Abstract
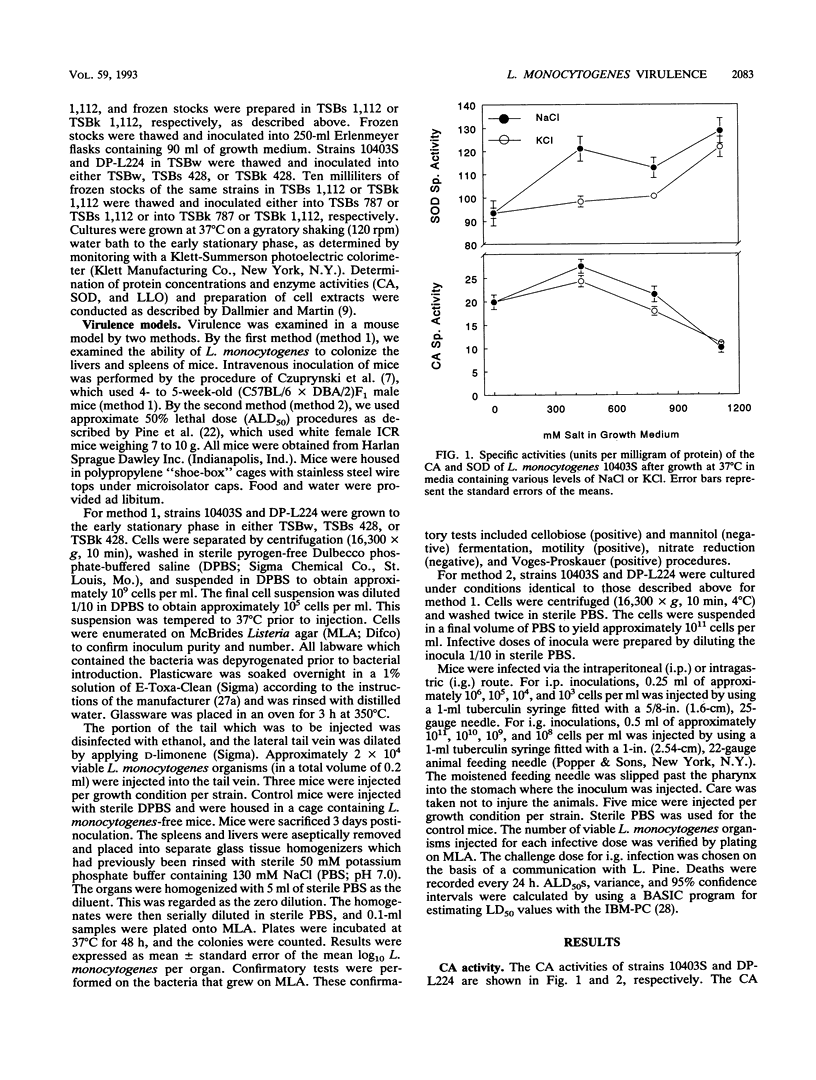
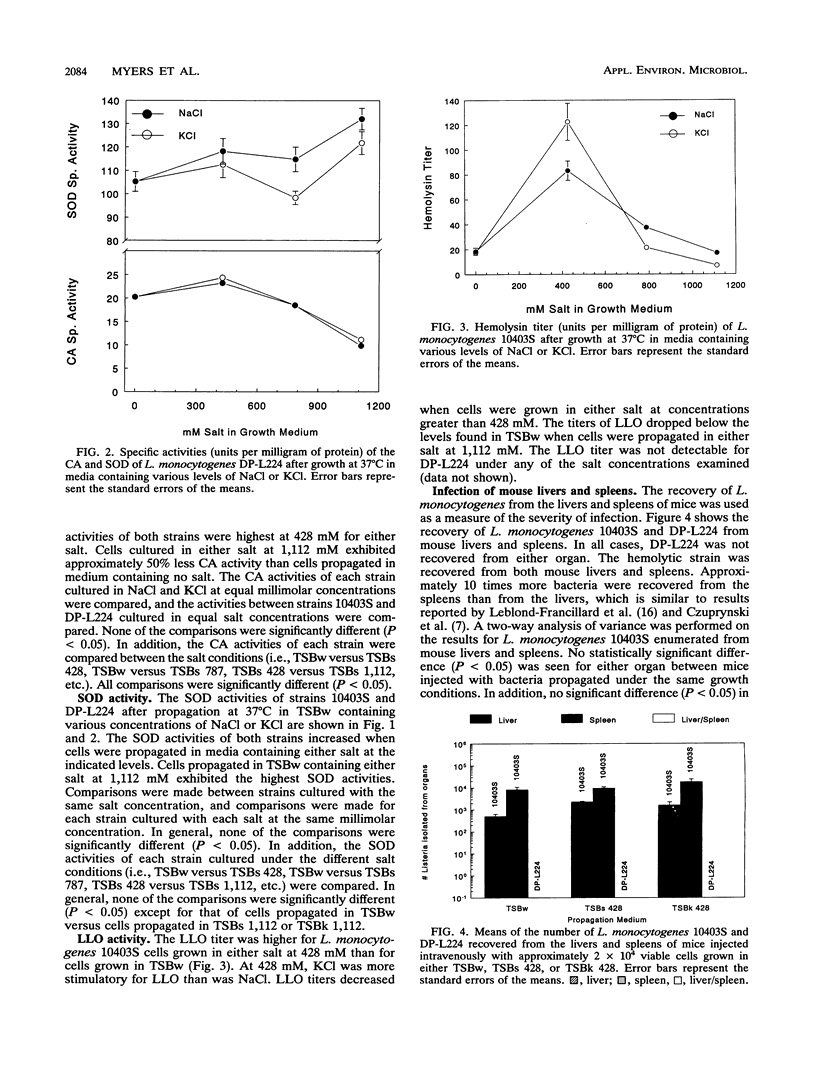
Virulence, as determined in a mouse model, and the virulence factor activities of catalase, superoxide dismutase, and listeriolysin O were examined in a parental strain (10403S) and in a nonhemolytic mutant strain (DP-L224) of Listeria monocytogenes. The cells were propagated in media containing various concentrations of sodium chloride or potassium chloride. Strains 10403S and DP-L224 exhibited significant increases in catalase activity and listeriolysin O activity when grown in medium containing either salt at 428 mM. The superoxide dismutase activities for both strains increased when they were grown in medium containing either salt. The superoxide dismutase activity was significantly increased only when cells were propagated in medium containing no salt compared with that when they were propagated in medium containing either salt at 1,112 mM. In addition, the listeriolysin O activity was highest for cells propagated in medium containing KCl at 428 mM, while the activity was significantly less for cells propagated in medium containing NaCl at an equal concentration. Virulence was examined in mouse livers and spleens after intravenous infection, and approximate 50% lethal doses were determined after intragastric and intraperitoneal infection. Each method of infection indicated that listeriolysin O is required for virulence, while growth in salt-containing medium or the production of higher levels of catalase, superoxide dismutase, and listeriolysin O do not appear to enhance the virulence of L. monocytogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audurier A., Pardon P., Marly J., Lantier F. Experimental infection of mice with Listeria monocytogenes and L. innocua. Ann Microbiol (Paris) 1980 Jul-Aug;131B(1):47–57. [PubMed] [Google Scholar]
- Chakraborty T., Goebel W. Recent developments in the study of virulence in Listeria monocytogenes. Curr Top Microbiol Immunol. 1988;138:41–58. [PubMed] [Google Scholar]
- Chakraborty T., Leimeister-Wächter M., Domann E., Hartl M., Goebel W., Nichterlein T., Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992 Jan;174(2):568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Vicente M. F., Mengaud J., Baquero F., Perez-Diaz J. C., Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989 Nov;57(11):3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart R. E., Foster B. G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985 Apr;151(4):721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Roll J. T. Growth at reduced temperatures increases the virulence of Listeria monocytogenes for intravenously but not intragastrically inoculated mice. Microb Pathog. 