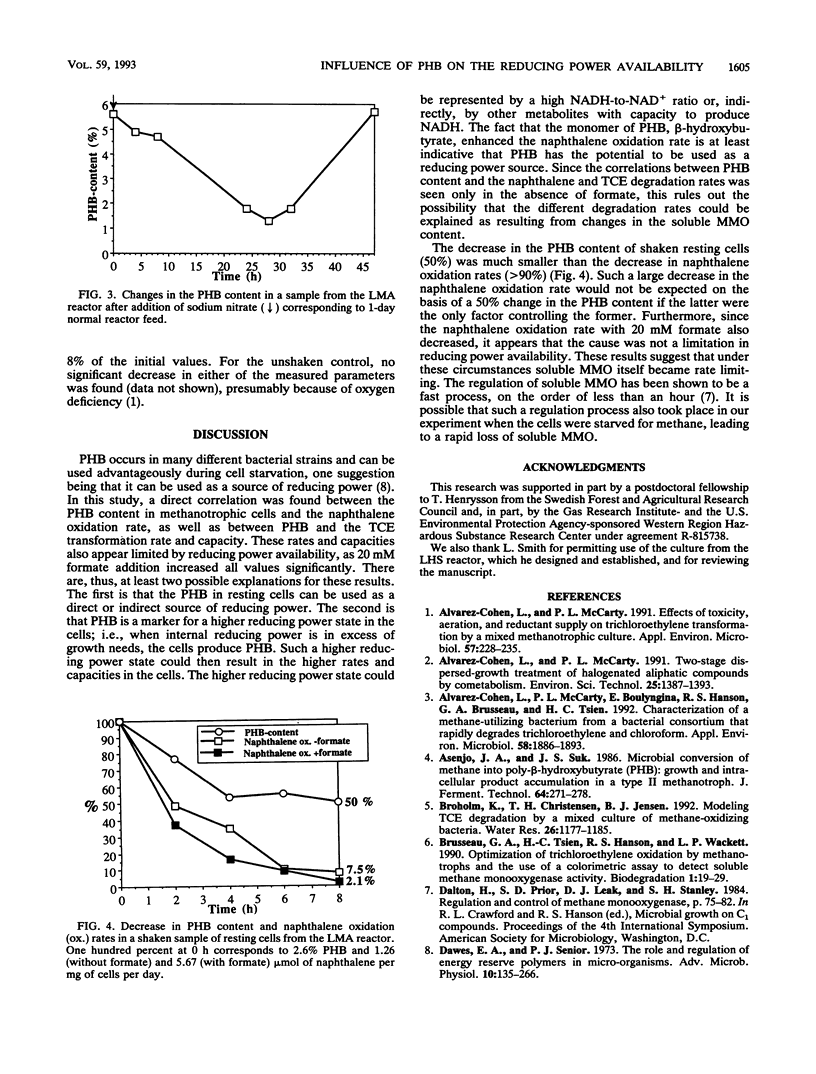
The role of the storage lipid poly-β-hydroxybutyrate (PHB) in trichloroethylene transformation by methanotrophic mixed cultures was investigated. Naphthalene oxidation rates were used to assay for soluble methane monooxygenase activity. The PHB content of methanotrophic cells grown in reactors varied diurnally as well as from day to day. A positive correlation between the amount of PHB in the cells and the naphthalene oxidation rate as well as between PHB and the trichloroethylene transformation rate and capacity was found. Addition of β-hydroxybutyrate increased the naphthalene oxidation rates significantly. PHB content in cells could be manipulated by incubation at different methane-to-nitrogen ratios. A positive correlation between the naphthalene oxidation rate and the PHB content after these incubations could be seen. Both the PHB content and the naphthalene oxidation rates decreased with time in resting methanotrophic cells exposed to oxygen. However, this decrease in the naphthalene oxidation rate cannot be explained by the decrease in the PHB content alone. Probably a deactivation of the methane monooxygenase itself is also involved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Cohen L., McCarty P. L., Boulygina E., Hanson R. S., Brusseau G. A., Tsien H. C. Characterization of a methane-utilizing bacterium from a bacterial consortium that rapidly degrades trichloroethylene and chloroform. Appl Environ Microbiol. 1992 Jun;58(6):1886–1893. doi: 10.1128/aem.58.6.1886-1893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusseau G. A., Tsien H. C., Hanson R. S., Wackett L. P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1(1):19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Fogel M. M., Taddeo A. R., Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986 Apr;51(4):720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- Henry S. M., Grbić-Galić D. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl Environ Microbiol. 1991 Jan;57(1):236–244. doi: 10.1128/aem.57.1.236-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLIO R. E., HARRINGTON A. A. Sudanophilic granules and lipid of Pseudomonas methanica. J Bacteriol. 1960 Sep;80:321–324. doi: 10.1128/jb.80.3.321-324.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldhuis C., Stegeman V., Veenhuis M. Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-beta-hydroxybutyric acid and its role in starvation. J Gen Microbiol. 1979 Jun;112(2):349–355. doi: 10.1099/00221287-112-2-349. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Oxidation of trichloroethylene by liver microsomal cytochrome P-450: evidence for chlorine migration in a transition state not involving trichloroethylene oxide. Biochemistry. 1982 Mar 2;21(5):1090–1097. doi: 10.1021/bi00534a041. [DOI] [PubMed] [Google Scholar]
- Oldenhuis R., Oedzes J. Y., van der Waarde J. J., Janssen D. B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991 Jan;57(1):7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., GOTTSCHALK G., VON BARTHA R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature. 1961 Jul 29;191:463–465. doi: 10.1038/191463a0. [DOI] [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Dawes E. A. A disk assay for poly- -hydroxybutyrate. Anal Biochem. 1973 Apr;52(2):607–613. doi: 10.1016/0003-2697(73)90067-5. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson B. H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985 Jan;49(1):242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



