Abstract
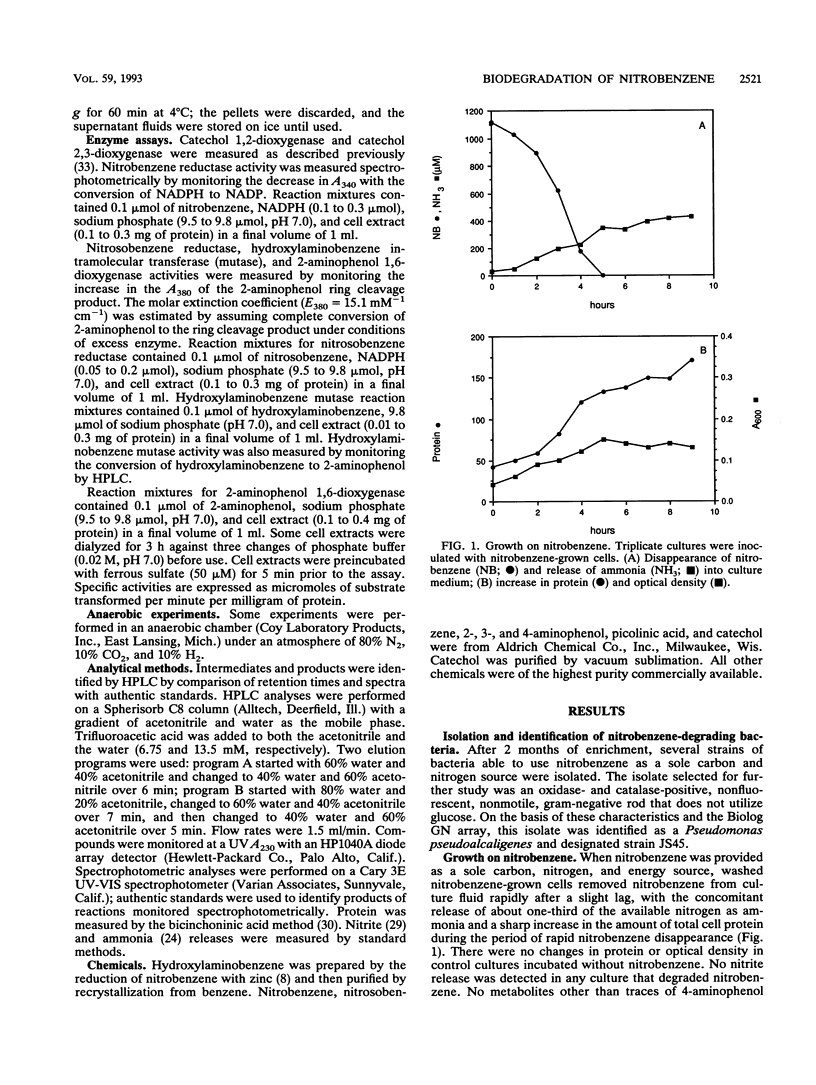
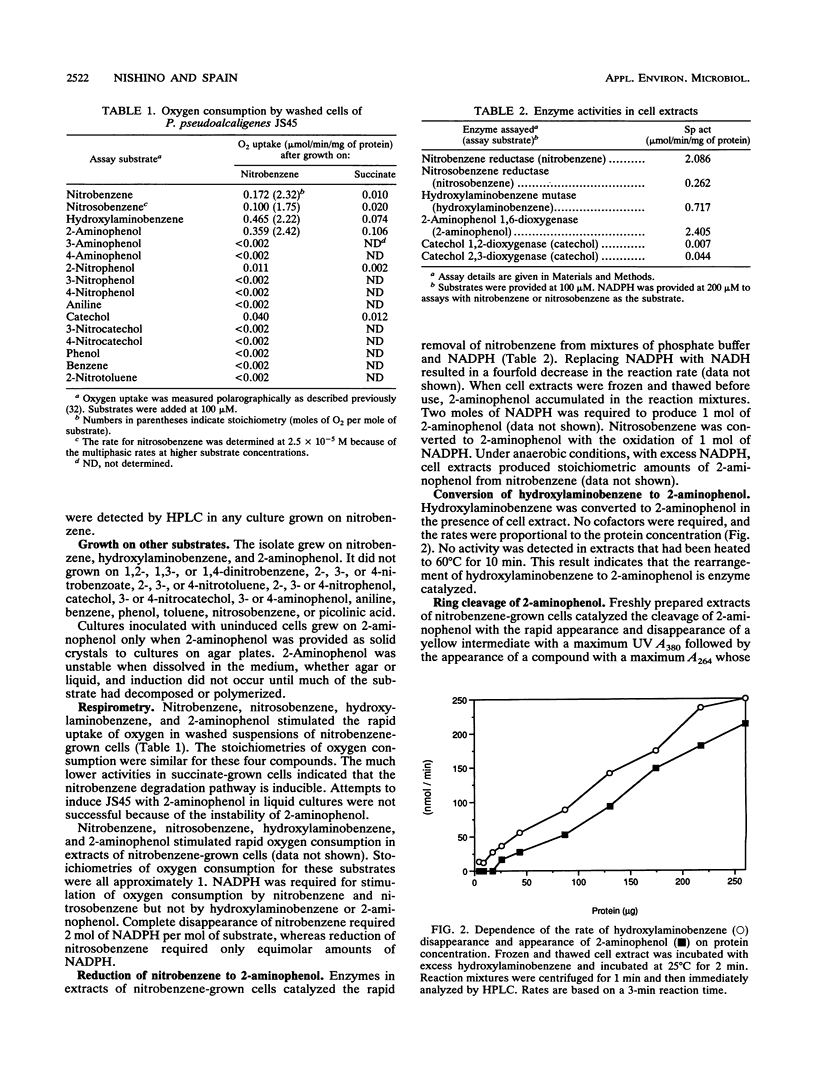
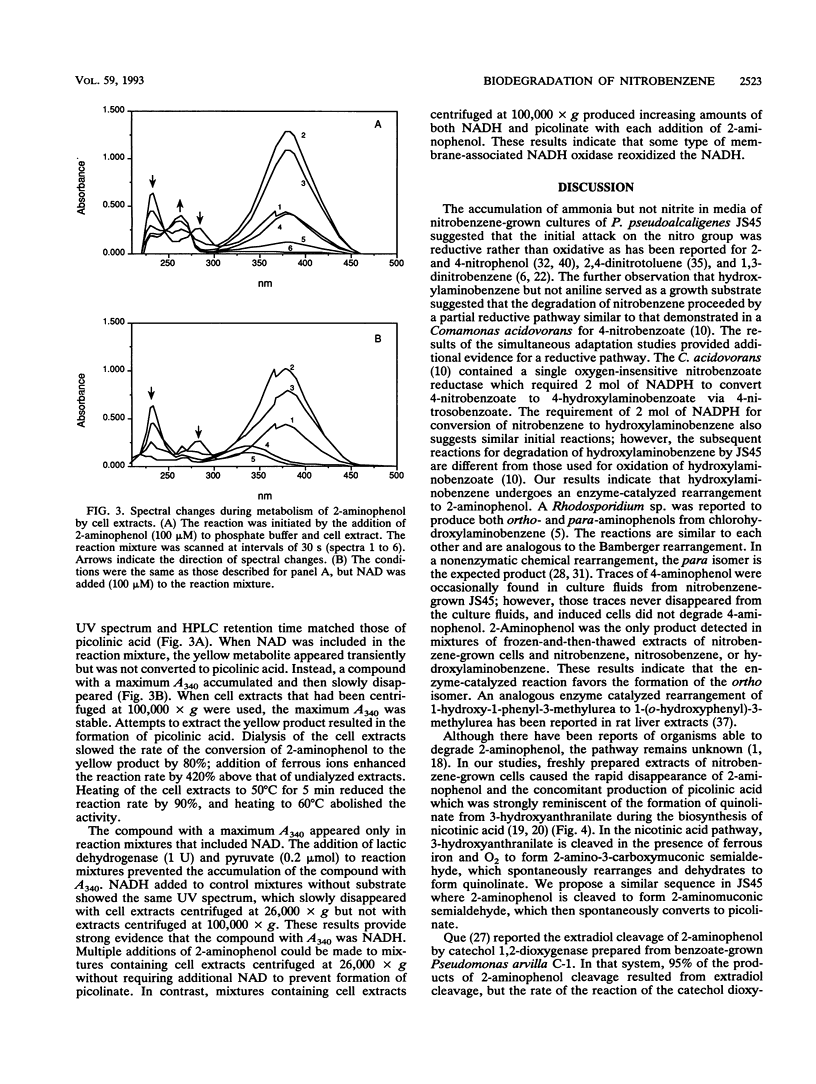
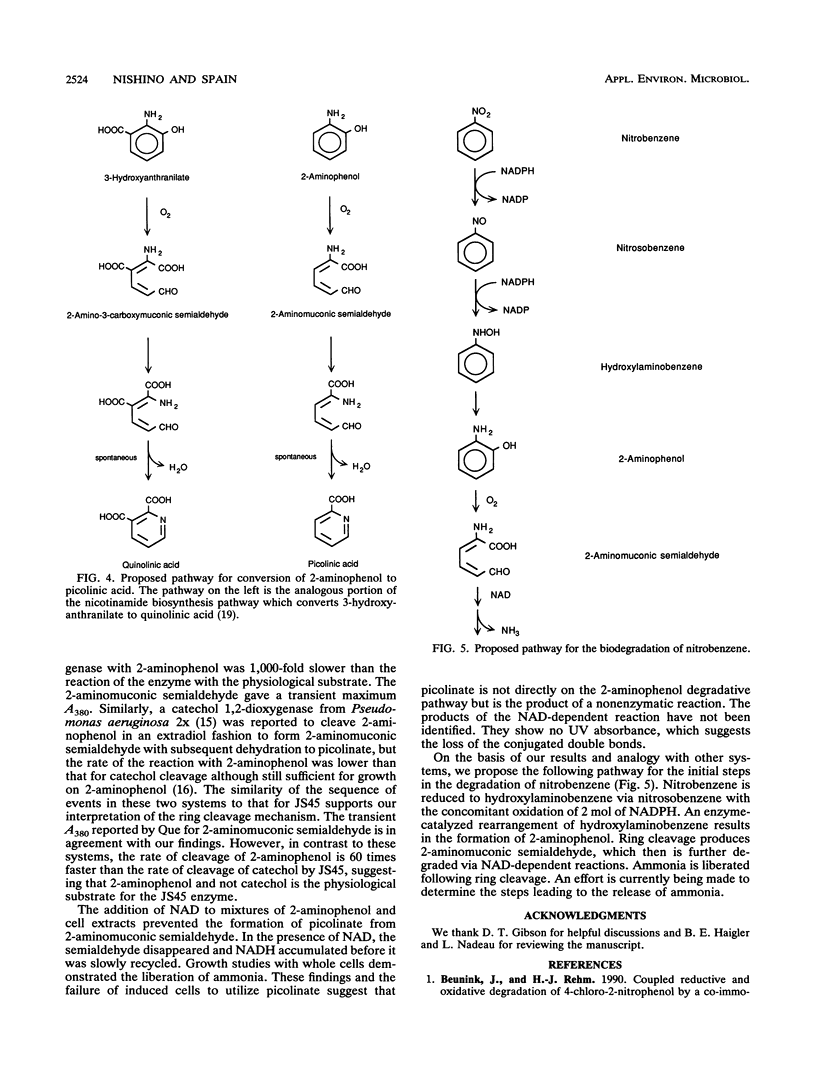
A Pseudomonas pseudoalcaligenes able to use nitrobenzene as the sole source of carbon, nitrogen, and energy was isolated from soil and groundwater contaminated with nitrobenzene. The range of aromatic substrates able to support growth was limited to nitrobenzene, hydroxylaminobenzene, and 2-aminophenol. Washed suspensions of nitrobenzene-grown cells removed nitrobenzene from culture fluids with the concomitant release of ammonia. Nitrobenzene, nitrosobenzene, hydroxylaminobenzene, and 2-aminophenol stimulated oxygen uptake in resting cells and in extracts of nitrobenzene-grown cells. Under aerobic and anaerobic conditions, crude extracts converted nitrobenzene to 2-aminophenol with oxidation of 2 mol of NADPH. Ring cleavage, which required ferrous iron, produced a transient yellow product with a maximum A380. In the presence of NAD, the product disappeared and NADH was produced. In the absence of NAD, the ring fission product was spontaneously converted to picolinic acid, which was not further metabolized. These results indicate that the catabolic pathway involves the reduction of nitrobenzene to nitrosobenzene and then to hydroxylaminobenzene; each of these steps requires 1 mol of