Abstract
The neural cell adhesion molecule (NCAM) and its polysialylated form (PSA-NCAM) contribute to long-term potentiation (LTP) in the CA1 hippocampus. Here we report that the deficient LTP found in slices prepared from NCAM knockout mice and in organotypic slice cultures treated with Endo-N, an enzyme that cleaves the PSA moiety of NCAM, can be rescued by brain-derived neurotrophic factor (BDNF). This effect is not reproduced by nerve growth factor, but can be obtained with high concentrations of NT4/5. The effect of BDNF cannot be accounted for by modifications of N-methyl-d-aspartate receptor-dependent responses or of high-frequency bursts. PSA-NCAM, however, could directly interact with BDNF. Exogenous application of PSA residues or recombinant PSA-NCAM also prevents LTP. Furthermore trkB phosphorylation, and thus BDNF signaling, is reduced in both NCAM knockout mice and Endo-N-treated slice cultures. These results suggest that one action of PSA-NCAM could be to sensitize pyramidal neurons to BDNF, thereby modulating activity-dependent synaptic plasticity.
The neural cell adhesion molecule (NCAM) and its polysialylated form (PSA-NCAM) have been implicated in many aspects of cellular and synaptic plasticity (1–6). They are likely to contribute to cell migration, neurite outgrowth, axon elongation, and synapse formation (6, 7). In addition, they recently were found to be required for the induction of long-term potentiation (LTP), an increase in synaptic efficacy believed to underlie learning and memory mechanisms. Interference with NCAM or PSA-NCAM was found in several studies to prevent induction of LTP. This result was obtained with antibodies directed against NCAM (1, 2), in transgenic mice lacking the NCAM gene (3, 4) and after specific removal of PSA on NCAM with the enzyme Endo-N (3, 5). Consistent with this finding, NCAM knockout mice and mice treated with the enzyme Endo-N also were found to be characterized by a learning deficit (5, 8). As PSA-NCAM is expressed at the synapse and because its expression is affected by neuronal and synaptic activity (3, 9), it has been proposed that PSA-NCAM could represent an important modulator of activity-induced plasticity in the hippocampus (7).
PSA is constituted of long chains of α2–8 sialic acids and is therefore highly negatively charged. A prevailing view is that the action of PSA-NCAM could be to interfere with the adhesive properties of other adhesion molecules, thus promoting remodeling of synaptic membranes (6, 10). Consistent with this interpretation, evidence has accumulated in recent years suggesting that LTP not only involves a change in synaptic function, but is also likely to be associated with remodeling of synaptic structures (11–14). In addition, several molecules involved during development in neurite outgrowth and synapse formation have been implicated in LTP. This is the case for various adhesion molecules (15, 16), including NCAM (7), for tissue plasminogen activator (17), an extracellular protease involved in many aspects of morphogenesis, and for growth factors (18, 19). Among these, brain-derived neurotrophic factor (BDNF) is certainly one interesting candidate for contributing to synaptic plasticity. Evidence obtained by using slice cultures prepared from BDNF-deficient mice showed that LTP was abolished in this model (20), but could be rescued by recombinant BDNF (21) or viral transfection with the BDNF gene (22). BDNF also has been implicated recently in fast modifications of synaptic transmission (23) and proposed to facilitate (24) or even trigger synaptic potentiation (ref. 25, but also see refs. 26–28). Although the contribution of these molecules to synaptic plasticity adds support to the idea that LTP involves both functional and structural modifications, the mechanisms through which these molecules participate to LTP remain unclear. The present study proposes one explanation concerning the role of adhesion molecules and namely provides evidence that the effects of PSA-NCAM on LTP could result from an interaction with the BDNF signaling cascade. It is proposed that the role of PSA-NCAM could be to sensitize pyramidal cells to the action of BDNF, and thus promote an activity-dependent regulation of the action of the growth factor on synaptic plasticity (29).
Methods
Preparation of Cultures and Slices.
