Abstract
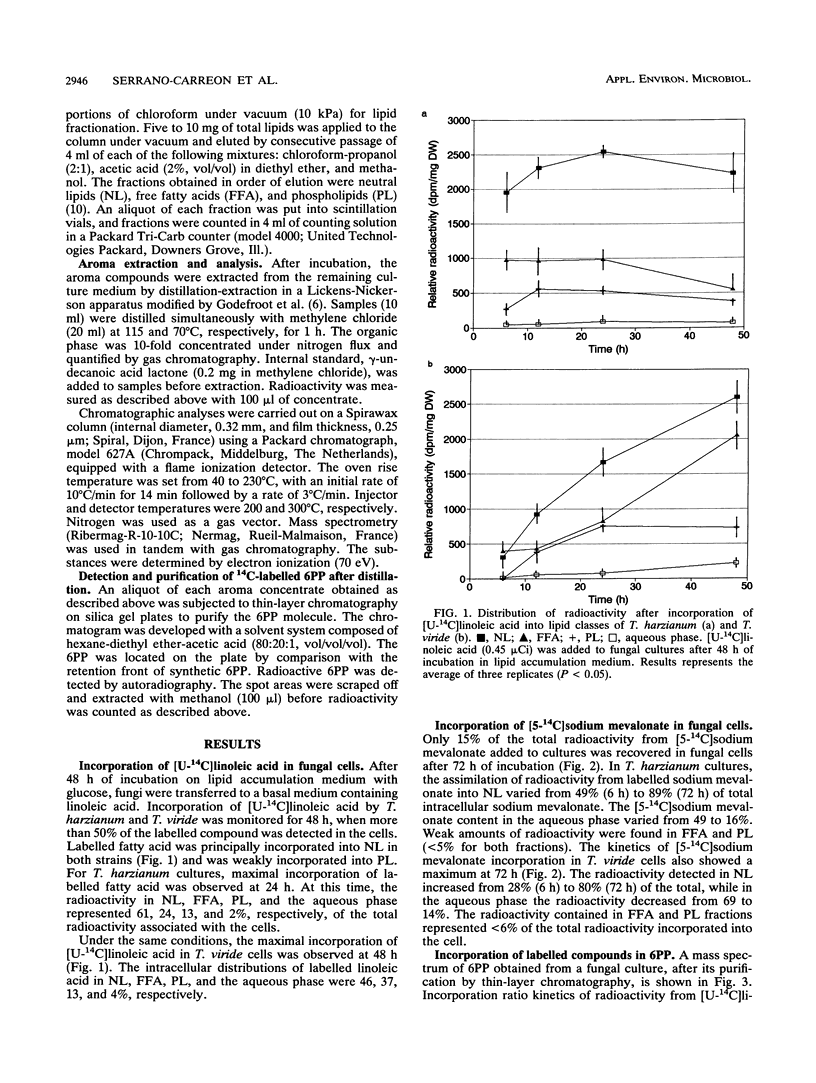
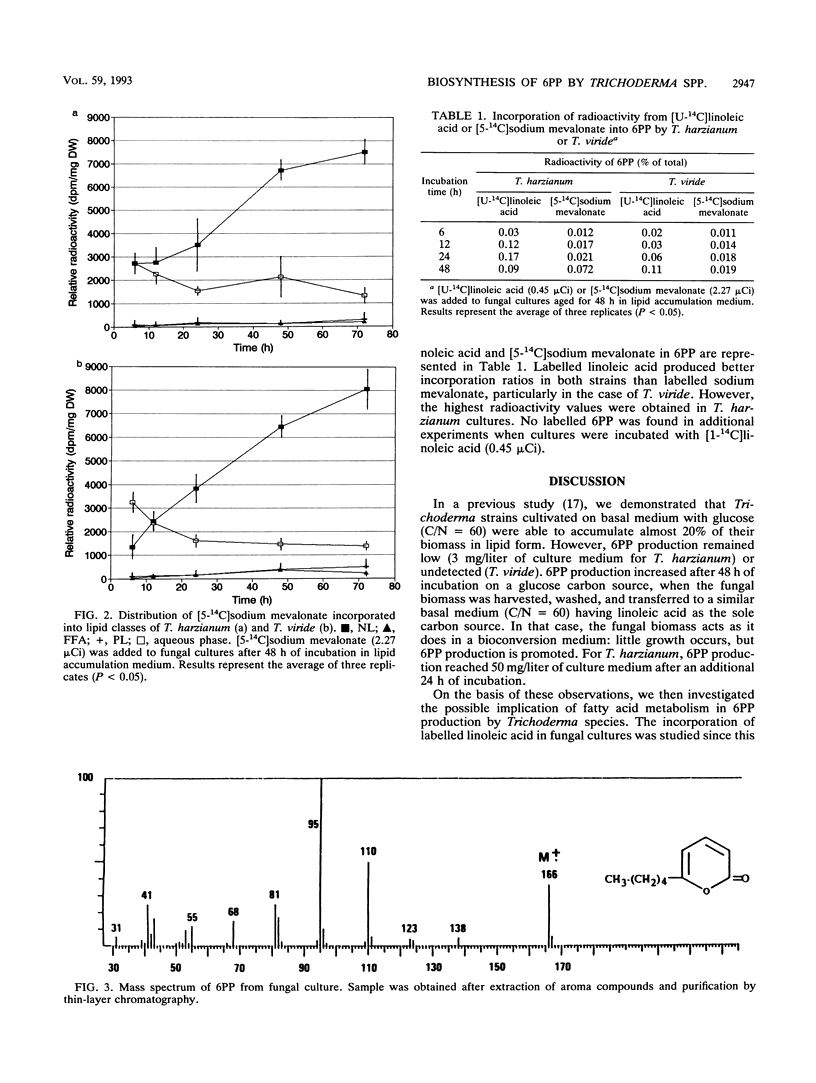
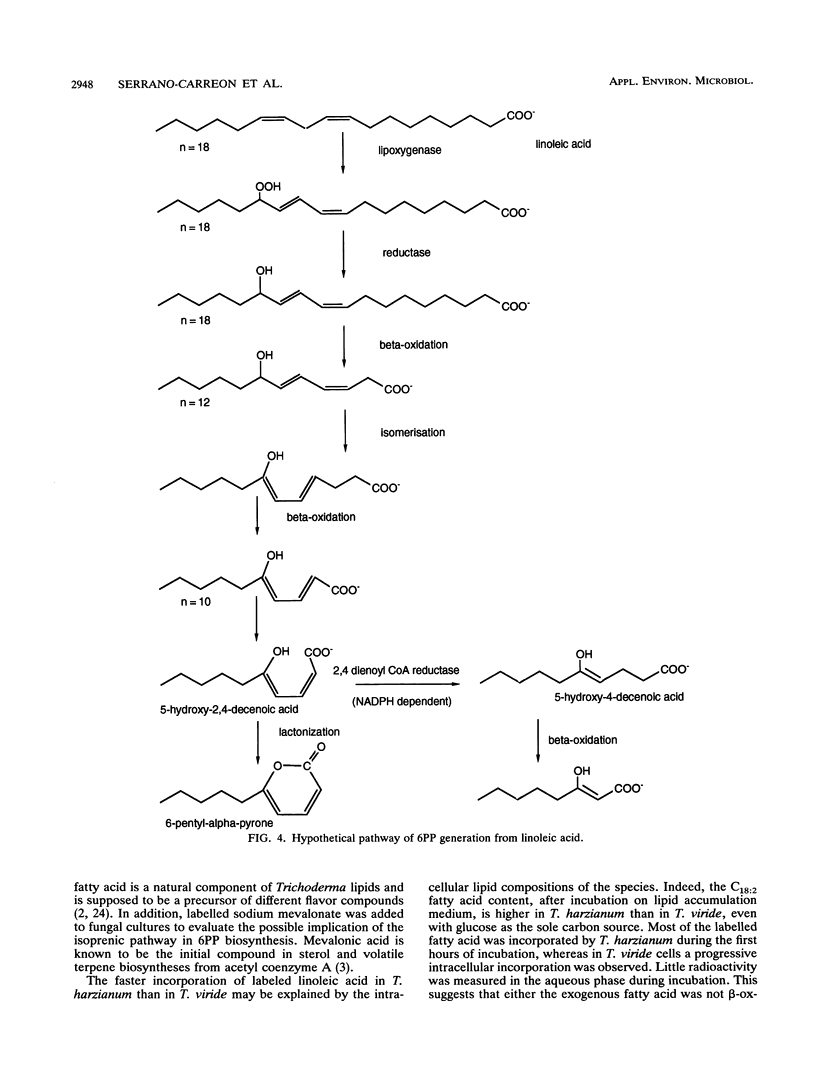
The understanding of the biosynthetic pathway of 6-pentyl-α-pyrone in Trichoderma species was achieved by using labelled linoleic acid or mevalonate as a tracer. Incubation of growing cultures of Trichoderma harzianum and T. viride with [U-14C]linoleic acid or [5-14C]sodium mevalonate revealed that both fungal strains were able to incorporate these labelled compounds (50 and 15%, respectively). Most intracellular radioactivity was found in the neutral lipid fraction. At the initial time of incubation, the radioactivity from [14C]linoleic acid was incorporated into 6-pentyl-α-pyrone more rapidly than that from [14C]mevalonate. No radioactivity incorporation was detected in 6-pentyl-α-pyrone when fungal cultures were incubated with [1-14C]linoleic acid. These results suggested that β-oxidation of linoleic acid was a probable main step in the biosynthetic pathway of 6-pentyl-α-pyrone in Trichoderma species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banthorpe D. V., Charlwood B. V., Francis M. J. The biosynthesis of monoterpenes. Sogo Kango. 