NADPH. An enzyme-mediated Bamberger-like rearrangement converts hydroxylaminobenzene to 2-aminophenol, which then undergoes meta ring cleavage to 2-aminomuconic semialdehyde. The mechanism for release of ammonia and subsequent metabolism are under investigation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beunink J., Rehm H. J. Coupled reductive and oxidative degradation of 4-chloro-2-nitrophenol by a co-immobilized mixed culture system. Appl Microbiol Biotechnol. 1990 Oct;34(1):108–115. doi: 10.1007/BF00170933. [DOI] [PubMed] [Google Scholar]
- Bruhn C., Lenke H., Knackmuss H. J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol. 1987 Jan;53(1):208–210. doi: 10.1128/aem.53.1.208-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN R. B. The microbial metabolism of nitro-aromatic compounds. J Gen Microbiol. 1958 Aug;19(1):1–14. doi: 10.1099/00221287-19-1-1. [DOI] [PubMed] [Google Scholar]
- Cain R. B. Utilization of anthranilic and nitrobenzoic acids by Nocardia opaca and a flavobacterium. J Gen Microbiol. 1966 Feb;42(2):219–235. doi: 10.1099/00221287-42-2-219. [DOI] [PubMed] [Google Scholar]
- Corbett M. D., Corbett B. R. Metabolism of 4-Chloronitrobenzene by the Yeast Rhodosporidium sp. Appl Environ Microbiol. 1981 Apr;41(4):942–949. doi: 10.1128/aem.41.4.942-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel O., Knackmuss H. J. Catabolism of 1,3-dinitrobenzene by Rhodococcus sp. QT-1. Arch Microbiol. 1991;157(1):76–79. doi: 10.1007/BF00245339. [DOI] [PubMed] [Google Scholar]