Organotypic hippocampal slice cultures were prepared from 7-day-old neonate Sprague–Dawley rats and maintained in culture for 10–15 days at the interface on a porous and transparent membrane as described (30). The culture medium was composed of 50% MEM, 25% horse serum, and 25% Hanks' solution buffered to pH 7.2 by addition of 5 mM Tris and 4 mM NaHCO3, with penicillin and streptomycin added. Cultures were kept in an incubator with 5% CO2 at 36°C for the first 4 days and then transferred at 33°C. Slice cultures usually were maintained for 10–15 days in culture before being tested. Hippocampal slices (400 μm thick) were prepared from adult mice lacking the NCAM gene (8). After decapitation and rapid dissection of the hippocampus, slices were cut by using a tissue chopper (McIlwain, Surrey, U.K.) and transferred in an interface recording chamber.
Materials.
D-AP5 (2-D, l-aminophosphonovalric acid), CNQX (6-cyano-7-nitroquinoxaline-2,3-dione), and bicuculline were purchased from Tocris Neuramin (Bristol, U.K.). Endo-N was purified from phage K1. Its activity was titrated to be 3,500 units/mg, with a concentration of protein of 1 mg/ml in the stock solution. The enzyme was diluted 500–2,000 times and applied on slice cultures for 12–15 h before carrying out electrophysiological recordings.
The recombinant PSA-NCAM-Fc chimera was produced by transfection of TE671 cells, a human rhabdosarcoma cell line that expresses PSA-NCAM on its surface. Cells were transfected with the pIG1-expressing plasmid encoding NCAM-Fc by using Lipofectamine (GIBCO/BRL) according to the manufacturer's intructions. The pIG1-NCAM plasmid was kindly provided by D. L. Simmons (Cell Adhesion Laboratory, Oxford, U.K.). Permanently transfected cell lines secreting PSA-NCAM-Fc into the culture medium were selected. For the present experiments, the same number of PSA-NCAM-Fc producing and control cells were cultured in DMEM alone for 3 days. Culture media were harvested, centrifuged, and concentrated approximately 10–15 times by using Centricon units (Millipore). PSA-NCAM-Fc concentration was estimated by Western blotting by comparison with signals given by purified chimera revealed with anti-human Fc antibody. Chimera concentration was estimated to be 80–100 μg/ml and was applied in the perfusion medium at a final concentration of 20–25 μg/ml.
Electrophysiology.
Recordings in both acutely dissected hippocampal slices and organotypic cultures were carried out by using an interface chamber with the tissue maintained under continuous perfusion with a medium containing 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4, 2.5 mM CaCl2, 22 mM NaHCO3, 10 mM d-glucose, and 2 mM ascorbic acid; the pH was 7.4 and the temperature 33°C. Stimulation was produced by placing one or two electrodes made of twisted nichrome wires on one or both sides of a recording pipette placed in the stratum radiatum of the CA1 area. The recording pipette was filled with medium (5 MOhm). Synaptic responses were recorded by using an Axoclamp 2A amplifier and the initial slope, amplitude, decay time, and area were analyzed on-line and stored for statistical analyses. LTP was induced by using theta burst stimulation (TBS), a pattern consisting of five bursts applied at 5 Hz with each burst being composed of four pulses at 100 Hz and the pattern of stimulation being repeated twice at 10-s interval. In all experiments, LTP was calculated as the increase in excitatory postsynaptic potential (EPSP) slope measured 25–30 min after stimulation versus baseline responses recorded during the 10–15 min immediately preceding high-frequency stimulation.
TrkB Receptor Phosphorylation Assay.
Twelve to 16 hippocampal organotypic slice cultures, treated or not with Endo-N, and six hippocampal slices or the whole hippocampus or cortex from NCAM knockout and wild-type mice were lysed at 4°C in a lysis buffer composed of 50 mM Hepes, 150 mM NaCl, 1% Triton X-100, 2 mM azide, 2 mM orthovanadate, 0.1 M PMSF, 1 mg/ml pepstatin, and 25 μg/ml aprotinin, pH 7.4. The lysate then was centrifuged at 4°C in a bench-top centrifuge at 10,000 rpm for 10 min. The protein concentration of the supernatant was determined (Bio-Rad protein assay kit) and 0.5–1 mg protein of each lysate was incubated with 10 μl monoclonal anti-phospho-tyrosine antibody (Upstate Biotechnology, Lake Placid, NY) overnight at 4°C and precipitated with A/G-Sepharose (Santa Cruz Biotechnology). The immunoprecipitate was boiled in a sample buffer [0.25% bromophenol blue/0.25% xylene cyanol/30% glycerol/20% 2× TBE (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3)] and separated in a 7.5% polyacrlyamide gel and subsequently transferred to a PVDF (Electran, BDH). Phosphorylated trkB receptors were detected by a monoclonal trkB antibody (1:500; Transduction Laboratories, Manhead, CA) followed by incubation with anti-mouse IgG (1:3,000; Bio-Rad). The immune complex was detected by ECL (Amersham Pharmacia) and exposed to autoradiographic film. The band intensities on autoradiograms were analyzed by densitometry.
