Abstract
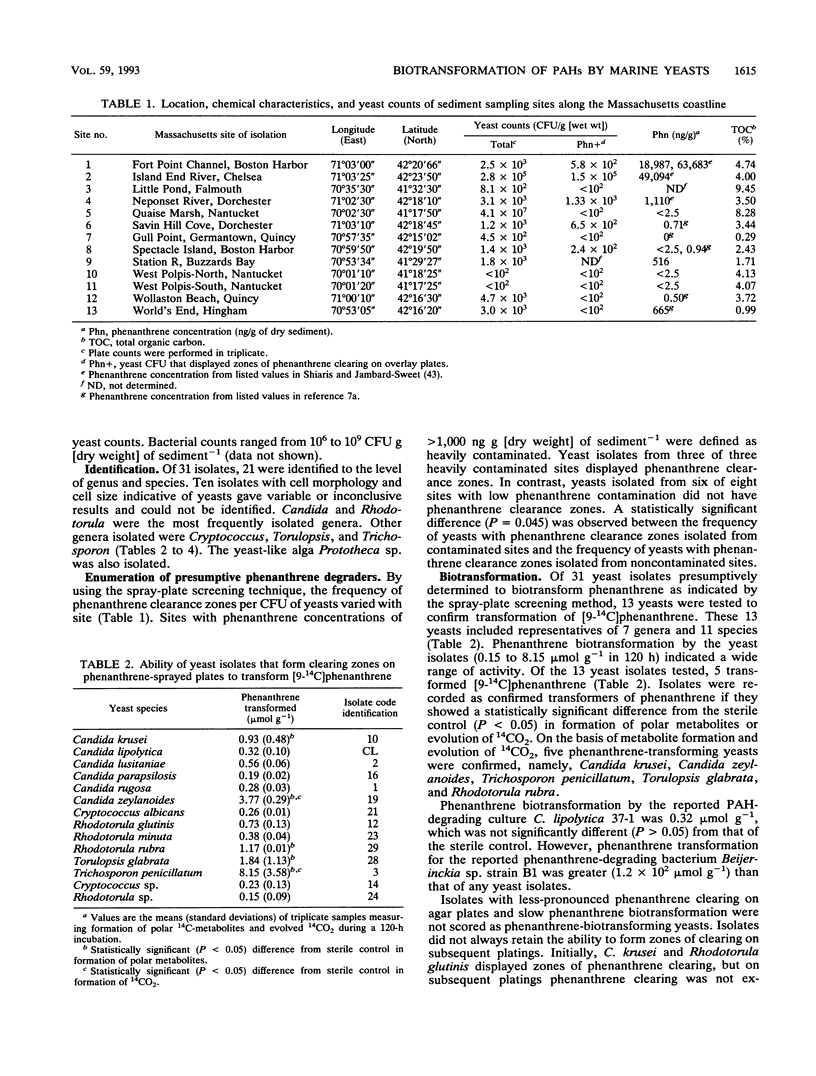
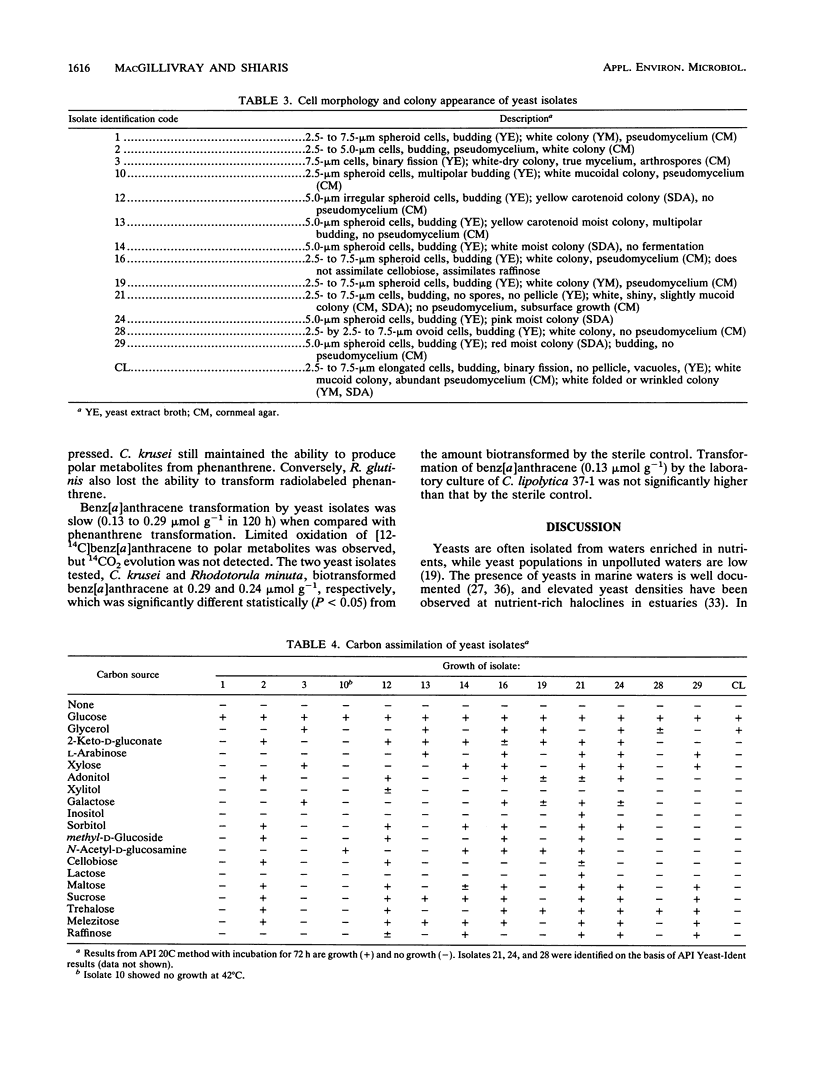
Yeast abundance in the sediments of 13 coastal sites in Massachusetts was quantified, and the potential of yeast isolates to biotransform polycyclic aromatic hydrocarbons (PAHs) was determined. Plate counts of yeasts varied between 10(2) to 10(7) CFU g (dry weight) of sediment-1. The most abundant genera isolated and identified included Candida, Cryptococcus, Rhodotorula, Torulopsis, and Trichosporon. More than 50% of the isolates from heavily contaminated sites transformed phenanthrene, as determined by spray-plate screening. The plate counts of phenanthrene-transforming yeasts correlated significantly to the sediment concentrations of phenanthrene. Transformation of [9-14C]phenanthrene and [12-14C]benz[a]anthracene by individual isolates varied greatly, ranging from 0.15 to 8.15 mumol of PAH g-1 in 120-h incubations. Of the isolated yeasts, Trichosporon penicillatum exhibited the greatest capacity for phenanthrene transformation. The ability to transform PAHs appears to be widespread among yeasts in coastal sediments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer J. E., Capone D. G. Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments. Appl Environ Microbiol. 1985 Jul;50(1):81–90. doi: 10.1128/aem.50.1.81-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücker M., Glatt H. R., Platt K. L., Avnir D., Ittah Y., Blum J., Oesch F. Mutagenicity of phenanthrene and phenanthrene K-region derivatives. Mutat Res. 1979 Apr;66(4):337–348. doi: 10.1016/0165-1218(79)90044-2. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Campbell W. L., Freeman J. P., Evans F. E. Identification of a novel metabolite in phenanthrene metabolism by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1989 Sep;55(9):2275–2279. doi: 10.1128/aem.55.9.2275-2279.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., White G. L., Heflich R. H. Fungal metabolism and detoxification of polycyclic aromatic hydrocarbons. Arch Microbiol. 1985 Nov;143(2):105–110. doi: 10.1007/BF00411031. