Abstract
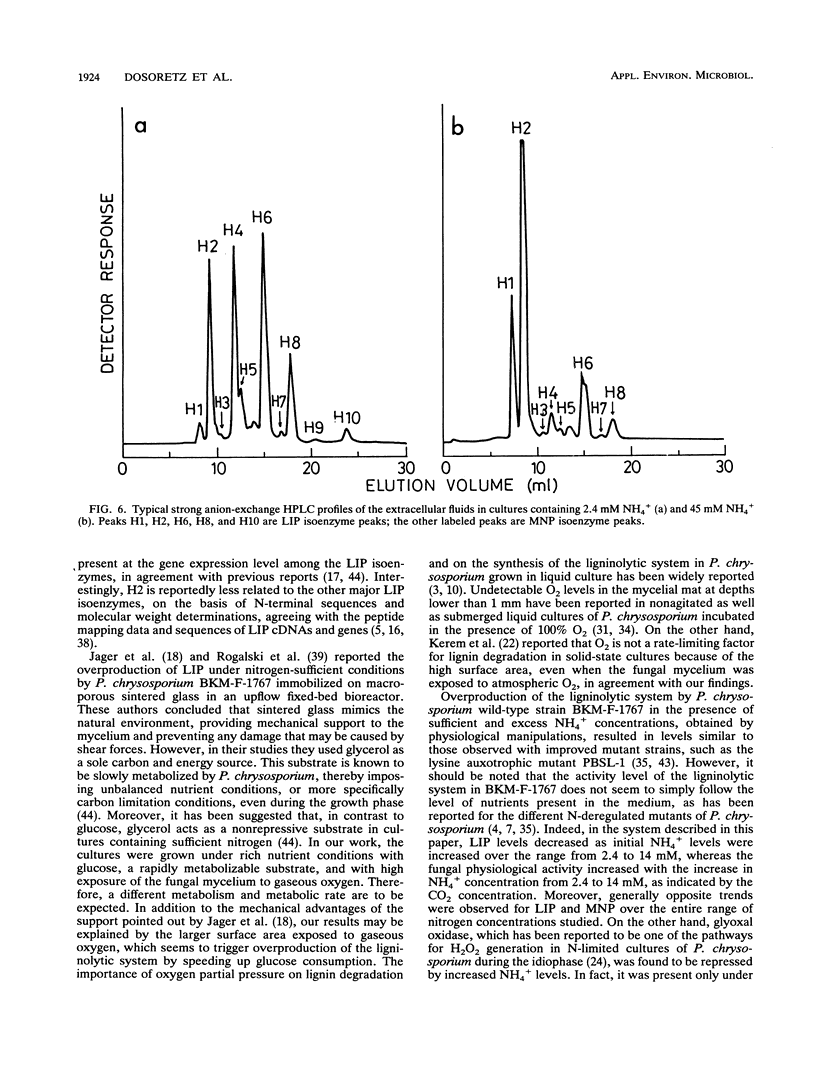
The ligninolytic enzymes synthesized by Phanerochaete chrysosporium BKM-F-1767 immobilized on polyurethane foam were characterized under limiting, sufficient, and excess nutrient conditions. The fungus was grown in a nonimmersed liquid culture system under conditions close to those occurring in nature, with nitrogen concentrations ranging from 2.4 to 60 mM. This nonimmersed liquid culture system consisted of fungal mycelium immobilized on porous pieces of polyurethane foam saturated with liquid medium and highly exposed to gaseous oxygen. Lignin peroxidase (LIP) activity decreased to almost undetectable levels as the initial NH4+ levels were increased over the range from 2.4 to 14 mM and then increased with additional increases in initial NH4+ concentration. At 45 mM NH4+, LIP was overproduced, reaching levels of 800 U/liter. In addition, almost simultaneous secretion of LIP and secretion of manganese-dependent lignin peroxidase were observed on the third day of incubation. Manganese-dependent lignin peroxidase activity was maximal under nitrogen limitation conditions (2.4 mM NH4+) and then decreased to 40 to 50% of the maximal level in the presence of sufficient or excess initial NH4+ concentrations. Overproduction of LIP in the presence of a sufficient nitrogen level (24 mM NH4+) and excess nitrogen levels (45 to 60 mM NH4+) seemed to occur as a response to carbon starvation after rapid glucose depletion. The NH4+ in the extracellular fluid reappeared as soon as glucose was depleted, and an almost complete loss of CO2 was observed, suggesting that an alternative energy source was generated by self-proteolysis of cell proteins.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Reddy C. A. Nitrogen-deregulated mutants of Phanerochaete chrysosporium--a lignin-degrading basidiomycete. Arch Microbiol. 1990;153(6):521–527. doi: 10.1007/BF00245259. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Andrawis A., Tien M. Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1988 Sep 15;155(2):626–633. doi: 10.1016/s0006-291x(88)80541-2. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. M., Pease E. A., Li J. K., Tien M. Production and characterization of recombinant lignin peroxidase isozyme H2 from Phanerochaete chrysosporium using recombinant baculovirus. Arch Biochem Biophys. 1992 Aug 1;296(2):660–666. doi: 10.1016/0003-9861(92)90624-6. [DOI] [PubMed] [Google Scholar]
- Kerem Z., Friesem D., Hadar Y. Lignocellulose Degradation during Solid-State Fermentation: Pleurotus ostreatus versus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Apr;58(4):1121–1127. doi: 10.1128/aem.58.4.1121-1127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kuan I. C., Tien M. Phosphorylation of lignin peroxidases from Phanerochaete chrysosporium. Identification of mannose 6-phosphate. J Biol Chem. 1989 Dec 5;264(34):20350–20355. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Michel F. C., Jr, Grulke E. A., Reddy C. A. Determination of the respiration kinetics for mycelial pellets of Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 May;58(5):1740–1745. doi: 10.1128/aem.58.5.1740-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kersten P. J., Kirk T. K. Selection and improvement of lignin-degrading microorganisms: potential strategy based on lignin model-amino Acid adducts. Appl Environ Microbiol. 1987 Feb;53(2):242–245. doi: 10.1128/aem.53.2.242-245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- Zitomer R. S., Lowry C. V. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol Rev. 1992 Mar;56(1):1–11. doi: 10.1128/mr.56.1.1-11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]