Results
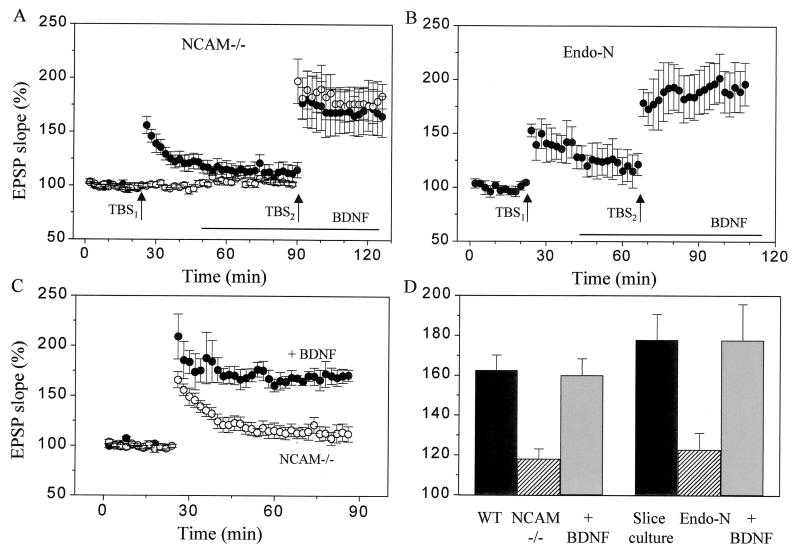
Application of high-frequency stimulation to a group of afferents in hippocampal slices prepared from NCAM-deficient mice induces only a decaying form of potentiation and no stable LTP (3, 4). This phenotype, however, can be reversed: treatment of slices prepared from NCAM knockout mice with 50–100 ng/ml of BDNF was capable of fully rescuing the LTP defect. In the experiments illustrated in Fig. 1A, TBS was applied to two independent groups of afferents in the CA1 area of NCAM-deficient hippocampal slices. Stimulation applied before BDNF treatment reproducibly resulted in a decaying potentiation (TBS1, ●). In contrast, restimulation of the same pathway 30 min after BDNF application (TBS2, ●) produced a markedly enhanced LTP (68 ± 18% versus 16 ± 6%, respectively, n = 5, P < 0.05). The LTP obtained in the presence of BDNF in NCAM-deficient slices was indistinguishable from the LTP obtained in wild-type animals (63 ± 8%; Fig. 1D). This effect of BDNF was not the result of the repeated application of high-frequency trains, because LTP also was restored when stimulation was applied to another naive input in the same slice (Fig. 1A, ○, 76 ± 14%, n = 5). Also, the LTP induced in the presence of BDNF in NCAM-deficient slices remained stable for at least 1 h as illustrated in Fig. 1C (n = 3). Note also that application of BDNF did not result in detectable modifications of the properties of excitatory transmission in these NCAM-deficient slices. We observed no significant changes in amplitude of either potentiated or naive pathways as well as no modifications in the ratio of facilitation evoked by using a paired-pulse interval of 50 ms (153 ± 6% versus 155 ± 6%). Responses elicited at a shorter interpulse interval (10 ms) were slightly, but not significantly, enhanced by BDNF application (ratio of slopes of second over first EPSP: 97.5 ± 5% versus 90.2 ± 4% after and before BDNF, respectively; n = 8). The values of LTP obtained in all experiments carried out in wild-type animals and NCAM knockout mice with or without treatment with BDNF are summarized in Fig. 1D.
Figure 1.
