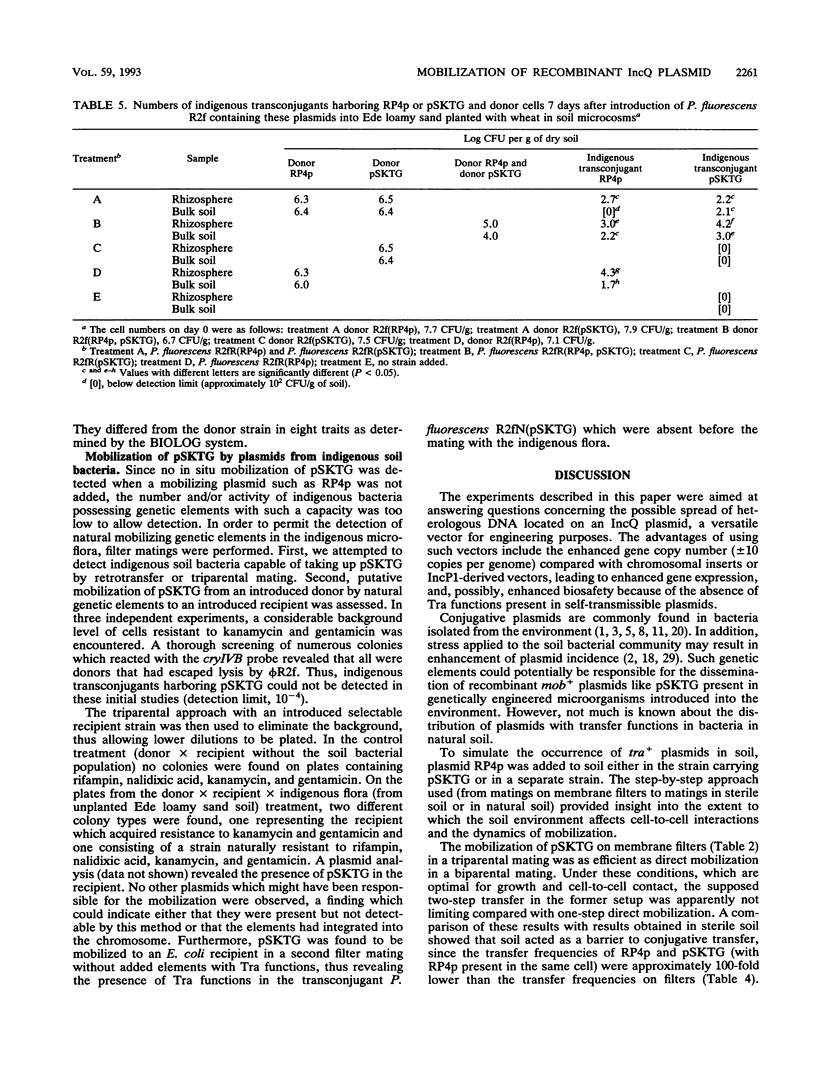
Abstract
Mobilization of a genetically engineered IncQ plasmid, pSKTG, was studied in vitro and in sterile and nonsterile soils. In biparental and triparental filter matings, the mobilization frequencies of pSKTG were identical, and the plasmid was mobilized only in the presence of self-transmissible plasmid RP4p. In sterile soil, mobilization was probably limited by reduced cell-to-cell contact, since the frequencies of mobilization were approximately 100-fold lower than the frequencies in the filter matings. The transfer frequency of pSKTG in sterile soil when RP4p was present in the same strain was about 100-fold higher than the transfer frequency when RP4p was present in a separate strain. In studies in natural soil, pSKTG was also found to be transferred to indigenous bacteria. However, natural mobilization by genetic elements present in the indigenous soil microflora could not be detected. In vitro studies of natural transfer suggested that such genetic elements occur in soil bacteria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton N. F., Day M. J., Bull A. T. Distribution of bacterial plasmids in clean and polluted sites in a South Wales river. Appl Environ Microbiol. 1982 Nov;44(5):1026–1029. doi: 10.1128/aem.44.5.1026-1029.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germida J. J., Khachatourians G. G. Transduction of Escherichia coli in soil. Can J Microbiol. 1988 Feb;34(2):190–193. doi: 10.1139/m88-035. [DOI] [PubMed] [Google Scholar]
- Hanson C. W., Martin W. J. Microwave oven for melting laboratory media. J Clin Microbiol. 1978 Apr;7(4):401–402. doi: 10.1128/jcm.7.4.401-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K. E., Weightman A. J., Fry J. C. Isolation and screening of plasmids from the epilithon which mobilize recombinant plasmid pD10. Appl Environ Microbiol. 1992 Apr;58(4):1292–1300. doi: 10.1128/aem.58.4.1292-1300.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna M., Stotzky G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Appl Environ Microbiol. 1992 Jun;58(6):1930–1939. doi: 10.1128/aem.58.6.1930-1939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M., Lejeune P., Sadouk A., Gerits J., Fabry L. Shuttle transfer (or retrotransfer) of chromosomal markers mediated by plasmid pULB113. Mol Gen Genet. 1987 Aug;209(1):61–70. doi: 10.1007/BF00329837. [DOI] [PubMed] [Google Scholar]
- Priefer U. B., Simon R., Pühler A. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J Bacteriol. 1985 Jul;163(1):324–330. doi: 10.1128/jb.163.1.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaume A., Angle J. S., Sadowsky M. J. Influence of soil variables on in situ plasmid transfer from Escherichia coli to Rhizobium fredii. Appl Environ Microbiol. 1989 Jul;55(7):1730–1734. doi: 10.1128/aem.55.7.1730-1734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Wang G. R., Salyers A. A. Evidence for natural transfer of a tetracycline resistance gene between bacteria from the human colon and bacteria from the bovine rumen. Appl Environ Microbiol. 1992 Apr;58(4):1313–1320. doi: 10.1128/aem.58.4.1313-1320.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E., van Elsas J. D., van Veen J. A., de Vos W. M. Detection of Plasmid Transfer from Pseudomonas fluorescens to Indigenous Bacteria in Soil by Using Bacteriophage phiR2f for Donor Counterselection. Appl Environ Microbiol. 1991 Dec;57(12):3482–3488. doi: 10.1128/aem.57.12.3482-3488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevors J. T., van Elsas J. D., van Overbeek L. S., Starodub M. E. Transport of a genetically engineered Pseudomonas fluorescens strain through a soil microcosm. Appl Environ Microbiol. 1990 Feb;56(2):401–408. doi: 10.1128/aem.56.2.401-408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham G. S., Atlas R. M. Plasmid frequency fluctuations in bacterial populations from chemically stressed soil communities. Appl Environ Microbiol. 1988 Sep;54(9):2192–2196. doi: 10.1128/aem.54.9.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