1989 Sep;7(3):213–223. doi: 10.1016/0882-4010(89)90057-0. [DOI] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988 Feb;54(2):581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase, superoxide dismutase, and hemolysin activities and heat susceptibility of Listeria monocytogenes after growth in media containing sodium chloride. Appl Environ Microbiol. 1990 Sep;56(9):2807–2810. doi: 10.1128/aem.56.9.2807-2810.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst J. The role of temperature factors in the epidemiology of listeriosis. Zentralbl Bakteriol Orig A. 1975 Sep;233(1):72–74. [PubMed] [Google Scholar]
- GIRARD K. F., SBARRA A. J., BARDAWIL W. A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963 Feb;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J Gen Microbiol. 1989 Mar;135(3):481–487. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- Kathariou S., Rocourt J., Hof H., Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections of mice. Infect Immun. 1988 Feb;56(2):534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. J., Postlethwaite R., MacGowan A. P. Listeria monocytogenes and its role in human infection. J Infect. 1988 Jul;17(1):7–28. doi: 10.1016/s0163-4453(88)92236-0. [DOI] [PubMed] [Google Scholar]
- Leblond-Francillard M., Gaillard J. L., Berche P. Loss of catalase activity in Tn1545-induced mutants does not reduce growth of Listeria monocytogenes in vivo. Infect Immun. 1989 Aug;57(8):2569–2573. doi: 10.1128/iai.57.8.2569-2573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol. 1992 Feb;174(3):947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980 May;28(2):516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Chenevert J., Geoffroy C., Gaillard J. L., Cossart P. Identification of the structural gene encoding the SH-activated hemolysin of Listeria monocytogenes: listeriolysin O is homologous to streptolysin O and pneumolysin. Infect Immun. 1987 Dec;55(12):3225–3227. doi: 10.1128/iai.55.12.3225-3227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Malcolm G. B., Plikaytis B. D. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect Immun. 1990 Sep;58(9):2940–2945. doi: 10.1128/iai.58.9.2940-2945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone J. R., Hassan H. M. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4269–4273. [PubMed] [Google Scholar]
- Shahamat M., Seaman A., Woodbine M. Survival of Listeria monocytogenes in high salt concentrations. Zentralbl Bakteriol A. 1980;246(4):506–511. [PubMed] [Google Scholar]
- Trevors J. T. A BASIC program for estimating LD50 values using the IBM-PC. Bull Environ Contam Toxicol. 1986 Jul;37(1):18–26. doi: 10.1007/BF01607723. [DOI] [PubMed] [Google Scholar]
- Udou T., Ichik'awa Y. Effect of sodium chloride on the activity and production of staphylococcal exonuclease. J Gen Microbiol. 1980 Jan;116(1):69–74. doi: 10.1099/00221287-116-1-69. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]