BDNF restores LTP in PSA-NCAM-deficient slices. (A) Induction of LTP using TBS in slices prepared from NCAM-deficient mice resulted in a decaying potentiation (●, TBS1). However, restimulation of the same pathway (●, TBS2) or of another independent, naive input (○, TBS2) after BDNF application (100 ng/ml, bar) restored LTP. Data are mean ± SEM of five experiments. (B) LTP induction in slice cultures treated with Endo-N, an enzyme that selectively cleaves the PSA moiety of NCAM. The defective LTP observed in Endo-N treated cultures (TBS1) was restored when stimulation was reapplied after BDNF application (100 ng/ml, TBS2). Data are mean ± SEM of four experiments. (C) The LTP induced by TBS in the presence of BDNF in NCAM-deficient slices remained stable for over 1 h. Data are mean ± SEM of 3–4 experiments. (D) Summary of the amount of LTP induced by TBS in the various conditions tested: slices of wild-type mice (WT, n = 3–6) and NCAM knockout mice recorded in the absence (NCAM −/−) or presence of BDNF (100 ng/ml, n = 7–10); nontreated organotypic slice cultures (n = 6), slice cultures treated for 12 h with Endo-N (n = 6), and slice cultures treated for 12 h with Endo-N but exposed for 30 min to 100 ng/ml BDNF (n = 7). The defective LTP observed in NCAM knockout mice and Endo-N-treated cultures is fully restored by BDNF.
Because elimination of the PSA moiety of NCAM by treatment with the enzyme Endo-N also resulted in a blockade of LTP (3, 5), we then tested whether BDNF similarly rescued the LTP defect observed under this condition. Fig. 1B shows that the decaying LTP found in organotypic slice cultures treated for 12 h with Endo-N (TBS1) was completely restored by BDNF (100 ng/ml) applied in the perfusion medium 30 min before restimulation of the same pathway (TBS2, 93 ± 19% versus 22 ± 8%, n = 4, P < 0.05). In six different experiments, the LTP obtained in Endo-N-treated organotypic cultures after BDNF application was comparable to that obtained in control slices, treated or not with BDNF (Fig. 1D). Thus BDNF also rescued the defect in synaptic plasticity associated with elimination of PSA on NCAM.
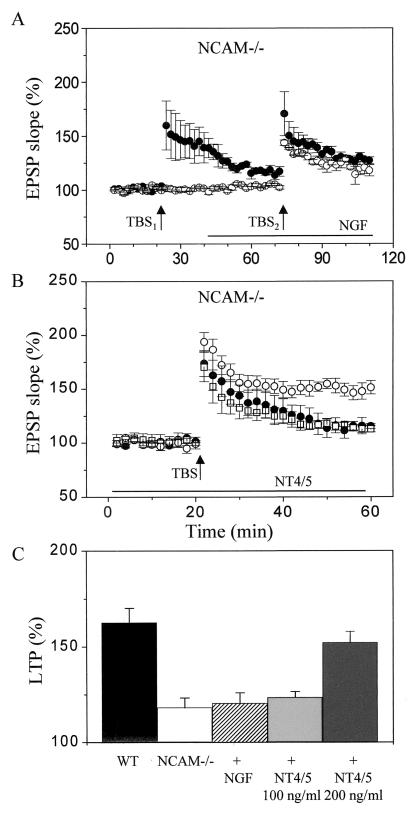
To test the specificity of the effect of BDNF, we then compared its action with those of other growth factors. Fig. 2A shows that in contrast to what has been obtained with BDNF, nerve growth factor (NGF) (100 ng/ml) did not reverse the deficient LTP obtained in NCAM knockout mice (21 ± 5% LTP in the presence of NGF versus 18 ± 5% in untreated slice cultures). Similarly, the defective LTP observed in Endo-N-treated slice cultures was not rescued by 100 ng/ml NGF (26 ± 6% versus 22 ± 8% LTP in the presence and absence of NGF, respectively; n = 3). NT4/5, at a concentration of 100 ng/ml (□ in Fig. 2B), also was unable to restore LTP (23 ± 3%, n = 6). However, at a higher concentration (200 ng/ml, ●, Fig. 2B), NT4/5 resulted in a significant enhancement in the amount of LTP induced by high-frequency stimulation (52 ± 5% versus 18 ± 5% in the presence and absence of NT4/5, respectively, n = 4–8, P < 0.05, or 63 ± 7% in wild-type animals). The amount of LTP obtained in NCAM-deficient animals under the various conditions tested are summarized in Fig. 3C.
Figure 2.
