Abstract
The phototrophic bacterium Rhodobacter capsulatus E1F1 photoreduced 2,4-dinitrophenol to 2-amino-4-nitrophenol by a nitrophenol reductase activity which was induced in the presence of nitrophenols and was repressed in ammonium-grown cells. The enzyme was located in the cytosol, required NAD(P)H as an electron donor, and used several nitrophenol derivatives as alternative substrates. The nitrophenol reductase was purified to electrophoretic homogeneity by a simple method. The enzyme was composed of two 27-kDa subunits, was inhibited by metal chelators, mercurial compounds, and Cu2+, and contained flavin mononucleotide and possibly nonheme iron as prosthetic groups. Purified enzyme also exhibited NAD(P)H diaphorase activity which used tetrazolium salt as an electron acceptor.
Full text
PDF

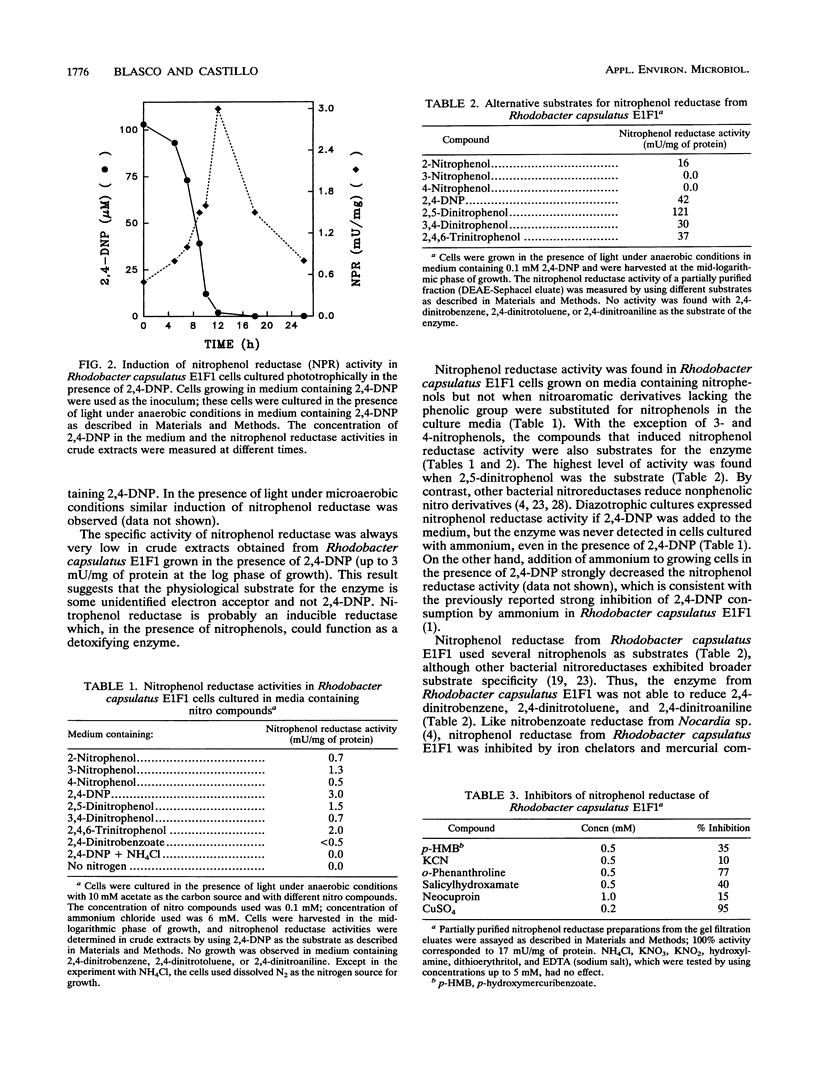
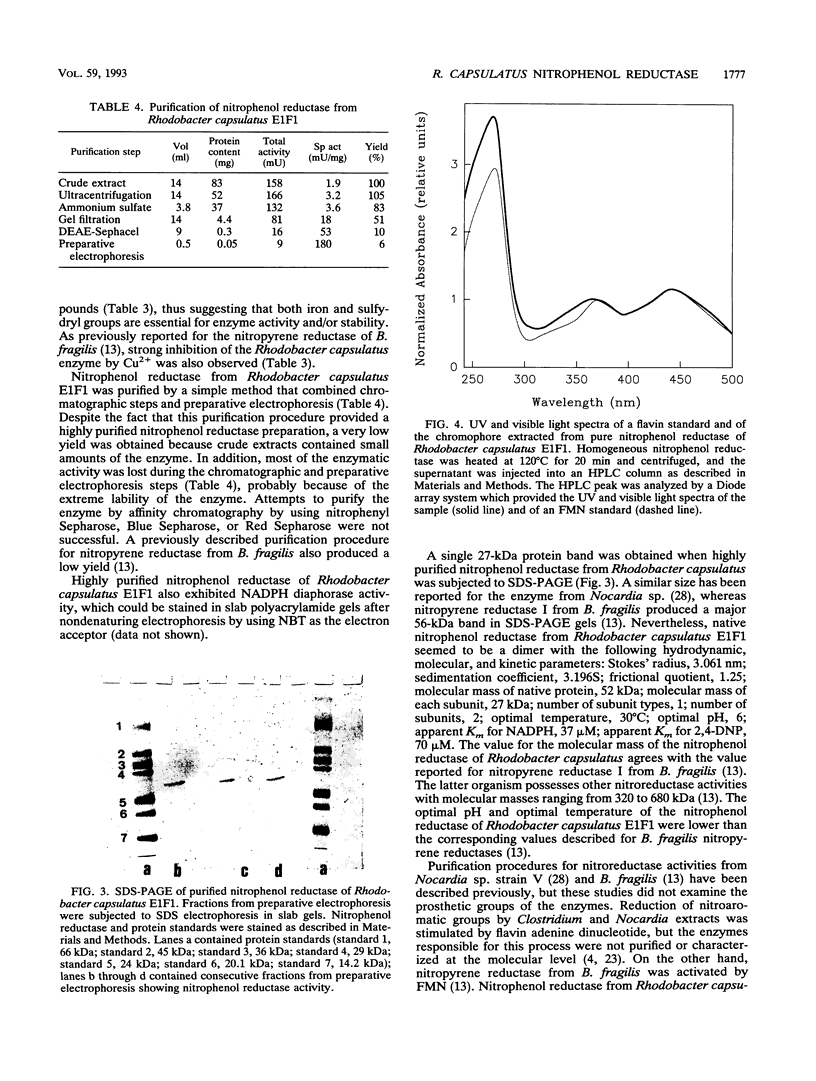

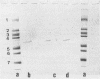
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasco R., Castillo F. Light-dependent degradation of nitrophenols by the phototrophic bacterium Rhodobacter capsulatus E1F1. Appl Environ Microbiol. 1992 Feb;58(2):690–695. doi: 10.1128/aem.58.2.690-695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. 2. Reduction of the nitro group. Biochem J. 1959 Oct;73:305–314. doi: 10.1042/bj0730305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo F. F., Ramírez J. M., Paneque A., Losada M. Ferredoxin and the dark and light reduction of dinitrophenol. Biochem Biophys Res Commun. 1966 Mar 8;22(5):547–553. doi: 10.1016/0006-291x(66)90309-3. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Spain J. C. Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways. Appl Environ Microbiol. 1991 Nov;57(11):3156–3162. doi: 10.1128/aem.57.11.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas L. E., Alexander M. Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol. 1983 Apr;45(4):1234–1241. doi: 10.1128/aem.45.4.1234-1241.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Gibson J. Uptake of benzoate by Rhodopseudomonas palustris grown anaerobically in light. J Bacteriol. 1986 Feb;165(2):504–509. doi: 10.1128/jb.165.2.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Kinouchi T., Ohnishi Y. Purification and characterization of 1-nitropyrene nitroreductases from Bacteroides fragilis. Appl Environ Microbiol. 1983 Sep;46(3):596–604. doi: 10.1128/aem.46.3.596-604.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenke H., Knackmuss H. J. Initial hydrogenation during catabolism of picric acid by Rhodococcus erythropolis HL 24-2. Appl Environ Microbiol. 