- Groenewegen P. E., Breeuwer P., van Helvoort J. M., Langenhoff A. A., de Vries F. P., de Bont J. A. Novel degradative pathway of 4-nitrobenzoate in Comamonas acidovorans NBA-10. J Gen Microbiol. 1992 Aug;138(Pt 8):1599–1605. doi: 10.1099/00221287-138-8-1599. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Spain J. C. Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways. Appl Environ Microbiol. 1991 Nov;57(11):3156–3162. doi: 10.1128/aem.57.11.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas L. E., Alexander M. Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol. 1983 Apr;45(4):1234–1241. doi: 10.1128/aem.45.4.1234-1241.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataeva I. A., Golovleva L. A. Catechol 2,3-dioxygenases from Pseudomonas aeruginosa 2x. Methods Enzymol. 1990;188:115–121. doi: 10.1016/0076-6879(90)88021-2. [DOI] [PubMed] [Google Scholar]
- Kataeva I. A., Golovleva L. A. Ekstradiol'noe rasshcheplenie 2-aminofenola pirokatekhazoi iz Pseudomonas aeruginosa 2X. Mikrobiol Zh. 1984 Nov-Dec;46(6):22–26. [PubMed] [Google Scholar]
- MEHLER A. H. Formation of picolinic and quinolinic acids following enzymatic oxidation of 3-hydroxyanthranilic acid. J Biol Chem. 1956 Jan;218(1):241–254. [PubMed] [Google Scholar]
- Patil S. S., Shinde V. M. Gas chromatographic studies on the biodegradation of nitrobenzene and 2,4-dinitrophenol in the nitrobenzene plant wastewater. Environ Pollut. 1989;57(3):235–250. doi: 10.1016/0269-7491(89)90015-8. [DOI] [PubMed] [Google Scholar]
- Que L., Jr Extradiol cleavage of o-aminophenol by pyrocatechase. Biochem Biophys Res Commun. 1978 Sep 14;84(1):123–129. doi: 10.1016/0006-291x(78)90272-3. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Pathway for Biodegradation of p-Nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991 Mar;57(3):812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Nishino S. F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1987 May;53(5):1010–1019. doi: 10.1128/aem.53.5.1010-1019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Wyss O., Gibson D. T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979 May 28;88(2):634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- Spanggord R. J., Spain J. C., Nishino S. F., Mortelmans K. E. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol. 1991 Nov;57(11):3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P., Timmis K. N. Influence of para-substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol. 1986 Aug;52(2):334–339. doi: 10.1128/aem.52.2.334-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


