Abstract
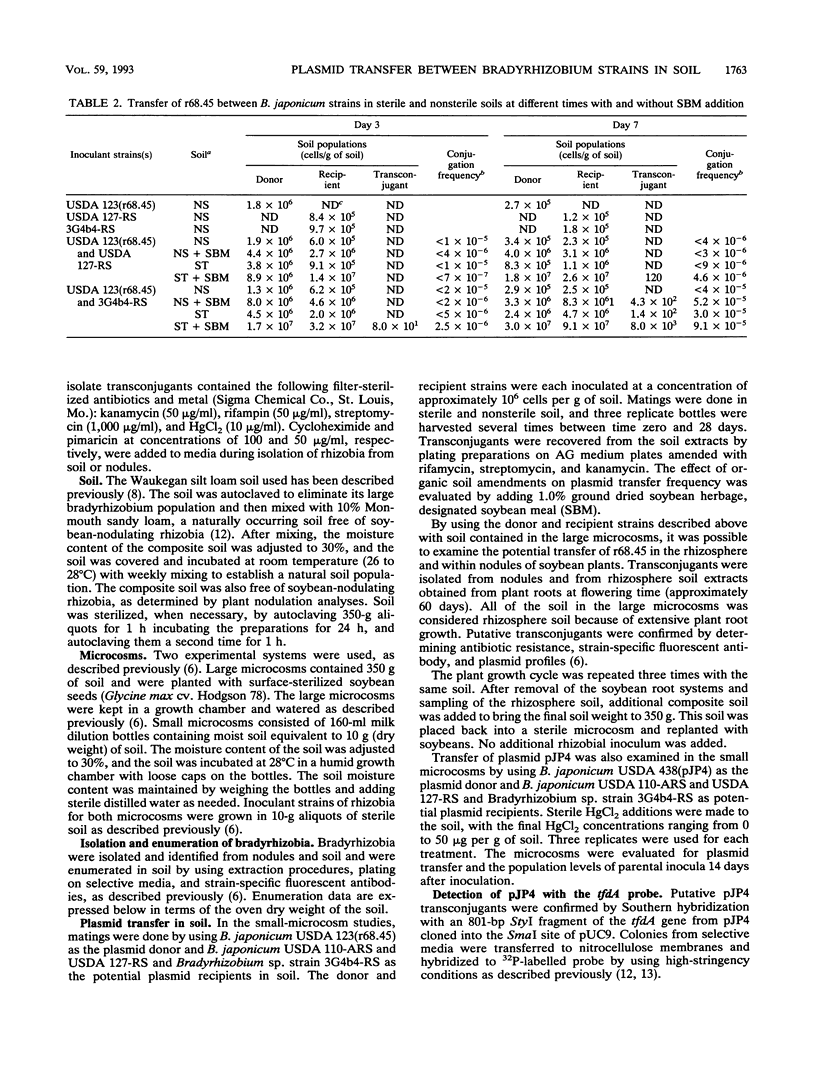
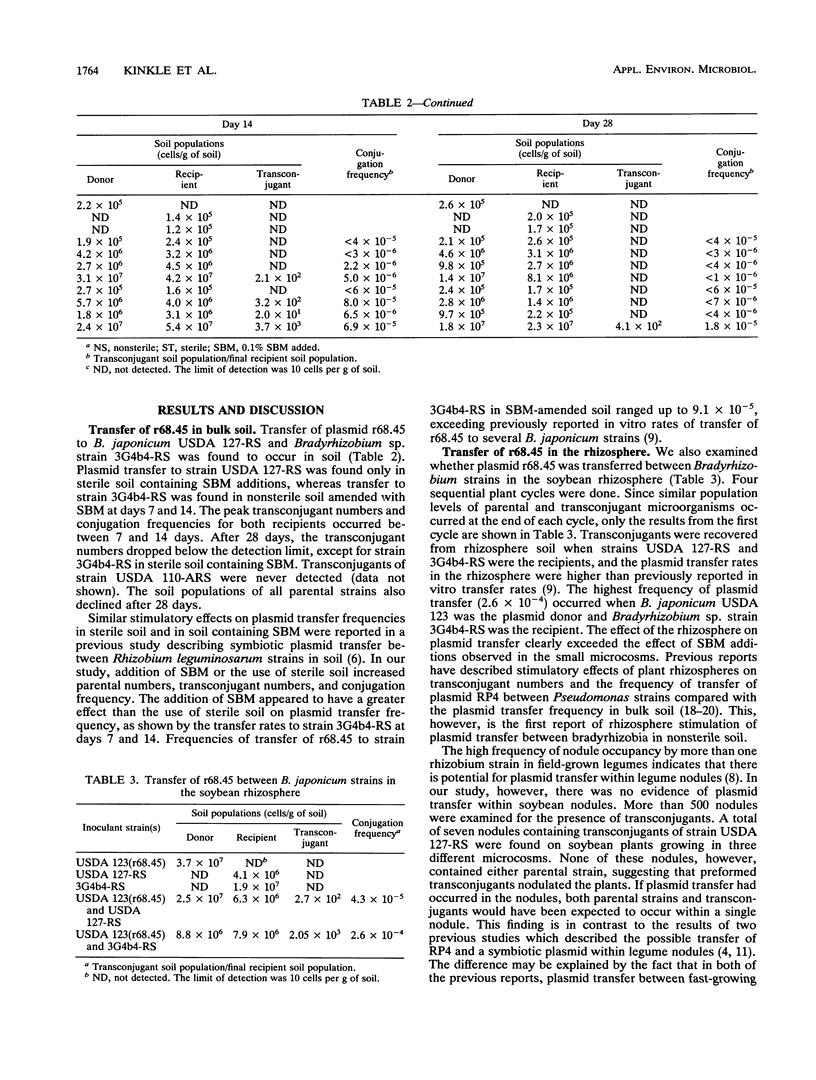
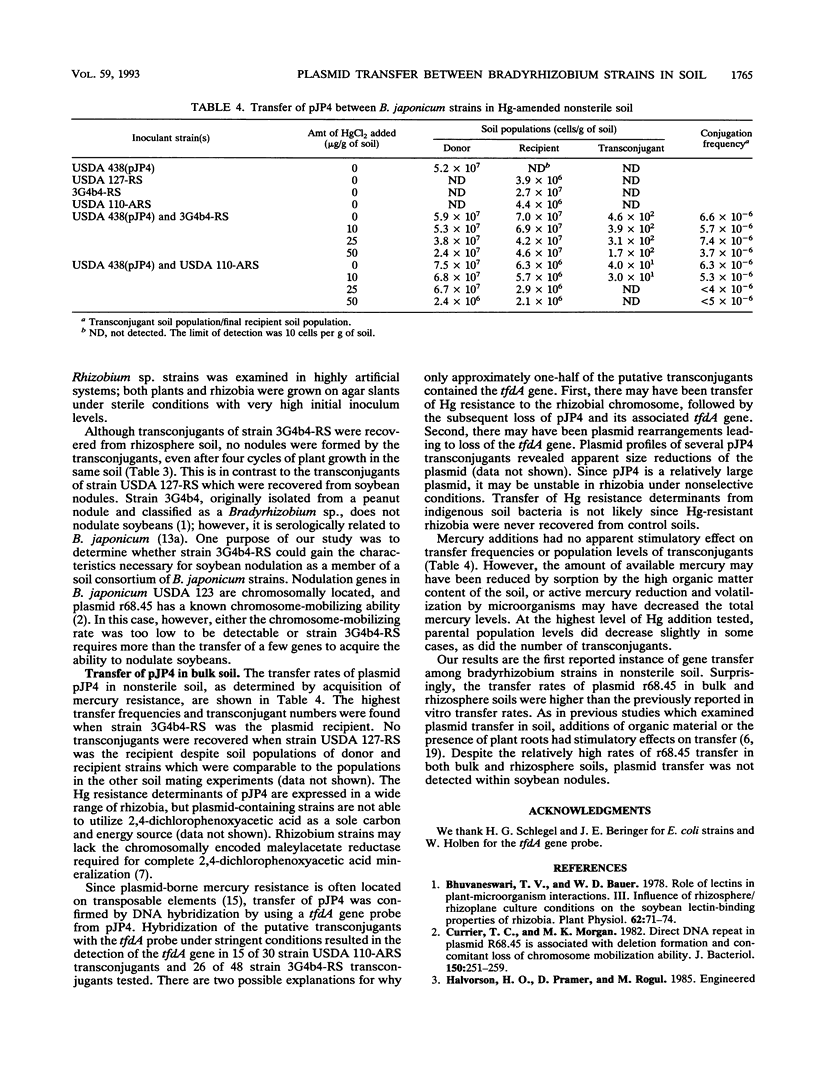
IncP plasmid r68.45, which carries several antibiotic resistance genes, and IncP plasmid pJP4, which contains genes for mercury resistance and 2,4-dichlorophenoxyacetic acid degradation, were evaluated for their ability to transfer to soil populations of rhizobia. Transfer of r68.45 was detected in nonsterile soil by using Bradyrhizobium japonicum USDA 123 as the plasmid donor and several Bradyrhizobium sp. strains as recipients. Plasmid transfer frequencies ranged up to 9.1 × 10-5 in soil amended with 0.1% soybean meal and were highest after 7 days with strain 3G4b4-RS as the recipient. Transconjugants were detected in 7 of 500 soybean nodules tested, but the absence of both parental strains in these nodules suggests that plasmid transfer had occurred in the soil, in the rhizosphere, or on the root surface. Transfer of degradative plasmid pJP4 was also evaluated in nonsterile soil by using B. japonicum USDA 438 as the plasmid donor and several Bradyrhizobium sp. strains as recipients. Plasmid pJP4 