Abstract
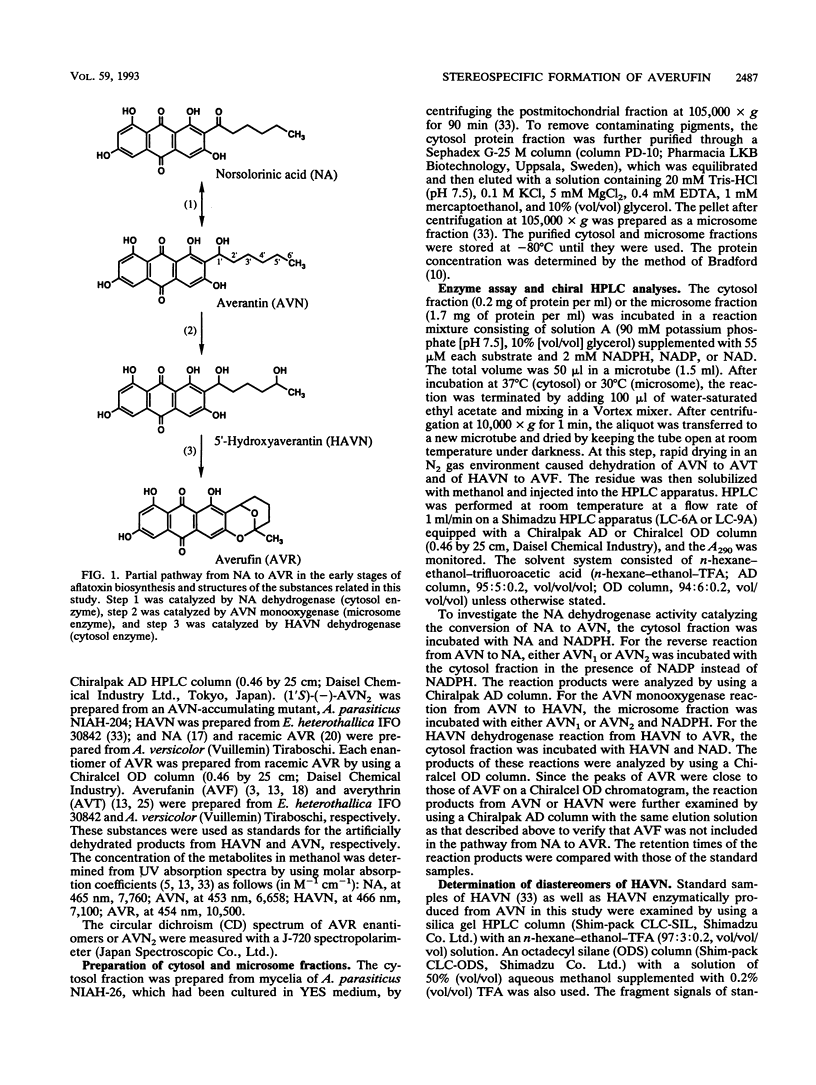
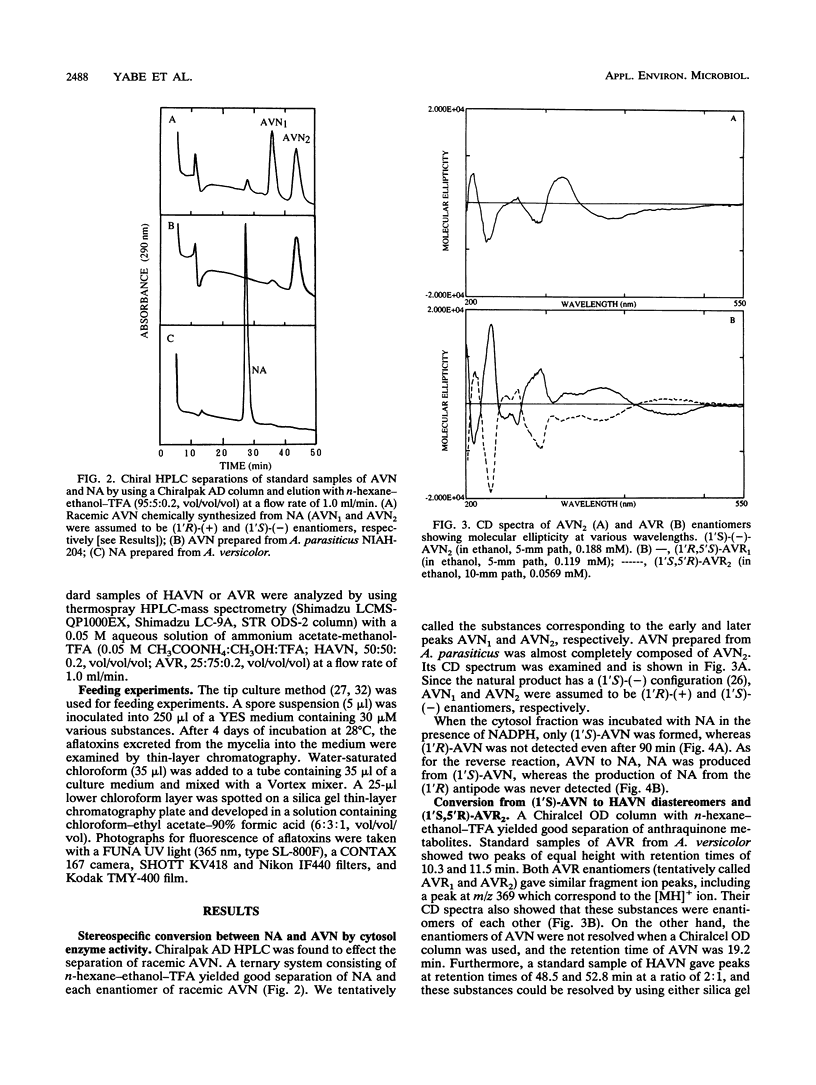
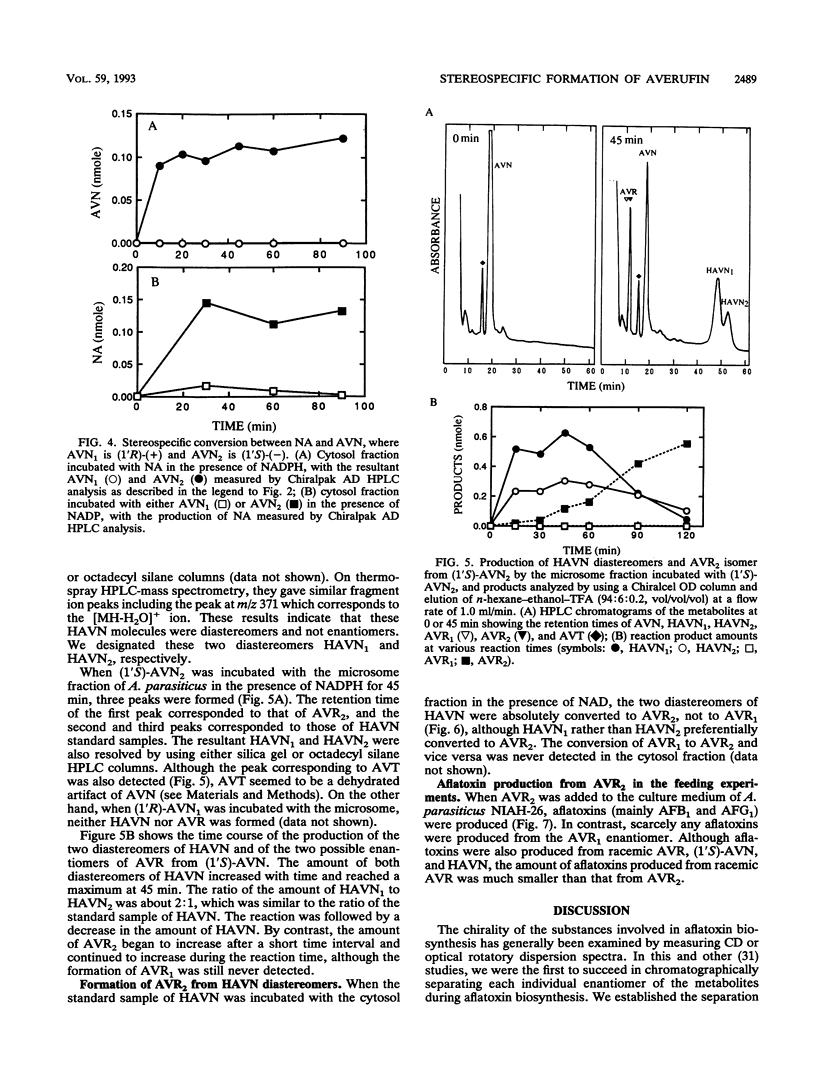
A reaction sequence, norsolorinic acid (NA)-->averantin (AVN)-->5'-hydroxyaverantin (HAVN)-->averufin (AVR), is the early part of a biosynthetic pathway for aflatoxins. In this study, we determined the stereochemical relationship among these metabolites by using chiral high-performance liquid chromatography. In cell-free experiments using the cytosol fraction of Aspergillus parasiticus NIAH-26, (1'S)-AVN was exclusively produced from NA in the presence of NADPH. Also, only (1'S)-AVN, and not (1'R)-AVN, served as a substrate for the reverse reaction from AVN to NA. When the microsome fraction of NIAH-26 was incubated with (1'S)-AVN in the presence of NADPH, two HAVN diastereomers and one AVR enantiomer were formed, whereas these substances were never produced from (1'R)-AVN. Moreover, (1'S,5'R)-AVR was exclusively formed from both HAVN diastereomers by the cytosol fraction in the presence of NAD. The feeding experiments using this mutant showed that aflatoxins were produced from (1'S,5'R)-AVR but not from (1'R,5'S)-AVR. These results indicate that the enzymes involved in this pathway show strict stereospecificity to their substrates and that the configuration of (1'S,5'R)-AVR leading to the formation of aflatoxins is due to the stereospecificity of NA dehydrogenase which catalyzes the reaction between (1'S)-AVN and NA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Chung C. H. Conversion of versiconal acetate to versiconal and versicolorin C in extracts from Aspergillus parasiticus. Mycopathologia. 