Abstract
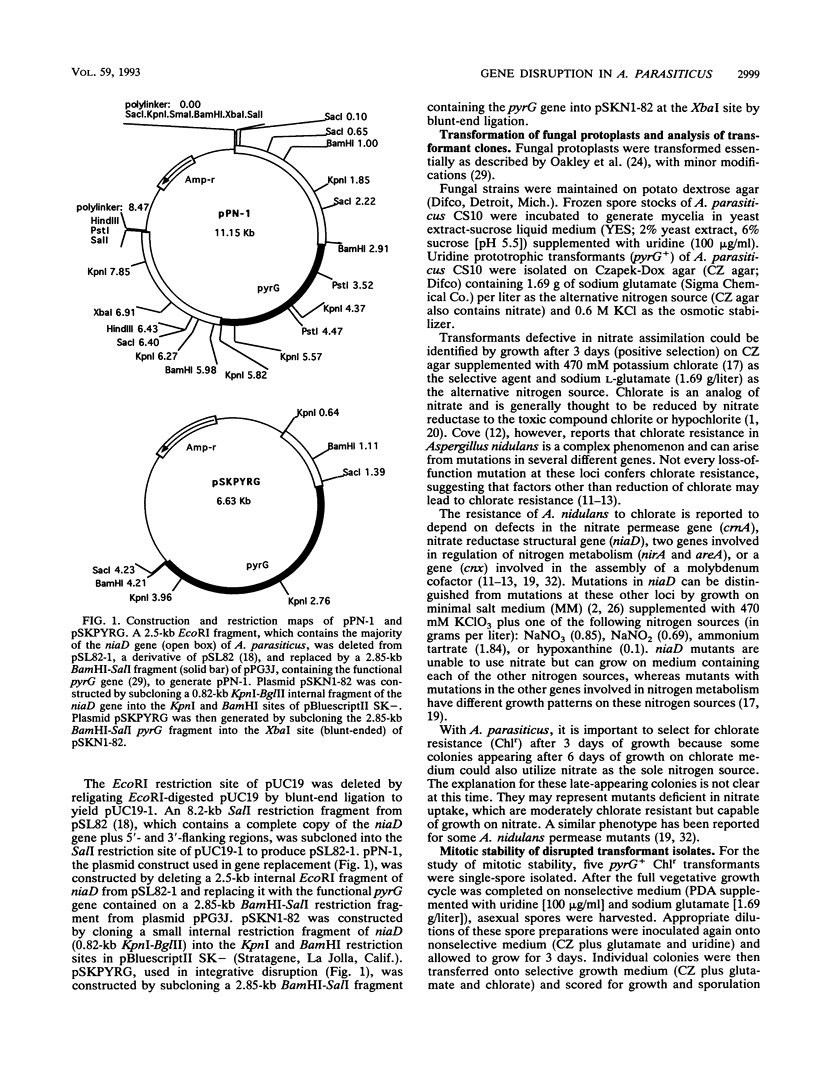
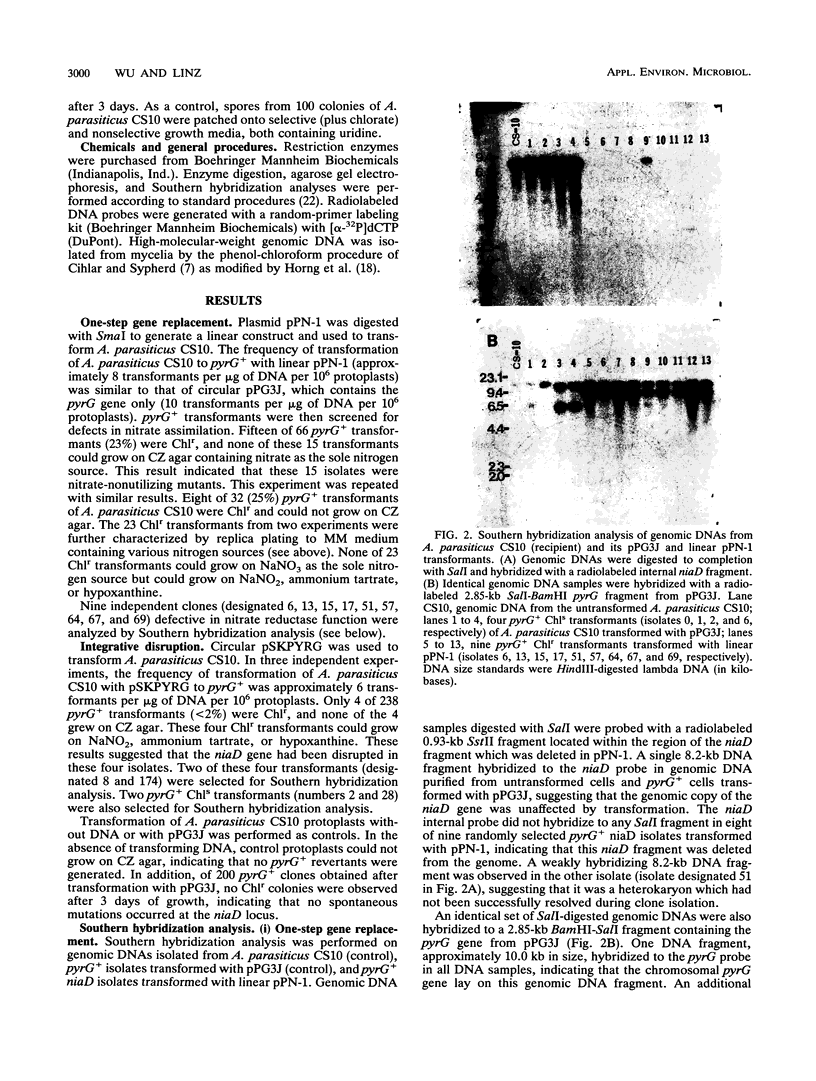
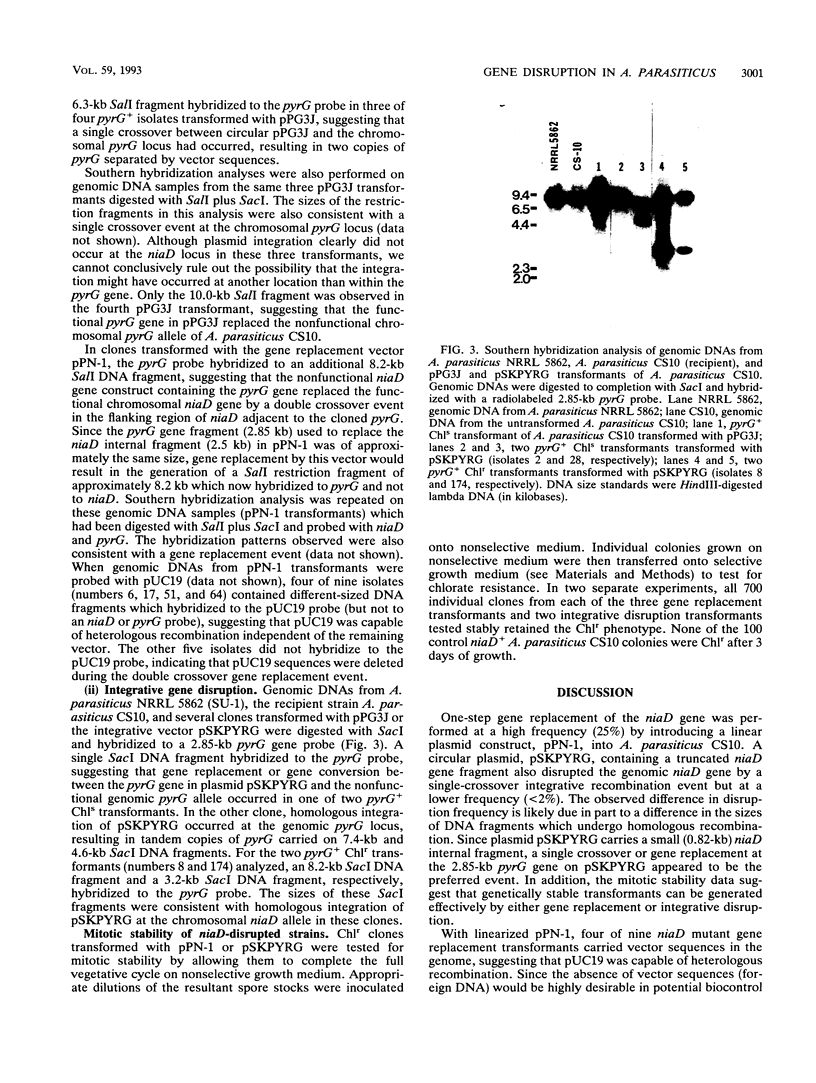
Functional disruption of the gene encoding nitrate reductase (niaD) in Aspergillus parasiticus was conducted by two strategies, one-step gene replacement and the integrative disruption. Plasmid pPN-1, in which an internal DNA fragment of the niaD gene was replaced by a functional gene encoding orotidine monophosphate decarboxylase (pyrG), was constructed. Plasmid pPN-1 was introduced in linear form into A. parasiticus CS10 (ver-1 wh-1 pyrG) by transformation. Approximately 25% of the uridine prototrophic transformants (pyrG+) were chlorate resistant (Chlr), demonstrating their inability to utilize nitrate as a sole nitrogen source. The genetic block in nitrate utilization was confirmed to occur in the niaD gene by the absence of growth of the A. parasiticus CS10 transformants on medium containing nitrate as the sole nitrogen source and the ability to grow on several alternative nitrogen sources. Southern hybridization analysis of Chlr transformants demonstrated that the resident niaD locus was replaced by the nonfunctional allele in pPN-1. To generate an integrative disruption vector (pSKPYRG), an internal fragment of the niaD gene was subcloned into a plasmid containing the pyrG gene as a selectable marker. Circular pSKPYRG was transformed into A. parasiticus CS10. Chlr pyrG+ transformants were screened for nitrate utilization and by Southern hybridization analysis. Integrative disruption of the genomic niaD gene occurred in less than 2% of the transformants. Three gene replacement disruption transformants and two integrative disruption transformants were tested for mitotic stability after growth under nonselective conditions. All five transformants were found to stably retain the Chlr phenotype after growth on nonselective medium.