was transferred only when strains USDA 110-ARS and 3G4b4-RS were the recipients. The plasmid transfer frequency was highest for strain 3G4b4-RS (up to 7.4 × 10-6). Mercury additions to soil, ranging from 10 to 50 μg/g of soil, did not affect population levels of parental strains or the plasmid transfer frequency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Morgan M. K. Direct DNA repeat in plasmid R68.45 is associated with deletion formation and concomitant loss of chromosome mobilization ability. J Bacteriol. 1982 Apr;150(1):251–259. doi: 10.1128/jb.150.1.251-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Jones R. A., Broder M. W., Stotzky G. Effects of genetically engineered microorganisms on nitrogen transformations and nitrogen-transforming microbial populations in soil. Appl Environ Microbiol. 1991 Nov;57(11):3212–3219. doi: 10.1128/aem.57.11.3212-3219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkle B. K., Schmidt E. L. Transfer of the Pea Symbiotic Plasmid pJB5JI in Nonsterile Soil. Appl Environ Microbiol. 1991 Nov;57(11):3264–3269. doi: 10.1128/aem.57.11.3264-3269.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukor J. J., Olsen R. H., Siak J. S. Recruitment of a chromosomally encoded maleylacetate reductase for degradation of 2,4-dichlorophenoxyacetic acid by plasmid pJP4. J Bacteriol. 1989 Jun;171(6):3385–3390. doi: 10.1128/jb.171.6.3385-3390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipke R., Wagner-Döbler I., Timmis K. N., Dwyer D. F. Survival and function of a genetically engineered Pseudomonad in aquatic sediment microcosms. Appl Environ Microbiol. 1992 Apr;58(4):1259–1265. doi: 10.1128/aem.58.4.1259-1265.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius-Güth Inge-M, Pühler Alfred, Simon Reinhard. Conjugal Transfer of Megaplasmid 2 between Rhizobium meliloti Strains in Alfalfa Nodules. Appl Environ Microbiol. 1990 Aug;56(8):2354–2359. doi: 10.1128/aem.56.8.2354-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Wellington E. M., Cresswell N., Saunders V. A. Growth and survival of streptomycete inoculants and extent of plasmid transfer in sterile and nonsterile soil. Appl Environ Microbiol. 1990 May;56(5):1413–1419. doi: 10.1128/aem.56.5.1413-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