Specificity of the effect of BDNF in restoring LTP in NCAM knockout mice. (A) Absence of effect of NGF (100 ng/ml) in reversing the LTP defect observed in NCAM knockout mice. The decaying potentiation recorded in slices from NCAM-deficient mice (●, TBS1) was unaltered by addition of NGF to the slice (bar) and either restimulation of the same pathway (●, TBS2) or induction of LTP on another naive input (○, TBS2). Data are mean ± SEM of four experiments. (B) Graph showing the amount of LTP induced in slices prepared from NCAM-deficient mice (●), slices from NCAM-deficient mice, but treated for 30 min either with 100 ng/ml NT4/5 (□) or 200 ng/ml NT4/5. Data are mean ± SEM of 3–4 experiments. (C) Summary of the amount of LTP obtained in slices prepared from wild-type mice (n = 7), NCAM-deficient mice (n = 6), NCAM-deficient mice treated for 30 min with either 100 ng/ml NGF (n = 6), 100 ng/ml NT4/5 (n = 6), or 200 ng/ml NT4/5 (n = 4).
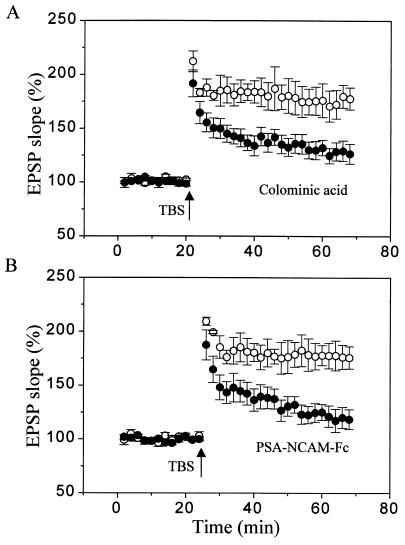
Figure 3.
Inhibition of LTP produced by exogenous application of colominic acid and recombinant PSA-NCAM. (A) Graph showing the LTP obtained in control hippocampal slices (○) and slices treated for 30 min before stimulation with 0.5 mg/ml of colominic acid (●), a mixture of α2–8-linked PSAs of various lengths. Data are mean ± SEM of seven experiments. (B) Graph showing a similar blockade of LTP produced by exogenous application for 30 min before stimulation of a recombinant PSA-NCAM-Fc chimera (20–25 μg/ml; ●), obtained by transfection of a tumoral cell line. The control LTP (○) was obtained in slices treated with the conditioned medium of transfected, nonsecreting cells. Data are mean ± SEM of five experiments.
We then investigated whether the capacity of BDNF to restore LTP in slices prepared from NCAM knockout mice or in Endo-N-treated slice cultures was caused by changes in the parameters controlling the induction of LTP. Control experiments carried out by measuring the area under the burst responses used to induce LTP before and after BDNF treatment showed that the summation of responses within the bursts was not affected by BDNF treatment (ratio of burst areas measured after versus before BDNF application of 99 ± 1%, 99 ± 3% and 101 ± 2% for the first, third, and fifth bursts, respectively, n = 4). Similarly, we did not detect any effect of BDNF on pure N-methyl-d-aspartate (NMDA) receptor-mediated synaptic responses recorded in NCAM knockout mice in the presence of low magnesium (0.1 mM), NBQX (2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline, 5 μM) to block AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and bicuculline (10 μM) to block inhibitory transmission (EPSP of 102 ± 4% after versus before BDNF application, n = 4). Thus, the rescue of LTP by BDNF in PSA-NCAM-deficient hippocampus was unlikely to reflect a detectable modulation of NMDA receptor-dependent induction mechanisms.
Another possibility was a direct interaction between PSA-NCAM and BDNF signaling cascades. PSA-NCAM is a highly negatively charged molecule that might bind BDNF or contribute to BDNF activation of its cell surface receptor. As illustrated in Fig. 3, treatment of hippocampal slices with either colominic acid, a mixture of α2–8-linked PSAs of various lengths, or with a recombinant PSA-NCAM-Fc chimera obtained through transfection of a human rhabdo-sarcoma cell line, reproduced the defect in LTP observed in NCAM knockout mice or Endo-N-treated cultures. The LTP obtained in the presence of 0.5 mg/ml colominic acid or 20–25 μg/ml soluble PSA-NCAM-Fc chimera was significantly reduced in comparison to control LTP or the LTP obtained in the presence of conditioned medium obtained from nonsecreting transfected cells (28 ± 8% and 21 ± 9% versus 75 ± 12% and 78 ± 11%, respectively, n = 5–7; P < 0.05). This result therefore indicated that addition of soluble PSA in the extracellular space is sufficient to alter LTP mechanisms, thereby pointing to the possibility of a direct interaction between PSA-NCAM and BDNF at the cell surface.
