Abstract
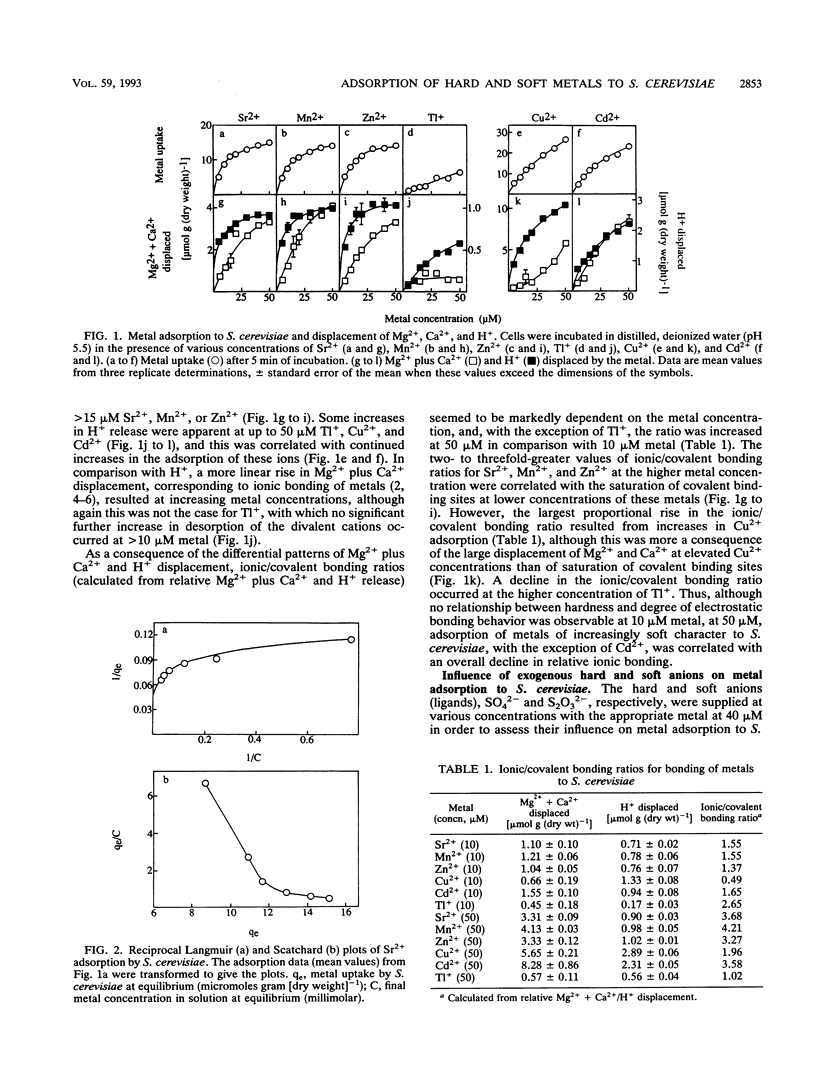
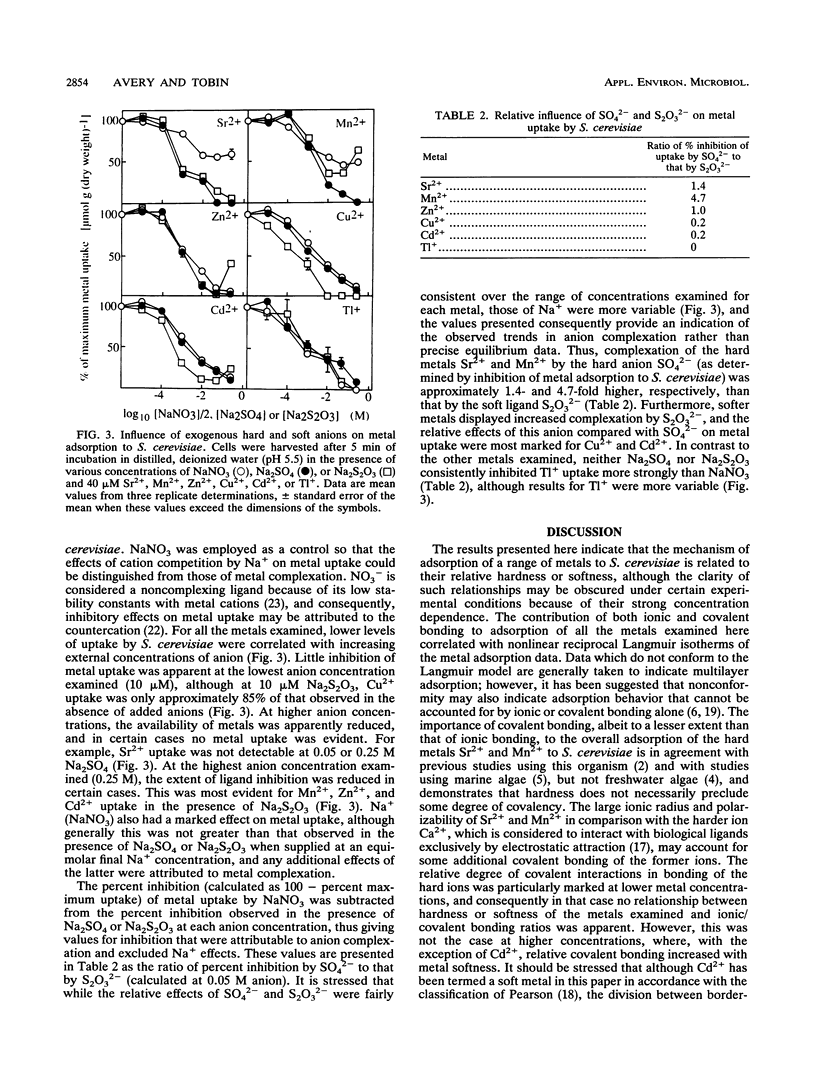
The applicability of the hard-and-soft principle of acids and bases in predicting metal adsorption characteristics in a biological context was investigated for metabolism-independent uptake of the metal ions Sr2+, Mn2+, Zn2+, Cu2+, Cd2+, and Tl+ by Saccharomyces cerevisiae. Metal adsorption increased with external metal concentration (5 to 50 microM), although some saturation of uptake of the harder ions examined, Sr2+, Mn2+, and Zn2+, was evident at the higher metal concentrations. Cation displacement experiments indicated that, with the exception of Tl+, relative covalent bonding (H+ displacement) of the metals was greater at low metal concentrations, while weaker electrostatic interactions (Mg2+ plus Ca2+ displacement) became increasingly important at higher concentrations. These results were correlated with curved Scatchard and reciprocal Langmuir plots of metal uptake data. Saturation of covalent binding sites was most marked for the hard metals, and consequently, although no relationship between metal hardness and ionic/covalent bonding ratios was evident at 10 microM metal, at 50 microM the ratio was generally higher for harder metals. Increasing inhibition of metal uptake at increasing external anion concentrations was partially attributed to the formation of