Abstract
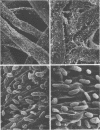
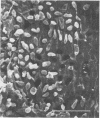
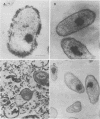
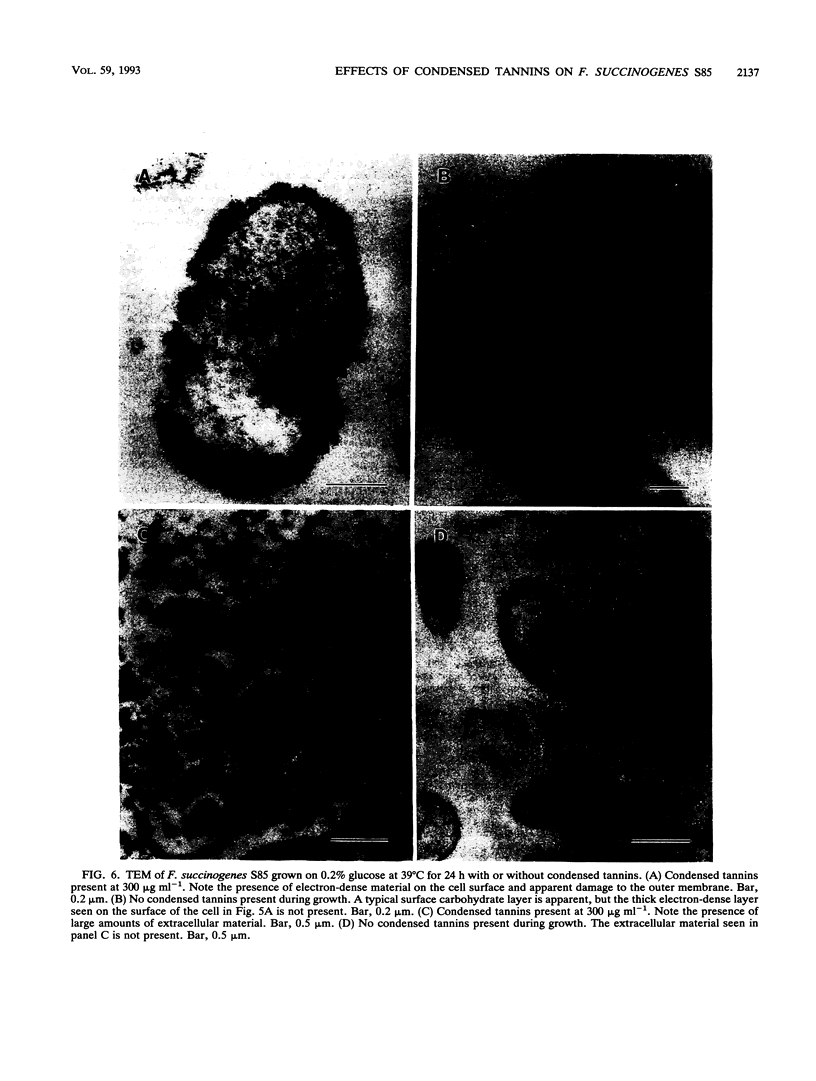
The effect of condensed tannins from birdsfoot trefoil (Lotus corniculatus L.) on the cellulolytic rumen bacterium Fibrobacter succinogenes S85 was examined. Condensed tannins inhibited endoglucanase activity in the extracellular culture fluid, at concentrations as low as 25 μg ml-1. In contrast, cell-associated endoglucanase activity increased in concentrations of condensed tannins between 100 and 300 μg ml-1. Inhibition of endoglucanase activity in both the extracellular and the cell-associated fractions was virtually complete at 400 μg of condensed tannins ml-1. Despite the sharp decline in extracellular endoglucanase activity with increasing concentrations of condensed tannins, filter paper digestion declined only moderately between 0 and 200 μg of condensed tannins ml-1. However, at 300 μg ml-1, filter paper digestion was dramatically reduced and at 400 μg ml-1, almost no filter paper was digested. F. succinogenes S85 was seen to form digestive grooves on the surface of cellulose, and at 200 μg ml-1, digestive pits were formed which penetrated into the interior of cellulose fibers. Cells grown with condensed tannins (100 to 300 μg ml-1) possessed large amounts of surface material, and although this material may have been capsular carbohydrate, its osmiophilic nature suggested that it had arisen from the formation of tannin-protein complexes on the cell surface. The presence of electron-dense extracellular material suggested that similar complexes were formed with extracellular protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry T. N., Duncan S. J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 1. Voluntary intake. Br J Nutr. 1984 May;51(3):485–491. doi: 10.1079/bjn19840054. [DOI] [PubMed] [Google Scholar]
- Barry T. N., Manley T. R., Duncan S. J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 4. Sites of carbohydrate and protein digestion as influenced by dietary reactive tannin concentration. Br J Nutr. 1986 Jan;55(1):123–137. doi: 10.1079/bjn19860016. [DOI] [PubMed] [Google Scholar]
- Biely P., Mislovicová D., Toman R. Soluble chromogenic substrates for the assay of endo-1,4-beta-xylanases and endo-1,4-beta-glucanases. Anal Biochem. 1985 Jan;144(1):142–146. doi: 10.1016/0003-2697(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Forsberg C. W. Factors affecting adhesion of Fibrobacter succinogenes subsp. succinogenes S85 and adherence-defective mutants to cellulose. Appl Environ Microbiol. 1989 Dec;55(12):3039–3044. doi: 10.1128/aem.55.12.3039-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- HENIS Y., TAGARI H., VOLCANI R. EFFECT OF WATER EXTRACTS OF CAROB PODS, TANNIC ACID, AND THEIR DERIVATIVES ON THE MORPHOLOGY AND GROWTH OF MICROORGANISMS. Appl Microbiol. 1964 May;12:204–209. doi: 10.1128/am.12.3.204-209.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Electron microscopic study of the methylcellulose-mediated detachment of cellulolytic rumen bacteria from cellulose fibers. Can J Microbiol. 1987 Mar;33(3):267–272. doi: 10.1139/m87-045. [DOI] [PubMed] [Google Scholar]
- Lyford S. J., Jr, Smart W. W., Jr, Bell T. A. Inhibition of rumen cellulose digestion by extracts of sericea lespedeza. J Anim Sci. 1967 May;26(3):632–637. doi: 10.2527/jas1967.263632x. [DOI] [PubMed] [Google Scholar]
- Makkar H. P., Singh B., Dawra R. K. Effect of tannin-rich leaves of oak (Quercus incana) on various microbial enzyme activities of the bovine rumen. Br J Nutr. 1988 Sep;60(2):287–296. doi: 10.1079/bjn19880100. [DOI] [PubMed] [Google Scholar]
- Matte A., Forsberg C. W. Purification, characterization, and mode of action of endoxylanases 1 and 2 from Fibrobacter succinogenes S85. Appl Environ Microbiol. 1992 Jan;58(1):157–168. doi: 10.1128/aem.58.1.157-168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Lam J., Forsberg C. W. Regulation and distribution of Fibrobacter succinogenes subsp. succinogenes S85 endoglucanases. Appl Environ Microbiol. 1990 May;56(5):1235–1244. doi: 10.1128/aem.56.5.1235-1244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]