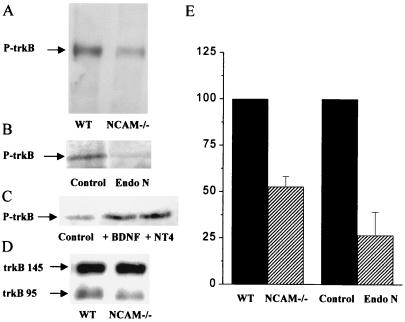
To further test this possibility, we then assessed the level of activation of the BDNF cascade by measuring the phosphorylation of trkB, the BDNF receptor, in both NCAM knockout mice and Endo-N-treated slice cultures. For these experiments, hippocampal slices or the hippocampus or cortex from NCAM knockout mice and Endo-N-treated and untreated hippocampal slice cultures were subjected to immunoprecipitation using a monoclonal phospho-tyrosine-specific antibody followed by immunoblotting using an anti-trkB receptor mAb. Results then were analyzed by densitometry of autoradiographic films. As illustrated in Fig. 4, the level of trkB phosphorylation measured in the hippocampus and cortex of NCAM knockout mice (Fig. 4A) and in Endo-N-treated slice cultures (Fig. 4B) was markedly lower than that observed in either wild-type animals or untreated slice cultures. The level of trkB phosphorylation measured by densitometry in three separate experiments for each condition and normalized for control values is summarized in Fig. 4E (53 ± 5% and 27 ± 13% in, respectively, NCAM knockout mice and Endo-N-treated cultures, n = 3, P < 0.05). As illustrated in Fig. 4C, the reduced phosphorylation of trkB observed in slices of NCAM-deficient mice could be reversed by addition of exogenous BDNF (100 ng/ml) or NT4/5 (200 ng/ml). Furthermore, Fig. 4D shows that the reduced level of trkB phosphorylation observed in NCAM knockout mice was not the result of a decrease in the total amount of receptor protein, because Western analyzes carried out using the anti-trkB receptor mAb without immunoprecipitation revealed no differences between transgenic and wild-type animals (101 ± 5%, n = 4, data normalized in each experiment to wild-type values). Finally, quantification of the ratio between truncated and not truncated forms of trkB also revealed no significant differences (ratio of 153 ± 33% and 147 ± 33% in transgenic and wild-type animals, respectively, n = 4). Thus, under the two conditions tested, the deficiency in PSA-NCAM was associated with a reduced level of BDNF receptor activation that was independent of the amount of receptor expressed at the cell surface.
Figure 4.
Reduced trkB phosphorylation in NCAM knockout mice and organotypic slice cultures treated with Endo-N. (A) Immunoblot obtained by using a monoclonal anti-trkB receptor antibody after immunoprecipitation with a monoclonal phosphospecific antibody recognizing phosphorylated trkB in wild-type (WT) and NCAM knockout mice (NCAM −/−). (B) Immunoblot obtained in the same way after immunoprecipitation with the antiphosphotyrosine antibody from control and Endo-N-treated slice cultures. (C) Immunoblot obtained after immunoprecipitation from slices prepared from NCAM-deficient mice in control conditions (Left), and after treatment for 30 min with either 100 ng/ml BDNF (Middle) or 200 ng/ml NT4/5 (Right). (D) Western blot carried out with a monoclonal trkB antibody before immunoprecipitation and showing that the amount of trkB receptor protein was comparable in wild-type (WT) and NCAM knockout mice (NCAM −/−). (E) Quantitative assessment by densitometry analysis of autoradiographic films of trkB phosphorylation in wild-type and NCAM knockout mice and in control and Endo-N-treated slice cultures as revealed in three different experiments for each condition. Differences are statistically significant (P < 0.05; n = 3; t test).
Discussion
The results of the present study provide evidence that the defective LTP observed in PSA-NCAM-deficient hippocampus can be selectively rescued by the growth factor BDNF and is linked to a reduced activation of the BDNF signaling cascade. This suggests a novel mechanism of action through which PSA-NCAM could modulate LTP and the possibility that this adhesion molecule sensitizes pyramidal cells to the action of BDNF.