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Yang S. K. Stereoselective metabolism of anthracene and phenanthrene by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1984 Jan;47(1):119–124. doi: 10.1128/aem.47.1.119-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale S. W., Dhawale S. S., Dean-Ross D. Degradation of phenanthrene by Phanerochaete chrysosporium occurs under ligninolytic as well as nonligninolytic conditions. Appl Environ Microbiol. 1992 Sep;58(9):3000–3006. doi: 10.1128/aem.58.9.3000-3006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Hambrick G. A., Delaune R. D., Patrick W. H. Effect of Estuarine Sediment pH and Oxidation-Reduction Potential on Microbial Hydrocarbon Degradation. Appl Environ Microbiol. 1980 Aug;40(2):365–369. doi: 10.1128/aem.40.2.365-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Gai W. Z., Green B., Moen M. A. Oxidative degradation of phenanthrene by the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jun;58(6):1832–1838. doi: 10.1128/aem.58.6.1832-1838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. H. Oxidation of naphthalene by Saccharomyces cerevisiae and Candida utilis. J Basic Microbiol. 1986;26(2):109–111. doi: 10.1002/jobm.3620260213. [DOI] [PubMed] [Google Scholar]
- Pinto A. S., Bomfim M. E., Hagler A. N., Angluster J. Metabolism of aromatic compounds by yeasts isolated from a polluted estuary in Rio de Janeiro, Brazil. Rev Bras Pesqui Med Biol. 1979 Sep;12(4-5):339–346. [PubMed] [Google Scholar]
- Pore R. S. Selective medium for the isolation of Prototheca. Appl Microbiol. 1973 Oct;26(4):648–649. doi: 10.1128/am.26.4.648-649.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiaris M. P., Cooney J. J. Replica plating method for estimating phenanthrene-utilizing and phenanthrene-cometabolizing microorganisms. Appl Environ Microbiol. 1983 Feb;45(2):706–710. doi: 10.1128/aem.45.2.706-710.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiaris M. P. Seasonal Biotransformation of Naphthalene, Phenanthrene, and Benzo[a]pyrene in Surficial Estuarine Sediments. Appl Environ Microbiol. 1989 Jun;55(6):1391–1399. doi: 10.1128/aem.55.6.1391-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992 Jan;9(1):53–61. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- Sutherland J. B., Selby A. L., Freeman J. P., Evans F. E., Cerniglia C. E. Metabolism of phenanthrene by Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Nov;57(11):3310–3316. doi: 10.1128/aem.57.11.3310-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