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barratt R. W., Johnson G. B., Ogata W. N. Wild-type and mutant stocks of Aspergillus nidulans. Genetics. 1965 Jul;52(1):233–246. doi: 10.1093/genetics/52.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. W., Goldblatt L. A. The isolation of mutants of Aspergillus flavus and A.parasiticus with altered aflatoxin producing ability. Sabouraudia. 1973 Nov;11(3):235–241. [PubMed] [Google Scholar]
- Chang P. K., Skory C. D., Linz J. E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992 Mar;21(3):231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- Chevalet L., Tiraby G., Cabane B., Loison G. Transformation of Aspergillus flavus: construction of urate oxidase-deficient mutants by gene disruption. Curr Genet. 1992 May;21(6):447–453. doi: 10.1007/BF00351654. [DOI] [PubMed] [Google Scholar]
- Cihlar R. L., Sypherd P. S. The organization of the ribosomal RNA genes in the fungus Mucor racemosus. Nucleic Acids Res. 1980 Feb 25;8(4):793–804. [PMC free article] [PubMed] [Google Scholar]
- Cove D. J. Chlorate toxicity in Aspergillus nidulans. Studies of mutants altered in nitrate assimilation. Mol Gen Genet. 1976 Jul 23;146(2):147–159. doi: 10.1007/BF00268083. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Cholorate toxicity in Aspergillus nidulans: the selection and characterisation of chlorate resistant mutants. Heredity (Edinb) 1976 Apr;36(2):191–203. doi: 10.1038/hdy.1976.24. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol Rev Camb Philos Soc. 1979 Aug;54(3):291–327. doi: 10.1111/j.1469-185x.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. Effect on aflatoxin production of competition between wild-type and mutant strains of Aspergillus parasiticus. Mycopathologia. 1987 Feb;97(2):93–96. doi: 10.1007/BF00436844. [DOI] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Bennett R. A., Wogan G. N. Metabolic activation of aflatoxin B1: patterns of DNA adduct formation, removal, and excretion in relation to carcinogenesis. Drug Metab Rev. 1982;13(4):581–602. doi: 10.3109/03602538209011088. [DOI] [PubMed] [Google Scholar]