The role of NCAM and PSA-NCAM in LTP has been clearly established in several studies using various approaches. Interference with NCAM, either through antibodies (1, 2) or by elimination of all isoforms of NCAM (3, 4), results in a deficient LTP. A key role in these effects appears to be played by PSA-NCAM, the polysialylated form of the molecule. Elimination of PSA on NCAM by the specific enzyme Endo-N reproduces the same phenotype as elimination of NCAM (3, 5). Also, PSA-NCAM expression at the cell surface is modulated by neuronal and synaptic activity (3, 9) and appears to be critical for properties of plasticity (6, 7). Finally, the present results indicate that inhibition of LTP also is obtained by exogenous application of either PSA or recombinant PSA-NCAM. It seems likely therefore that the highly negatively charged polysialic residues attached to NCAM significantly contribute to the role of this adhesion molecule in LTP.
The mechanisms through which PSA-NCAM or other adhesion molecules participate to synaptic plasticity remain unclear. The present study provides evidence that, in the case of PSA-NCAM, an important mechanism could be through modulation of BDNF signaling. BDNF plays an important role in many aspects of development and plasticity in the central nervous system and recently has been implicated in LTP and rapid modifications of synaptic function (18, 19). Neuronal activity and particularly high-frequency stimulation enhances the expression of BDNF mRNA (31), a deficiency in BDNF or blockade of trkB results in a blockade of LTP (20, 32), and application of BDNF facilitates LTP induction (24). It has even been reported that direct application of BDNF could result in synaptic potentiation (25). This effect, however, was not reproduced in slices by all studies (24, 26–28) and was not observed here. Thus, although BDNF appears to be required for LTP induction, application of the growth factor is most likely not sufficient to trigger synaptic potentiation.
BDNF was found here to restore LTP in PSA-NCAM-deficient hippocampus. Interestingly, NT4/5, which also activates trkB, reproduced the effects of BDNF, but only at higher concentrations. The reason for this difference is unknown, but it might involve functional interactions such as those recently reported between trkB and the p75 receptor (33, 34). With regard to BDNF, several mechanisms can be considered to explain its action on LTP. In dissociated cell cultures, BDNF has been reported to modulate NMDA receptor function (35) through a modification of its glycine sensitivity (36). However, under conditions where glycine is present, such as in slices and slice cultures, BDNF action on NMDA receptors was not observed (36). Consistent with this, we did not detect any effect of BDNF on pure NMDA receptor-mediated synaptic responses. BDNF also has been proposed to affect presynaptic properties (23) and in particular paired-pulse facilitation elicited at high frequency (27, 37). This effect could influence the summation of responses during high-frequency trains and thus facilitate LTP induction. We compared here high-frequency bursts used to induce LTP before and after application of BDNF, but did not detect any significant difference that could have accounted for its facilitating action on LTP. We conclude therefore that the ability of BDNF to restore LTP in PSA-NCAM-deficient hippocampus is unlikely to involve a modulation of LTP induction mechanisms.
A more likely explanation, however, is a direct interaction between PSA-NCAM and BDNF signaling cascades. In particular, the present results suggest that the LTP defect observed in PSA-NCAM-deficient slices could result from a deficient activation of BDNF signaling. The studies by Kortje et al. (20) have shown that even a reduction of BDNF levels such as found in BDNF heterozygous mutant mice is sufficient to result in a deficient LTP. Furthermore, the defect could be rescued by exogenous application of BDNF (21) as was also observed here in PSA-NCAM-deficient slices. Consistent with this interpretation, we found that trkB phosphorylation was indeed altered in both NCAM knockout mice and slice cultures treated with Endo-N, not because of a down-regulation of the receptor but because of a reduced activation of trkB. Thus, interference with PSA-NCAM expression at the cell surface somehow affected BDNF activation of its receptor. Furthermore, recent evidence from experiments carried out by using an in vitro gel filtration assay show that the highly negatively charged PSA-NCAM can selectively bind BDNF and thus modulate its signaling. Finally, this interpretation is also consistent with the observation that bath application of soluble PSA or recombinant PSA-NCAM-Fc chimera, probably through binding of BDNF, reproduces the same LTP defect as that observed in PSA-NCAM-deficient slices and BDNF knockout mice. Taken together, these results support the hypothesis that one important role of PSA-NCAM could be to enhance or facilitate BDNF activation of its receptor, and thereby sensitize pyramidal cells to the action of the growth factor. Because PSA-NCAM is expressed at the cell surface and at synapses in an activity-dependent manner (3, 9), this mechanism could represent a way to amplify or selectively enhance the action of BDNF at active sites or active synapses. This might be particularly important for mechanisms of synaptic plasticity and LTP where the synaptic changes exhibit some input specificity. This mechanism also could be involved in the activity-dependent effects of BDNF that have been reported recently (29). It is also interesting to note that a similar interaction between NCAM and the fibroblast growth factor has been described in a context of neurite outgrowth (38). These observations open the possibility that in addition to their proadhesive or antiadhesive properties (6), adhesion molecules could contribute to signaling cascades by interacting with and modulating the action of growth factors (39).