1990 Apr;110(1):31–35. doi: 10.1007/BF00442767. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Christensen S. B. New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol. 1983;29:53–92. doi: 10.1016/s0065-2164(08)70354-x. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar D., Cleveland T. E., Kingston D. G. Enzymological evidence for separate pathways for aflatoxin B1 and B2 biosynthesis. Biochemistry. 1991 Apr 30;30(17):4343–4350. doi: 10.1021/bi00231a033. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., McCormick S. P., Lee L. S., Hill R. A. Identification of O-methylsterigmatocystin as an aflatoxin B1 and G1 precursor in Aspergillus parasiticus. Appl Environ Microbiol. 1987 May;53(5):1028–1033. doi: 10.1128/aem.53.5.1028-1033.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw J. H., Roberts J. C., Roffey P. Studies in mycological chemistry. XIX. "Product B" (averantin) [1,3,6,8-tetrahydroxy-2-(1-hydroxyhexyl)anthraquinone], a pigment from Aspergillus versicolor (Vuillemin) Tiraboschi. J Chem Soc Perkin 1. 1966;9:855–857. doi: 10.1039/j39660000855. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F. The affinity purification and characterization of a dehydrogenase from Aspergillus parasiticus involved in aflatoxin B1 biosynthesis. Prep Biochem. 