- Horng J. S., Linz J. E., Pestka J. J. Cloning and characterization of the trpC gene from an aflatoxigenic strain of Aspergillus parasiticus. Appl Environ Microbiol. 1989 Oct;55(10):2561–2568. doi: 10.1128/aem.55.10.2561-2568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie S. T., Wilkinson J. Q., Crawford N. M. Effect of Chlorate Treatment on Nitrate Reductase and Nitrite Reductase Gene Expression in Arabidopsis thaliana. Plant Physiol. 1991 Nov;97(3):873–879. doi: 10.1104/pp.97.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara E. B., Timberlake W. E. Molecular characterization of the Aspergillus nidulans yA locus. Genetics. 1989 Feb;121(2):249–254. doi: 10.1093/genetics/121.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E., Mitchell B. L., Oakley C. E., Carmona C., Gray G. L., May G. S. Cloning, mapping and molecular analysis of the pyrG (orotidine-5'-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 1987;61(3):385–399. doi: 10.1016/0378-1119(87)90201-0. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark A. A. Mutagenicity and carcinogenicity of mycotoxins: DNA binding as a possible mode of action. Annu Rev Microbiol. 1980;34:235–262. doi: 10.1146/annurev.mi.34.100180.001315. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Marshall M. A. Genetic engineering of filamentous fungi. Science. 1989 Jun 16;244(4910):1313–1317. doi: 10.1126/science.2525275. [DOI] [PubMed] [Google Scholar]
- Tomsett A. B., Cove D. J. Deletion mapping of the niiA niaD gene region of Aspergillus nidulans. Genet Res. 1979 Aug;34(1):19–32. doi: 10.1017/s001667230001925x. [DOI] [PubMed] [Google Scholar]