Acknowledgments
We thank M. Moosmayer and L. Parisi for the preparation of the cultures and S. Chliate for technical assistance. This work was supported by grants from the Swiss Science Foundation to D.M., the priority program PNR38 to D.M. and J.Z.K., and European Economic Community Biotech grants to G.R. and J.Z.K and to D.M.
Abbreviations
- NCAM
neural cell adhesion molecule
- BDNF
brain-derived neurotrophic factor
- PSA
polysialic acid
- LTP
long-term potentiation
- TBS
theta burst stimulation
- EPSP
excitatory postsynaptic potential
- NGF
nerve growth factor
- NMDA
N-methyl-d-aspartate
References
- 1.Lüthi A, Laurent J P, Figurov A, Muller D, Schachner M. Nature (London) 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 2.Ronn L C B, Bock E, Lindenmann D, Jahnsen H. Brain Res. 1995;677:145–151. doi: 10.1016/0006-8993(95)00147-i. [DOI] [PubMed] [Google Scholar]
- 3.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss J Z. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 4.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J D, Lledo P M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker C G, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Rutishauser U, Landmesser L. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 7.Kiss J Z, Rougon G. Curr Opin Neurobiol. 1997;7:640–646. doi: 10.1016/s0959-4388(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 8.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S. Nature (London) 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 9.Kiss J Z, Wang C, Olive S, Rougon G, Lang J, Baetens D, Harry D, Pralong W F. EMBO J. 1994;13:5284–5292. doi: 10.1002/j.1460-2075.1994.tb06862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutishauser U, Acheson A, Hall A K, Mann D M, Sunshine J. Science. 1988;240:53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- 11.Muller D. Rev Neurosci. 1997;8:77–93. doi: 10.1515/revneuro.1997.8.2.77. [DOI] [PubMed] [Google Scholar]
- 12.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 13.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 14.Toni N, Buchs P-A, Nikonenko I, Bron C R, Muller D. Nature (London) 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 15.Tang L, Hung C P, Schuman E M. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 16.Staubli U, Chun D, Lynch G. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y Y, Bach M E, Lipp H P, Zhuo M, Wolfer D P, Hawkins R D, Schoonjans L, Kandel E R, Godfraind J M, Mulligan R, et al. Proc Natl Acad Sci USA. 1996;93:8699–7004. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu B, Figurov A. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 20.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 22.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine E S, Black I B, Plummer M R. Adv Pharmacol. 1998;42:921–924. doi: 10.1016/s1054-3589(08)60897-2. [DOI] [PubMed] [Google Scholar]
- 24.Figurov A, Pozzo Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 25.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottschalk W, Pozzo-Miller L D, Figurov A, Lu B. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frerking M, Malenka R C, Nicoll R A. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 29.Boulanger L, Poo M M. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- 30.Stoppini L, Buchs P-A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 31.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Kolbeck R, Barde Y A, Bonhoeffer T, Kossel A. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibel M, Hoppe E, Barde Y A. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman W J, Greene L A. Exp Cell Res. 1999;253:131–142. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- 35.Levine E S, Crozier R A, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis C R, Xiong Z G, Plant J R, Churchill D, Lu W Y, MacVicar B A, MacDonald J F. J Neurophysiol. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- 37.Pozzo-Miller L D, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z H, Lu B. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saffell J L, Williams E J, Mason I J, Walsh F S, Doherty P. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 39.Doherty P, Walsh F S. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]