metal-anion complexes. Inhibitory effects of the hard anion SO42(-) were most marked for uptake of the hard metals Sr2+ and Mn2+, whereas greater relative effects on adsorption of the softer cations Cu2+ and Cd2+ were correlated with complexation by the soft anion S2O32(-). Inhibition of uptake of the borderline metal Zn2+ by SO42(-) and that by S2O32(-) were approximately equal.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery S. V., Tobin J. M. Mechanisms of strontium uptake by laboratory and brewing strains of Saccharomyces cerevisiae. Appl Environ Microbiol. 1992 Dec;58(12):3883–3889. doi: 10.1128/aem.58.12.3883-3889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd G. M. Metals and microorganisms: a problem of definition. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):197–203. doi: 10.1111/j.1574-6968.1992.tb14040.x. [DOI] [PubMed] [Google Scholar]
- Macaskie L. E. The application of biotechnology to the treatment of wastes produced from the nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol. 1991;11(1):41–112. doi: 10.3109/07388559109069183. [DOI] [PubMed] [Google Scholar]
- Skowroński T., Szubińska S., Jakubowski M., Pawlik B. Cadmium availability to the cyanobacterium Synechocystis aquatilis in solutions containing chloride. Environ Pollut. 1992;76(2):163–167. doi: 10.1016/0269-7491(92)90104-i. [DOI] [PubMed] [Google Scholar]