1992 Sep;58(9):2933–2937. doi: 10.1128/aem.58.9.2933-2937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADHOSINGH C. The metabolic detoxication of 2,4-dinitrophenol by Fusarium oxysporum. Can J Microbiol. 1961 Aug;7:553–567. doi: 10.1139/m61-065. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCormick N. G., Cornell J. H., Kaplan A. M. Identification of biotransformation products from 2,4-dinitrotoluene. Appl Environ Microbiol. 1978 May;35(5):945–948. doi: 10.1128/aem.35.5.945-948.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Gurevich P., Henis Y. Reduction of nitrosubstituted aromatic compounds by the halophilic anaerobic eubacteria Haloanaerobium praevalens and Sporohalobacter marismortui. Appl Environ Microbiol. 1991 Nov;57(11):3367–3370. doi: 10.1128/aem.57.11.3367-3370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafil F., Franklin W., Heflich R. H., Cerniglia C. E. Reduction of nitroaromatic compounds by anaerobic bacteria isolated from the human gastrointestinal tract. Appl Environ Microbiol. 1991 Apr;57(4):962–968. doi: 10.1128/aem.57.4.962-968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. B., Spain J. C., Haddock J. D., Gibson D. T. Oxidation of nitrotoluenes by toluene dioxygenase: evidence for a monooxygenase reaction. Appl Environ Microbiol. 1992 Aug;58(8):2643–2648. doi: 10.1128/aem.58.8.2643-2648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Lorimer G. H., Hall R. L., Borchers R., Bailey J. L. Reduced nicotinamide adenine dinucleotide-nitrate reductase of Chlorella vulgaris. Purification, prosthetic groups, and molecular properties. J Biol Chem. 1975 Jun 10;250(11):4120–4127. [PubMed] [Google Scholar]
- VILLANUEVA J. R. THE PURIFICATION OF A NITRO-REDUCTASE OF NOCARDIA V. J Biol Chem. 1964 Mar;239:773–776. [PubMed] [Google Scholar]
- Wang C. S., Raper J. R. Isozyme patterns and sexual morphogenesis in Schizophyllum. Proc Natl Acad Sci U S A. 1970 Jul;66(3):882–889. doi: 10.1073/pnas.66.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Wright G. E., Madigan M. T. Photocatabolism of Aromatic Compounds by the Phototrophic Purple Bacterium Rhodomicrobium vannielii. Appl Environ Microbiol. 1991 Jul;57(7):2069–2073. doi: 10.1128/aem.57.7.2069-2073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P., Timmis K. N. Influence of para-substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol. 1986 Aug;52(2):334–339. doi: 10.1128/aem.52.2.334-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]