1972 Apr;72(2):115–155. [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- Jensen E. C., Ogg C., Nickerson K. W. Lipoxygenase inhibitors shift the yeast/mycelium dimorphism in Ceratocystis ulmi. Appl Environ Microbiol. 1992 Aug;58(8):2505–2508. doi: 10.1128/aem.58.8.2505-2508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y., Salem N., Jr Separation of lipid classes by solid phase extraction. J Lipid Res. 1990 Dec;31(12):2285–2289. [PubMed] [Google Scholar]
- Maga J. A. Lactones in foods. CRC Crit Rev Food Sci Nutr. 1976 Sep;8(1):1–56. doi: 10.1080/10408397609527216. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Beppu T., Arima K. Crystallization and positional specificity of hydroperoxidation of Fusarium lipoxygenase. Biochim Biophys Acta. 1978 Sep 28;530(3):439–450. [PubMed] [Google Scholar]
- Mizugaki M., Uchiyama M., Okui S. Metabolism of hydroxy fatty acids. V. Metabolic conversion of homoricinoleic and homoricinelaidic acids by Escherichia coli K 12. J Biochem. 1965 Sep;58(3):273–278. doi: 10.1093/oxfordjournals.jbchem.a128198. [DOI] [PubMed] [Google Scholar]
- OKUI S., UCHIYAMA M., MIZUGAKI M. METABOLISM OF HYDROXY FATTY ACIDS. II. INTERMEDIATES OF THE OXIDATIVE BREAKDOWN OF RICINOLEIC ACID BY GENUS CANDIDA. J Biochem. 1963 Dec;54:536–540. doi: 10.1093/oxfordjournals.jbchem.a127827. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]