1991;21(2-3):125–140. doi: 10.1080/10826069108018008. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Wan C. C., Billington J. A. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):121–126. doi: 10.1007/BF00707548. [DOI] [PubMed] [Google Scholar]
- ROBERTS J. C., ROFFEY P. STUDIES IN MYCOLOGICAL CHEMISTRY. XVII. AVERYTHRIN, AN ANTHRAQUINONOID PIGMENT FROM ASPERGILLUS VERSICOLOR (VUILLEMIN) TIRABOSCHI. J Chem Soc. 1965 Jun;33:3666–3672. doi: 10.1039/jr9650003666. [DOI] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl Environ Microbiol. 1988 Aug;54(8):2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hashimoto J., Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989 Sep;55(9):2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Hamasaki T. Stereochemistry during aflatoxin biosynthesis: cyclase reaction in the conversion of versiconal to versicolorin B and racemization of versiconal hemiacetal acetate. Appl Environ Microbiol. 1993 Aug;59(8):2493–2500. doi: 10.1128/aem.59.8.2493-2500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Nakamura H., Ando Y., Terakado N., Nakajima H., Hamasaki T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl Environ Microbiol. 1988 Aug;54(8):2096–2100. doi: 10.1128/aem.54.8.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Nakamura Y., Nakajima H., Ando Y., Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 1991 May;57(5):1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]